乳铁蛋白对神经病理性痛大鼠脊髓小胶质细胞活化的影响研究
2017-01-16宗川曰
王 军,薛 红,宗川曰,宗 毅
·基础医学· ·论著·
乳铁蛋白对神经病理性痛大鼠脊髓小胶质细胞活化的影响研究
王 军,薛 红,宗川曰,宗 毅
目的 探讨乳铁蛋白对神经病理性痛大鼠脊髓背角小胶质细胞活化的影响。方法 雄性SD大鼠32只,体质量200~220 g,按照数字表法随机分为4组(对照组、神经病理性痛组、米诺环素组、乳铁蛋白组),每组8只。对照组大鼠仅分离坐骨神经,不结扎,肌鞘内注射生理盐水8 μl;其余3组大鼠采用结扎坐骨神经的方法制备神经病理性痛模型。神经病理性痛组鞘内仅注射生理盐水8 μl;乳铁蛋白组鞘内注射乳铁蛋白100 μg,米诺环素组鞘内注射米诺环素(小胶质细胞特异性活化抑制剂)100 μg。给药后每隔30 min以热刺激法测试大鼠热缩爪潜伏期,共180 min,测量7次后,常规处死大鼠取脊髓背角,采用免疫荧光法检测小胶质细胞特异性标记物Iba-1的表达,并进行统计学分析。结果 与对照组比较,神经病理性痛组缩爪潜伏期明显延长,差异有统计学意义(P<0.05);与神经病理性痛组相比,米诺环素组和乳铁蛋白组缩爪潜伏期延长,差异有统计学意义(P<0.05);而米诺环素组与乳铁蛋白组爪潜伏期比较差异无统计学意义(P>0.05),可间接证明乳铁蛋白可通过抑制脊髓小胶质细胞活化减轻大鼠神经病理性痛。与对照组比较,神经病理性痛组、米诺环素组和乳铁蛋白组脊髓背角Iba-1表达明显减少,差异有统计学意义(P<0.05);与神经病理性痛组相比,米诺环素组和乳铁蛋白组脊髓背角Iba-1表达也明显减少,差异有统计学意义(P<0.05);而米诺环素组与乳铁蛋白组缩爪潜伏期比较,差异无统计学意义(P>0.05),可直接证明乳铁蛋白可通过抑制脊髓小胶质细胞活化减轻大鼠神经病理性痛。结论 乳铁蛋白可通过抑制大鼠脊髓小胶质细胞活化减轻神经病理性痛,对临床麻醉镇痛过程有一定的指导价值。
乳铁蛋白;神经病理性痛;小胶质细胞
乳铁蛋白广泛存在于哺乳动物的体液中,具有抗炎、抗肿瘤及免疫调节等多种作用[1]。有研究[2-3]显示,乳铁蛋白对神经病理性痛大鼠的镇痛作用与大鼠脊髓背角一氧化氮(NO)-环GMP依赖性蛋白激酶(PKG)信号传导通路相关,但具体的机制仍不清楚。NO存在于神经元及神经胶质细胞中,且与小胶质细胞的活化相关。本研究拟探讨乳铁蛋白对神经病理性痛模型大鼠脊髓小胶质细胞活化的影响,为研究乳铁蛋白的镇痛机制提供实验依据。现报道如下。
1 材料与方法
1.1 材料 米诺环素(小胶质细胞特异性活化抑制剂)、牛乳铁蛋白(美国Sigma公司),兔抗Iba-1抗体(日本Wako公司),FITC标志的羊抗兔抗体(美国Cruz公司);热刺激仪7370(意大利Basile公司),荧光定量PCR仪(美国ABI公司),凝胶成像系统(美国Image公司)。冰冻切片机、激光共聚焦显微镜(中国Leica分公司)。
1.2 动物选择及分组 健康雄性SD大鼠32只,体质量200~220 g,由徐州医学院动物中心提供,动物证编号:20151123291。单笼饲养,饲养室温度为20~26℃,光照时间8:00-22:00,大鼠自由进食及饮水。按照数字表法随机分为4组,每组8只,对照组大鼠仅分离坐骨神经,不结扎,肌鞘内注射生理盐水8 μl,其余3组大鼠采用结扎坐骨神经的方法制备神经病理性痛大鼠模型。神经病理性痛组鞘内仅注射生理盐水8 μl;乳铁蛋白组肌鞘内注射乳铁蛋白100 μg,米诺环素组鞘内注射米诺环素100 μg。给药后每隔30 min以热刺激法测试大鼠热缩爪潜伏期,共180 min,测量7次后,处死大鼠取脊髓背角,采用免疫荧光法检测大鼠脊髓背角小胶质细胞标记物Iba-1的表达。
1.3 神经病理性大鼠模型的制备 大鼠神经病理性痛模型的制备参照文献[4]的方法。腹腔注射戊巴比妥钠(50 mg/kg)麻醉大鼠后,常规消毒左后肢,切开皮肤、皮下组织,钝性分离肌肉,于股骨后暴露坐骨神经主干,光学显微镜下用4-0铬制肠线松扎5处,间隔1 mm,结扎线松紧度以引起大鼠腿部轻微抽搐但不影响坐骨神经主干神经外膜的血运为度,然后逐层依次缝合,依照参照文献[4]介绍的方法行鞘内置管。
1.4 大鼠缩爪潜伏期的测定 参照文献[5]介绍的缩爪潜伏期测定方法,将SD大鼠放置于热测试仪玻璃表面,测试前让大鼠微适应25 min后,移动玻璃下方的热刺激源(强光柱),采用微电子计时器记录大鼠后爪光源聚焦至由于疼痛而回缩爪的时间,即为一个缩爪潜伏期。整个实验过程中,光柱照射强度不变,并调整后爪回缩潜伏期为10 s。为避免大鼠足底被烫伤,热刺激源刺激最长时间为30 s。分别在给药后0、30、60、90、120、150、180 min时记录大鼠缩爪潜伏期,每一时间点重复测定3次,取平均值。
1.5 大鼠脊髓背角Iba-1表达水平的测定 在最后1次大鼠缩爪潜伏期测定结束后,腹腔注射戊巴比妥钠(50 mg/kg)麻醉大鼠后,从主动脉依次灌注多聚甲醛和生理盐水,取大鼠脊髓L4-5置于多聚甲醛溶液中固定3.5 h,转入25%蔗糖溶液中,4℃恒温过夜。待组织沉底后,沉淀,连续冰冻切片,片厚25 μm,浸泡在0.01 mol/L PB液中。PBS液清洗3次后,在5%小牛血清中加入0.3% TritonX-100,16℃孵育35 min。加入兔抗Iba-1抗体(一抗,1∶100),4℃孵育24~48 h,PBS液再冲洗3次后,滴加的羊抗兔IgG(FITC标记,二抗,1:100),16℃孵育2.0 h,PBS液再冲洗3次。三酰甘油与PBS(1:1)混合液封片,对照组用PBS液代替一、二抗。每只大鼠取L4-5节段脊髓切片3张,荧光染色后,采用Leica激光共聚焦显微镜进行观察,并测量切片中部每0.4 mm×0.4 mm范围的像素密度,重复测定3次,取平均值。采用Image Pro P 5.0图像统计软件,计算切片的荧光光密度,转化成定量数据后表达Iba-1在大鼠脊髓背角小胶质细胞的活性。
1.6 统计学处理 采用SPSS 15.0统计软件进行数据处理,计量资料以均数±标准差(x±s)表示,组间均数比较采用t检验。以P<0.05表示为差异有统计学意义。
2 结果
2.1 各组大鼠热刺激缩爪潜伏期的比较 与对照组比较,神经病理性痛组缩爪潜伏期明显延长,差异有统计学意义(P<0.05);与神经病理性痛组相比,米诺环素组和乳铁蛋白组部分时间点缩爪潜伏期明显延长,差异有统计学意义(P<0.05);米诺环素组与乳铁蛋白组缩爪潜伏期比较,差异无统计学意义(P>0.05)。见表1。
表1 各组大鼠热刺激缩爪潜伏期的比较(s,x±s)
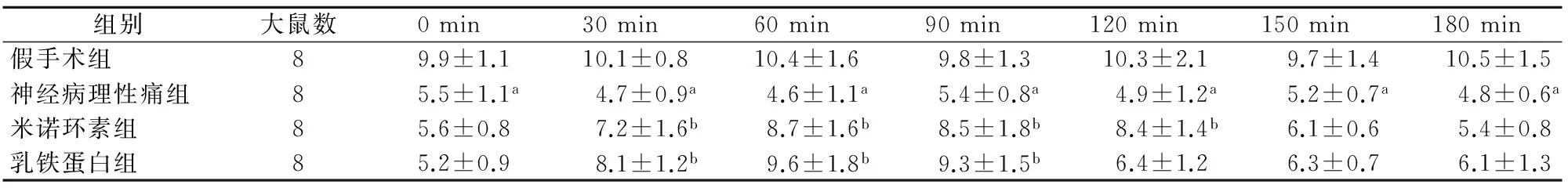
组别大鼠数0min30min60min90min120min150min180min假手术组89.9±1.110.1±0.810.4±1.69.8±1.310.3±2.19.7±1.410.5±1.5神经病理性痛组85.5±1.1a4.7±0.9a4.6±1.1a5.4±0.8a4.9±1.2a5.2±0.7a4.8±0.6a米诺环素组85.6±0.87.2±1.6b8.7±1.6b8.5±1.8b8.4±1.4b6.1±0.65.4±0.8乳铁蛋白组85.2±0.98.1±1.2b9.6±1.8b9.3±1.5b6.4±1.26.3±0.76.1±1.3
注:与假手术组比较aP<0.05;与神经病理性痛组比较bP<0.05化。本研究结果显示,与对照组比较,神经病理性痛组缩爪潜伏期明显延长,差异有统计学意义;与神经病理性痛组相比,米诺环素组和乳铁蛋白组缩爪潜伏期延长,而米诺环素组与乳铁蛋白组爪潜伏期延长比较差异无统计学意义,可间接证明乳铁蛋白可通过抑制脊髓小胶质细胞活化减轻大鼠神经病理性痛。与对照组比较,神经病理性痛组、米诺环素组和乳铁蛋白组脊髓背角Iba-1表达明显减少,差异有统计学意义;与神经病理性痛组相比,米诺环素组和乳铁蛋白组脊髓背角Iba-1表达也明显减少,差异有统计学意义,而米诺环素组与乳铁蛋白组缩爪潜伏期比较,差异无统计学意义,提示乳铁蛋白可通过抑制脊髓小胶质细胞活化减轻大鼠神经病理性痛。
综上所述,乳铁蛋白可通过抑制NO的合成,减少炎性介质,进一步减少脊髓背角小胶质细胞的活化水平,减轻大鼠神经病理性痛,可对临床麻醉镇痛有一定的借鉴意义。
[1] Brock JH. The physiology of lactoferrin[J]. Bioch Biol Cellul, 2002, 80(1): 1-6. DOI:10.1139/001-212.
[2] Wang J, Zhang LC, Lv YW, et al. Involvement of the nitric oxide-cyclic GMP-protein kinase G-K+ channel pathway in the antihyperalgesic effects of bovine lactoferrin in a model of neuropathic pain[J]. Brain Res, 2008, 1209: 1-7. DOI:10.1016/j.brainres.2008.03.004.
[3] 王军,居从金,颜学军,等. 乳铁蛋白对神经病理性痛大鼠脊髓背角PKG活性的影响[J]. 中华麻醉学杂志, 2010, 30(12): 1456-1458. DOI:10.3760/cma.j.issn.0254-1416.2010.12.015.
[4] Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man[J]. Pain, 1988, 33(1): 87-107. DOI:10.1016/0304-3959(88)90209-6.
[5] Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space[J]. Physiol Behav, 1976, 17(6): 1031-1036. DOI:10.1016/0031-9384(76)90029-9.
[6] Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia[J]. Pain, 1988, 32(1): 77-88. DOI:10.1016/0304-3959(88)90026-7.
[7] Mika J, Obara I, Przewlocka B. The role of nociceptin and dynorphin in chronic pain: implications of neuro-glial interaction[J]. Neuropeptides, 2011, 45(4): 247-261. DOI:10.1016/j.npep.2011.03.002.
[8] Kuboyama K, Tsuda M, Tsutsui M, et al. Reduced spinal microglial activation and neuropathic pain after nerve injury in mice lacking all three nitric oxide synthases[J]. Molecul Pain, 2011, 7(1): 50. DOI:10.1186/1744-8069-7-50.
(本文编辑:王映红)
2.2 各组大鼠脊髓背角Iba-1表达水平比较 与对照组比较,神经病理性痛组、米诺环素组和乳铁蛋白组脊髓背角Iba-1表达明显减少,差异有统计学意义(P<0.05);与神经病理性痛组比较,米诺环素组和乳铁蛋白组脊髓背角Iba-1表达也明显减少,差异有统计学意义(P<0.05)。而米诺环素组与乳铁蛋白组Iba-1得表达量比较,差异无统计学意义(P>0.05)。见图1、表2。
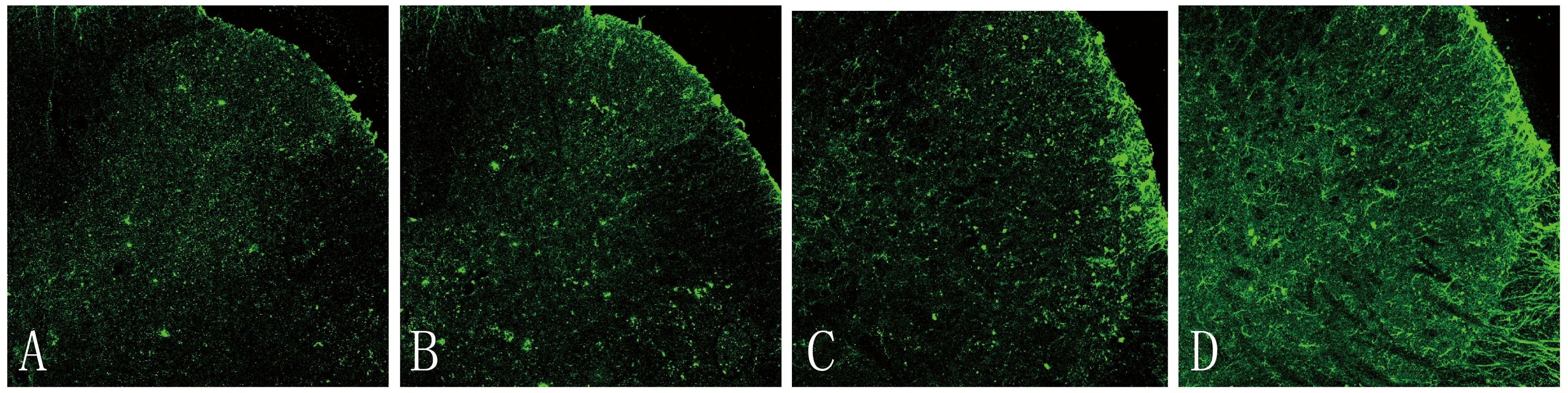
注:A为对照组,B为神经病理性痛组,C为米诺环素组,D为乳铁蛋白组图1 各组大鼠脊髓背角Iba-1表达的荧光图(荧光染色×100)
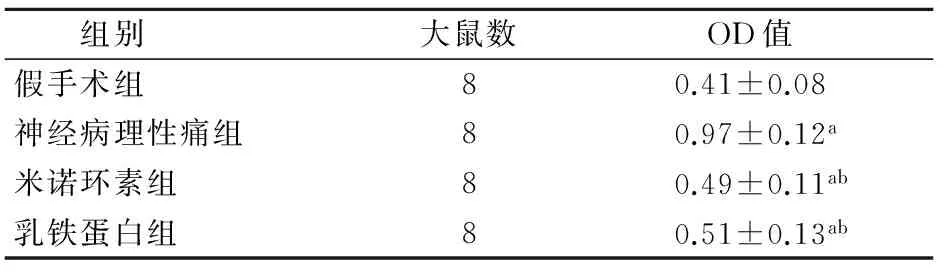
组别大鼠数OD值假手术组80.41±0.08神经病理性痛组80.97±0.12a米诺环素组80.49±0.11ab乳铁蛋白组80.51±0.13ab
注:与假手术组比较aP<0.05;与神经病理性痛组比较bP<0.05
3 讨论
乳铁蛋白的镇痛效果与神经病理性痛大鼠脊髓背角环GMP依赖性PKG信号传导导通路有关,但确切作用机制有待于进一步研究。NO广泛存在于神经元及神经小胶质细胞中,且与脊髓背角小胶质细胞活化密切相关。本实验参照文献[5],采用结扎坐骨神经的方法制备大鼠神经病理性痛模型已经广泛应用于疼痛学实验,此模型作用效果与人类周围神经损伤诱发的神经病理性疼痛的临床症状和行为学相似,因此,在实验中该方法制备大鼠神经病理性痛模型具有较好的可行性。同时本研究结果显示,与对照组比较,神经病理性痛组结扎坐骨神经后PWL明显缩短,提示大鼠神经病理性痛模型制备成功。
神经元由神经胶质细胞对提供营养作用和支撑其结构,伤害性刺激信号产生后,中枢神经系统产生部分免疫因子参与多信号的传递,免疫因子的生成和释放导致神经病理性痛的形成,如脊髓背角小胶质细胞的活化可引起炎症,可进一步对神经病理性痛进行调节,乳铁蛋白与神经病理性痛也密切相关[5-6]。多项研究[2-3,7-8]表明,NO合酶缺失的大鼠神经损伤后脊髓背角小胶质细胞活化明显减少,神经病理性痛大鼠临床症状也相应减轻,从而抑制NO的合成,进一步减少脊髓背角小胶质细胞的活化,从而减轻神经病理性痛。而乳铁蛋白在神经病理性痛大鼠的镇痛作用机制与脊髓背角NO-环GMP依赖性PKG信号传导通路密切相关,因此,可以推测,在神经病理性痛大鼠的镇痛作用中,乳铁蛋白通过与NO相关信号传导通路抑制脊髓背角小胶质细胞活
Effects of lactoferrin on microglial activation in the spinal cord in the rat model of neuropathic pain
Wang Jun, Xue Hong, Zong Chuanyue, Zong Yi
(DepartmentofAnesthesiology,SecondPeople′sHospitalofHuaianCity,Huaian223002,China)
Objective To investigate the effects of lactoferrin on microglial activation in the spinal cord in the rat model of neuropathic pain (NP).MethodsThirty-two male SD rats with the weight of 200-220 g were randomly divided into 4 groups, i.e. the control group, the neuropathic pain group, the minocycline group and the lactoferrin group, each consisting of 8 animals. The sciatic nerve was only exposed but not ligated in the animals of the control group, and 8 μl of normal saline were injected intrathecally. The neuropathic pain model was produced by placing loosely constrictive ligatures around the common sciatic nerve in the animals of the other groups. Eight μl of normal saline were injected intrathecally in the animals of the neuropathic pain group. One hundred μl of lactoferrin were given intrathecally in the animals of the lactoferrin group, while the same amount of minocycline was also given intrathecally in the animals of the minocycline group. Following medication, the paw withdrawal latency (PWL) was detected every 30 minutes by using thermal nociceptive stimulus with a total of 180 minutes. After detections for 7 times, the rats were sacrificed for the collection of spinal dorsal horn. The expression of Iba-1 was determined by immunofluorescence, and then statistical analyses were made accordingly.ResultsPWL for the animals of the neuropathic pain group was obviously prolonged, as compared with that of the control group, and statistical significance could be seen, when comparisons were made between them(P<0.05). PWL for the animals of both the minocycline group and the lactoferrin group was prolonged, as compared with that of the neuropathic pain group, also with statistical significance(P<0.05). No statistical significance could be noted in PWL for the animals of the minocycline group and the lactoferrin group, when comparisons were made between them(P>0.05). This was an indirect indication that lactoferrin could alleviate neuropathic pain through the inhibition of the activation of spinal microglia. The expression levels of Iba-1 in the animals of the minocycline group and the lactoferrin group, as well as the neuropathic pain group, were significantly decreased, and statistical significance could be seen, when it was compared with that of the control group(P<0.05). The expression levels of Iba-1 in the animals of the minocycline group and the lactoferrin group were also significantly decreased, as compared with that of the neuropathic pain group, also with statistical significance(P<0.05). No statistical significance was shown in PWL, when comparisons were made between the minocycline group and the lactoferrin group(P>0.05). This was also an indirect indication that lactoferrin could alleviate neuropathic pain through the inhibition of the activation of spinal microglia.ConclusionLactoferrin could alleviate neuropathic pain through the inhibition of the activation of spinal microglia, which had certain medical value in the clinical practice of anesthesia.
Lactoferrin; Neuropathic pain; Microglia
223002 江苏 淮安,淮安市第二人民医院麻醉科
R614
A
10.3969/j.issn.1009-0754.2016.06.007
2016-03-22)
