病毒穿越血脑屏障的两种方式与可能机制
2017-01-05朱耐伟朱勇喆戚中田
朱耐伟,朱勇喆,戚中田
第二军医大学微生物学教研室,上海市医学生物防护重点实验室, 上海 200433
·综述·
病毒穿越血脑屏障的两种方式与可能机制
朱耐伟,朱勇喆,戚中田
第二军医大学微生物学教研室,上海市医学生物防护重点实验室, 上海 200433
血脑屏障是维持中枢神经系统内环境稳定的重要结构,限制血液中大多数病原体的入侵;但有些病毒可穿越血脑屏障入侵中枢神经系统,导致神经功能障碍及炎症性疾病。目前认为,病毒可通过细胞和细胞间隙两种方式穿越血脑屏障,前者为直接感染脑微血管内皮细胞和跨细胞途径,后者为破坏内皮细胞间紧密连接及“特洛伊木马”途径。本文就近年来病毒穿越血脑屏障的途径和机制进行综述。
病毒;血脑屏障;机制
血脑屏障(blood-brain barrier,BBB)介于血液与脑组织之间,是维持中枢神经系统内环境稳定的重要组织结构,为抵御病原体入侵的天然屏障。然而,有些病毒可通过不同的途径和机制穿越血脑屏障入侵脑部,破坏神经细胞与脑内微环境,引起中枢神经系统感染。这些病毒包括人类免疫缺陷病毒(human immunodeficiency virus,HIV)、麻疹病毒(measles virus)、脊髓灰质炎病毒(poliovirus,PV)、乙型脑炎病毒(Japanese encephalitis virus,JEV)和西尼罗病毒(West Nile virus,WNV)[1],是全球脑炎发病和致死的重要原因之一[2]。本文就近年来病毒穿越血脑屏障的方式和机制进行综述。
1 血脑屏障的结构特点
1885年,Enrlich等[3]发现染料并不能经静脉注射进入中枢神经系统。1909年,Goldmann等[3]认为脑内存在一种结构性屏障。直到20世纪中期,Reese和Karnovsky[4]在电镜下发现了血脑屏障的超微结构特征:由脑微血管内皮细胞(brain microvascular endothelial cell,BMEC)及其细胞间的紧密连接(tight junction)、完整的基膜(basement membrane)、周细胞(pericyte)、小胶质细胞(microglial cell)及星形胶质细胞脚板(astrocytic endfeet)围成的神经胶质膜构成[3-4](图1)。其中BMEC是血脑屏障的主要结构,其顶端面与脑微血管中的血流接触,基底面则与脑内相通,BMEC间的紧密连接分子限制细胞旁路途径的物质转运。此外,周细胞调控血管形成及血管的完整性;小胶质细胞在病原体刺激下可释放细胞因子,清除病原体;星形胶质细胞可分泌可溶性因子促进紧密连接和屏障的完整性[5-7]。这些结构使病毒不能随意侵入中枢神经系统,引起脑内感染。
图1 血脑屏障的结构特点
Fig.1 Structural characteristics of the blood-brain barrier
2 病毒穿越血脑屏障的途径
病毒穿越血脑屏障是中枢神经系统感染发生的前提。当病毒穿过血脑屏障后,病毒表面蛋白与脑内细胞表面蛋白或受体相互作用,通过炎症因子和病毒自身蛋白导致神经细胞变性坏死,引起意识障碍、惊厥等脑炎症状[8]。血脑屏障作为天然固有屏障,极大限制了各种病原体入脑的概率[9]。目前认为病毒可通过以下两种方式穿越血脑屏障。
2.1 病毒通过细胞穿越血脑屏障
2.1.1 直接感染BMEC BMEC是血脑屏障的主要结构,感染BMEC是病毒进入中枢神经系统的必经之路之一(图2A);小胶质细胞与神经胶质细胞作为血脑屏障的组成,病毒也可通过感染这些细胞进入中枢神经系统。Chapagain等[10]发现人多瘤病毒JC(human polyomavirus JC,JCV)可感染人脑微血管内皮细胞(human brain microvascular endothelial cell,HBMEC),感染3 d后可在胞内检测到病毒载量;而感染10 d后则可通过激光共聚焦显微镜观察到HBMEC表达JCV早期蛋白标记T抗原。释放的子代病毒具有感染性并可在敏感细胞系中有效复制,因此推测JCV在体内可通过感染HBMEC来穿越血脑屏障,进一步感染神经细胞,引起进行性多灶性白质脑病。Afonso等[11]发现人嗜T细胞病毒1型(human T lymphotropic virus type 1,HTLV-1)可在HBMEC内复制并释放子代病毒,提示内皮细胞感染可能使子代病毒进入中枢神经系统,造成神经元变性与坏死,引起HTLV-1相关脊髓病和热带痉挛性瘫痪病。
Coyne等[12]发现PV可通过结合PV受体,继而激活酪氨酸激酶和RhoA GTP酶,经内吞方式侵入HBMEC后复制增殖,提示血源性病毒可能通过感染HBMEC来穿越血脑屏障从而进入中枢神经系统。
Casiraghi等[13]发现EB病毒(Epstein-Barr virus, EBV)可感染HBMEC,使细胞因子及趋化因子升高,从而诱导血脑屏障破坏,导致自身反应性淋巴细胞入脑,损伤神经元,引起多发性硬化症。
Lai等[14]发现HBMEC对JEV易感,在细胞内仅能少量复制,但不会引起细胞死亡和破坏屏障的完整性。Verma等[15]发现WNV可感染HBMEC并在其内复制,但未观察到细胞死亡。
Mankowski等[16]通过对猴免疫缺陷病毒(simian immunodeficiency virus,SIV)感染的猴脑组织进行免疫组化分析,在血管内皮处检测到病毒RNA,并通过电镜在内皮细胞中观察到病毒颗粒,提示SIV可感染BMEC并穿越血脑屏障。HIV的多种病毒蛋白可与HBMEC相互作用,从而实现对血脑屏障完整性的破坏[17]。例如,包膜蛋白gp120在血管内皮细胞处可增强脂质的过氧化作用,上调白细胞介素1β(interleukin 1β,IL-1β)和诱导型一氧化氮合酶(inducible nitric oxide synthase,iNOS),从而破坏血脑屏障[18]。病毒反式激活因子(trans-activating factor, Tat)含有使其顺利通过血脑屏障的跨膜结构域[19],且可通过CD40配体依赖途径增加血脑屏障渗透性[20];Tat还可破坏血脑屏障处的周细胞,破坏血脑屏障的完整性[21]。负调节因子(negative regulatory factor, Nef)则可通过促凋亡基因如含半胱氨酸的天冬氨酸蛋白水解酶6(cysteinyl aspartate specific proteinase 6,caspase-6)、caspase-8、caspase-9及caspase-10,诱导HBMEC凋亡,以改变血脑屏障的完整性,有利于HIV入侵中枢神经系统[18]。
2.1.2 跨细胞途径 血脑屏障在正常情况下是非渗透性的,但其存在多种特殊的跨细胞转运机制[22],如内吞与转胞吞机制,为病毒入侵中枢神经系统提供了可能途径(图2B)。HTLV-1颗粒可经内吞作用进入内皮细胞,并从基底面释放出来[23];HIV可通过BMEC的巨胞饮作用跨越血脑屏障[24-25];JEV可通过胞内囊泡在脑内皮细胞和周细胞中进行转运[26];而WNV的病毒样颗粒(virus-like particle,VLP)则可从内皮细胞的顶端转移到基底部[27],Verma等[15]应用体外血脑屏障模型(由体外培养的单层HBMEC构成),发现WNV在不破坏血脑屏障完整性的前提下可穿越血脑屏障,提示其可能通过跨细胞途径侵入中枢神经系统。然而,上述结果均来自体外实验,而BMEC在体内与体外环境中有明显区别,该机制仍需体内实验来证实。
2.2 病毒通过细胞间隙穿越血脑屏障
2.2.1 破坏内皮细胞间紧密连接 BMEC间的紧密连接是血脑屏障完整性的结构与功能基础,其调控物质及限制病原体通过细胞旁路途径进入中枢神经系统[28]。紧密连接由多种跨膜蛋白组成,如闭合蛋白(occludin)、封闭蛋白(claudin)及连接黏附分子等,这些跨膜蛋白又通过紧密连接分子(zonula occluden 1,ZO-1)等蛋白复合体固定在内皮细胞上[29-30]。某些病毒感染时可导致紧密连接破坏,血脑屏障通透性增加,病毒穿越血脑屏障引起中枢神经系统感染(图2C)。
鼠腺病毒1型(mouse adenovirus type 1,MAV-1)感染HBMEC可引起明显的组织病理学变化,增加血脑屏障通透性。虽然MAV-1感染会引起小鼠脑组织炎性细胞浸润,但其对血脑屏障的破坏并不依赖炎症反应,因为炎症反应减轻后血脑屏障的破坏程度并未降低[31-32]。Gralinski等[33]认为,MAV-1感染HBMEC后可能激发了宿主的天然免疫反应,并通过基质金属蛋白酶(matrix metalloproteinase,MMP)降解了紧密连接蛋白,导致细胞紧密连接处的occludin及claudin-5降低,增加了血脑屏障的渗透性。
Chang等[34]发现JEV感染星形胶质细胞后可引起血管内皮生长因子(vascular endothelial growth factor,VEGF)、IL-6及MMP-2和MMP-9释放,通过内皮细胞的Jak2/STAT3信号途径及泛素蛋白酶体导致ZO-1和claudin-5降解,提示星形胶质细胞的感染可能破坏了血脑屏障的完整性。
WNV直接感染HBMEC并不会引起细胞间紧密连接蛋白发生变化[15];但将感染WNV的星形胶质细胞上清液加入体外血脑屏障模型中,可引起HBMEC间紧密连接蛋白降解,从而破坏血脑屏障的完整性[35]。进一步的分子机制研究发现,WNV感染后,星形胶质细胞分泌的MMP-1和MMP-3可直接消化紧密连接蛋白,或通过激活MMP-9对紧密连接蛋白造成破坏,使WNV更易进入脑部,感染中枢神经系统[35-36]。
HTLV-1可诱导ZO-1的重排,改变紧密连接的分布。进一步研究发现,HTLV-1的蛋白Tax可与ZO-1的PDZ结构域(PZD95、Discs-large、ZO-1三者结构域的简称)相互作用[37-38]。此外,HTLV-1感染的淋巴细胞还可通过释放IL-1α和肿瘤坏死因子α(tumor necrosis factor α,TNF-α)改变紧密连接的结构,以增加细胞旁路的通透性,使病毒穿越血脑屏障[39-40]。
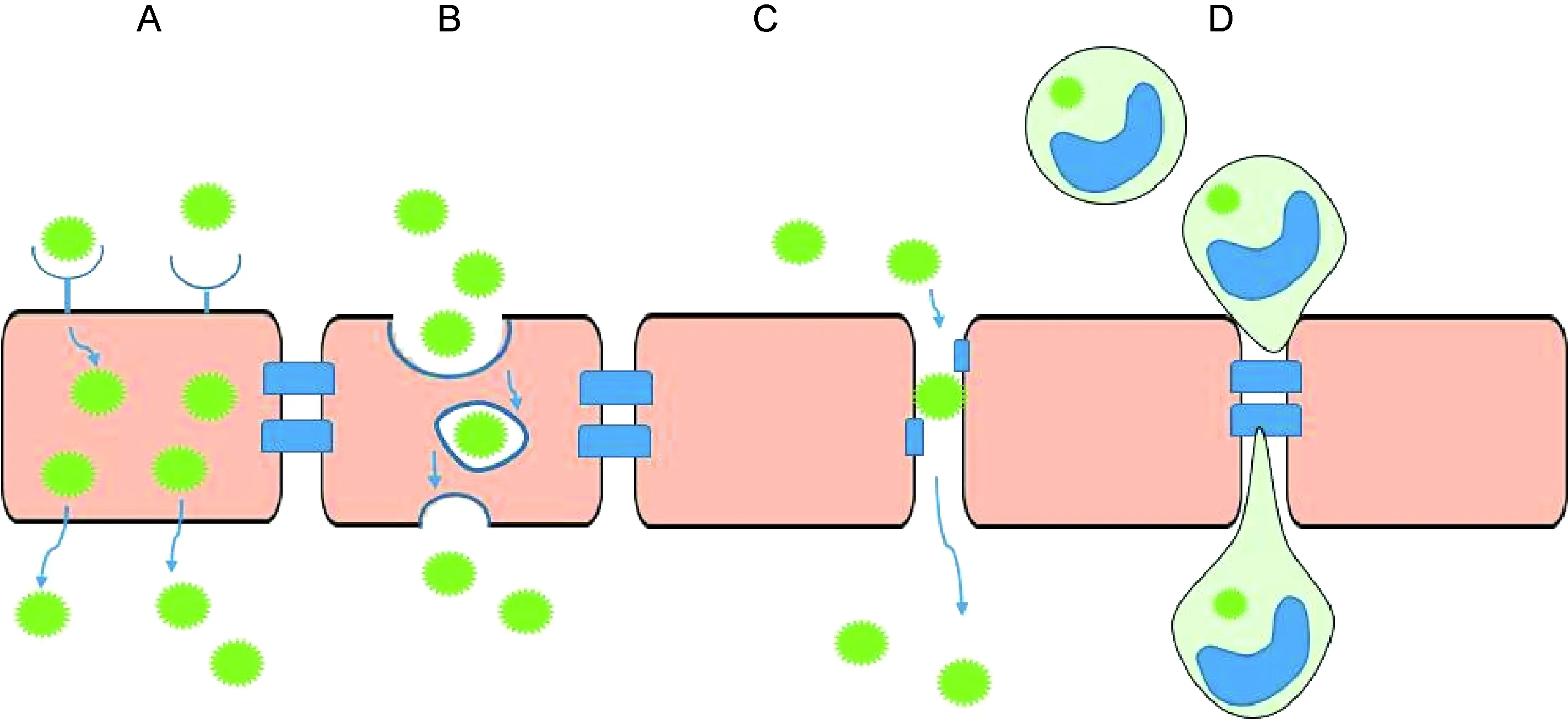
A: Direct infection of the brain microvascular endothelial cells. B: Transcellular pathway. C: Breaching of the tight junction between endothelial cells. D: The “Trojan horse” pathway.
图2 病毒穿越血脑屏障的途径
Fig.2 Pathways of virus crossing the blood-brain barrier
在体外血脑屏障模型中,感染HIV的单核细胞可引起MMP-2和MMP-9增加,以及ZO-1、occludin、claudin-5下降,导致血脑屏障渗透性增加。Dallasta等[41]也观察到类似现象,HIV脑炎患者的皮质下白质区、灰质区及基底节均有ZO-1蛋白的缺失。此外,HIV蛋白可通过多种途径影响紧密连接,如包膜蛋白gp120可通过酪氨酸磷酸化激活局部黏着斑激酶(focal adhesion kinase,FAK)途径,从而下调ZO-1、ZO-2及occludin等[42-43];同时gp120可增加MMP-2和MMP-9表达,继而破坏claudin-5形成[44]。病毒蛋白R(viral protein R,Vpr)则可通过感染星形胶质细胞,分泌趋化因子和炎症因子来破坏血脑屏障间的紧密连接,增加血脑屏障的渗透性[18]。Tat一方面可激活BMEC的氧化还原通路和转录因子,并通过刺激蛋白激酶C(protein kinase C,PKC)和Ras/丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)途径破坏细胞内氧化还原反应,导致紧密连接蛋白变性和失活[45];另一方面可通过ras同源基因家族成员A (ras homolog gene family,member A,RhoA)/Rho相关卷曲螺旋蛋白激酶(Rho-associated coiled-coil containing protein kinase,ROCK)途径或上调MMP-9表达来下调或降解occludin,从而破坏紧密连接蛋白的完整性[18]。此外,Tat还可引起紧密连接蛋白的磷酸化,破坏紧密连接蛋白复合体的聚合,增加内皮细胞的渗透性[46]。Raymond等[47]发现小胶质细胞来源的Nef+外泌体可明显下调体外血脑屏障模型中ZO-1的表达,提示来自小胶质细胞的感染HIV的外泌体可能参与破坏血脑屏障的完整性和渗透性。
血脑屏障处细胞因子种类与其效应之间的平衡是复杂的。多数情况下,病毒感染BMEC可增加促炎症细胞因子如TNF-α、IL-1β、IL-6、λ干扰素(interferon λ,IFN-λ)等释放,破坏BMEC间的紧密连接,从而破坏血脑屏障的完整性。星形胶质细胞、周细胞、BMEC及白细胞等均可产生上述细胞因子。不同的细胞因子其作用机制各不相同,对血脑屏障的作用也不同。MMP在病毒感染时可直接降解紧密连接,破坏血脑屏障,加速病毒感染中枢神经系统[3];而Ⅰ型干扰素(如IFN-β)和Ⅲ型干扰素(IFN-λ)可稳定紧密连接,同时激活BMEC中的TAM(Tyro3、Axl和 Mertk三者的统称)受体以协同干扰素的作用,从而增强紧密连接的稳定性,维持血脑屏障的完整性[3,48]。
2.2.2 “特洛伊木马”途径 病毒可通过“特洛伊木马”途径入侵中枢神经系统,即携带病毒的白细胞穿越血脑屏障(图2D)。单核细胞或巨噬细胞的感染被认为是免疫缺陷病毒穿越血脑屏障的主要机制,包括SIV和HIV[49-50]。SIV或HIV感染时,大量CD16+单核细胞在外周扩增,该单核细胞与趋化因子结合后能促进HIV或SIV感染的单核细胞与BMEC接触与黏附。Clay等[50]在追踪荧光素标记的单核细胞时发现,感染SIV的单核细胞快速定位于脑血管周围和脉络丛,提示其穿越了中枢神经系统屏障。脑内和脑脊液内检测到SIV出现的时间与单核细胞入侵中枢神经系统的时间相一致,提示单核细胞介导了SIV的神经感染。Alexaki等[49]证明脑组织中CD16+单核细胞出现在HIV感染部位,进一步研究发现外周来源的CD16+单核细胞比其他来源的单核细胞更易感染HIV。单核细胞对中枢神经系统的入侵可能由血管周围巨噬细胞的更替和再分布引起,也可能是CC趋化因子配体2(CC chemokine ligand 2,CCL2)、MMP和促炎症细胞因子升高而使血管渗透性增加导致[51-52]。此外,Tat可诱发内皮细胞上的黏附分子及星形胶质细胞分泌趋化因子,促进单核细胞迁移至中枢神经系统[53]。
除免疫缺陷病毒外,JCV也可通过挟持白细胞加速入侵中枢神经系统。JCV在体外模型中可从B细胞转移到胶质细胞,后者是体内感染时的主要靶细胞;同时,体内感染JCV时,在外周血B细胞中可检测到JCV[10,54]。但B细胞是否在体内作为“特洛伊木马”用于病毒的入侵和扩散,仍未十分明确。
Garcia-Tapia等[55]在体内实验中发现,WNV可通过朗格罕斯细胞从感染部位转移到引流淋巴结,进一步感染单核细胞和部分CD4+淋巴细胞。此外,淋巴细胞趋化因子和单核细胞趋化因子可将外周单核细胞聚集到中枢神经系统的血管周围区域。这些聚集的单核细胞可产生促炎症因子,破坏血脑屏障的完整性,导致更多白细胞进入中枢神经系统。Dai等[56]和Wang等[57]虽然均在脑内检测到WNV抗原与进入中枢神经系统的白细胞共定位,但尚未有证据表明外周感染WNV的白细胞可进入中枢神经系统。
虽然白细胞可抵御病毒感染,但其也可扮演“特洛伊木马”将病毒携带到机体其他部位。尽管血脑屏障可精密调控免疫细胞进入中枢神经系统,但病毒仍能在免疫监测下利用血源性途径通过感染的白细胞入侵中枢神经系统。
3 病毒穿越血脑屏障的机制
病毒穿越血脑屏障的机制尚未明确,但与病毒结构蛋白和细胞受体的相互作用、受体介导的病毒内吞即病毒-细胞膜融合等过程相关[58]。JEV编码的包膜蛋白E位于病毒颗粒表面,是JEV致病与免疫的主要分子。Luca等[59]通过结构生物学方法解析E蛋白的晶体结构,发现包膜蛋白含有3个结构域,其中结构域Ⅱ介导JEV与细胞膜融合,结构域Ⅲ识别细胞表面的受体,结构域Ⅰ负责连接结构域Ⅱ和Ⅲ[60]。Li等[61]发现结构域Ⅲ中的loop多肽(loop3)可抑制JEV与细胞的结合。Yun等[62]将JEV疫苗株SA-14-14-2包膜蛋白的244位甘氨酸替换为谷氨酸后,可使小鼠产生严重的神经系统症状,提示包膜蛋白对JEV的嗜神经性具有重要影响。Liu等[58]发现包膜蛋白144位组氨酸、258位谷氨酰胺、319位组氨酸和410位苏氨酸均参与病毒与细胞膜的融合,为JEV入侵阶段的关键位点。Ferguson等[63]发现塞姆利基森林脑炎病毒(Semliki Forest virus,SFV)包膜蛋白E2中162位和247位氨基酸决定了其穿越血脑屏障的能力。因此,病毒包膜蛋白中某个或某几个关键氨基酸位点可能介导病毒穿越血脑屏障,但其机制仍需进一步研究。
4 结语
某些病毒可侵入中枢神经系统导致脑炎,而血脑屏障的存在极大限制了病毒入脑引起感染。虽然病毒穿越血脑屏障的机制尚不十分明确,但目前认为病毒可通过直接感染BMEC、破坏内皮细胞间紧密连接、“特洛伊木马”途径及跨细胞途径穿越血脑屏障。因此,病毒穿越血脑屏障进入中枢神经系统是多因素、多途径的过程。仍需通过体内和体外实验,阐明介导病毒穿越血脑屏障的细胞分子及关键病毒蛋白与氨基酸位点,深入研究穿越血脑屏障过程中病毒自身与细胞分子间的相互关系,为恢复血脑屏障的完整性及阻止病毒入侵中枢神经系统提供新的理论依据和干预策略。
[1] Swanson PA, McGavern DB. Viral diseases of the central nervous system [J]. Curr Opin Virol, 2015, 11: 44-54.
[2] Stahl JP, Mailles A. What is new about epidemiology of acute infectious encephalitis? [J]. Curr Opin Neurol, 2014, 27(3): 337-341.
[3] Miner JJ, Diamond MS. Mechanisms of restriction of viral neuroinvasion at the blood-brain barrier [J]. Curr Opin Immunol, 2016, 38: 18-23.
[4] Engelhardt B. Development of the blood-brain barrier [J]. Cell Tissue Res, 2003, 314(1): 119-129.
[5] Xia L, Ju T, Westmuckett A, An G, Ivanciu L, McDaniel JM, Lupu F, Cummings RD, McEver RP. Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans [J]. J Cell Biol, 2004, 164(3): 451-459.
[6] Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease [J]. Pharmacol Rev, 2005, 57(2): 173-185.
[7] Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of microglia in central nervous system infections [J]. Clin Microbiol Rev, 2004, 17(4): 942-964.
[8] Kovalevich J, Langford D. Neuronal toxicity in HIV CNS disease [J]. Future Virol, 2012, 7(7): 687-698.
[9] Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system [J]. Cell Host Microbe, 2013, 13(4): 379-393.
[10] Chapagain ML, Verma S, Mercier F, Yanagihara R, Nerurkar VR. Polyomavirus JC infects human brain microvascular endothelial cells independent of serotonin receptor 2A [J]. Virology, 2007, 364(1): 55-63.
[11] Afonso PV, Ozden S, Cumont MC, Seilhean D,Cartier L, Rezaie P, Mason S, Lambert S, Huerre M, Gessain A, Couraud PO, Pique C, Ceccaldi PE, Romero IA. Alteration of blood-brain barrier integrity by retroviral infection [J]. PLoS Pathog, 2008, 4(11): e1000205.
[12] Coyne CB, Kim KS, Bergelson JM. Poliovirus entry into human brain microvascular cells requires receptor-induced activation of SHP-2 [J]. EMBO J, 2007, 26(17): 4016-4028.
[13] Casiraghi C, Dorovini-Zis K, Horwitz MS. Epstein-Barr virus infection of human brain microvessel endothelial cells: a novel role in multiple sclerosis [J]. J Neuroimmunol, 2011, 230(1-2): 173-177.
[14] Lai CY, Ou YC, Chang CY, Pan HC, Chang CJ, Liao SL, Su HL, Chen CJ. Endothelial Japanese encephalitis virus infection enhances migration and adhesion of leukocytes to brain microvascular endothelia via MEK-dependent expression of ICAM1 and the CINC and RANTES chemokines [J]. J Neurochem, 2012, 123(2): 250-261.
[15] Verma S, Lo Y, Chapagain M, Lum S, Kumar M, Gurjav U, Luo H, Nakatsuka A, Nerurkar VR. West Nile virus infection modulates human brain microvascular endothelial cells tight junction proteins and cell adhesion molecules: transmigration across the in vitro blood-brain barrier [J]. Virology, 2009, 385(2): 425-433.
[16] Mankowski JL, Spelman JP, Ressetar HG, Strandberg JD, Laterra J, Carter DL, Clements JE, Zink MC. Neurovirulent simian immunodeficiency virus replicates productively in endothelial cells of the central nervous system in vivo and in vitro [J]. J Virol, 1994, 68(12): 8202-8208.
[17] Spindler KR, Hsu TH. Viral disruption of the blood brain barrier [J]. Trends Microbiol, 2012, 20(6): 282-290.
[18] Atluri VS, Hidalgo M, Samikkannu T, Kurapati KR, Jayant RD, Sagar V, Nair MP. Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update [J]. Front Cell Neurosci, 2015, 9: 212. doi: 10.3389/fncel.2015.00212.
[19] Cooper I, Sasson K, Teichberg VI, Schnaider-Beeri M, Fridkin M, Shechter Y. Peptide derived from HIV-1 Tat protein destabilizes a monolayer of endothelial cells in an in vitro model of the blood-brain barrier and allows permeation of high molecular weight proteins [J]. J Biol Chem, 2012, 287(53): 44676-44683.
[20] Davidson DC, Hirschman MP, Sun A, Singh MV, Kasischke K, Maggirwar SB. Excess soluble CD40L contributes to blood brain barrier permeability in vivo: implications for HIV-associated neurocognitive disorders [J]. PLoS One, 2012, 7(12): e51793. doi: 10.1371/journal.pone.0051793.
[21] Niu F, Yao H, Liao K, Buch S. HIV Tat 101-mediated loss of pericytes at the blood-brain barrier involves PDGF-BB [J]. Ther Targets Neurol Dis, 2015, 2(1): e471. doi: 10.14800/ttnd.471.
[22] Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier [J]. Neurobiol Dis, 2010, 37(1): 13-25.
[23] Romero IA, Prevost MC, Perret E, Adamson P, Greenwood J, Couraud PO, Ozden S. Interactions between brain endothelial cells and human T-cell leukemia virus type 1-infected lymphocytes: mechanisms of viral entry into the central nervous system [J]. J Virol, 2000, 74(13): 6021-6030.
[24] Maréchal V, Prevost MC, Petit C, Perret E, Heard JM, Schwartz O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis [J]. J Virol, 2001, 75(22): 11166-11177.
[25] Liu NQ, Lossinsky AS, Popik W, Li X, Gujuluva C, Kriederman B, Roberts J, Pushkarsky T, Bukrinsky M, Witte M, Weinand M, Fiala M. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway [J]. J Virol, 2002, 76(13): 6689-6700.
[26] Liou ML, Hsu CY. Japanese encephalitis virus is transported across the cerebral blood vessels by endocytosis in mouse brain [J]. Cell Tissue Res, 1998, 293(3): 389-394.
[27] Hasebe R, Suzuki T, Makino Y, Igarashi M, Yamanouchi S, Maeda A, Horiuchi M, Sawa H, Kimura T. Transcellular transport of West Nile virus-like particles across human endothelial cells depends on residues 156 and 159 of envelope protein [J]. BMC Microbiol, 2010, 10: 165.doi: 10.1186/1471-2180-10-165.
[28] Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation [J]. Vascul Pharmacol, 2002, 38(6): 323-337.
[29] Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function [J]. Int Rev Cytol, 2006, 248: 261-298.
[30] de Lange EC. The physiological characteristics and transcytosis mechanisms of the blood-brain barrier (BBB) [J]. Curr Pharm Biotechnol, 2012, 13(12): 2319-2327.
[31] Kajon AE, Brown CC, Spindler KR. Distribution of mouse adenovirus type 1 in intraperitoneally and intranasally infected adult outbred mice [J]. J Virol, 1998, 72(2): 1219-1223.
[32] Ashley SL, Welton AR, Harwood KM, Van Rooijen N, Spindler KR. Mouse adenovirus type 1 infection of macrophages [J]. Virology, 2009, 390(2): 307-314.
[33] Gralinski LE, Ashley SL, Dixon SD, Spindler KR. Mouse adenovirus type 1-induced breakdown of the blood-brain barrier [J]. J Virol, 2009, 83(18): 9398-9410.
[34] Chang CY, Li JR, Chen WY, Ou YC, Lai CY, Hu YH, Wu CC, Chang CJ, Chen CJ. Disruption of in vitro endothelial barrier integrity by Japanese encephalitis virus-infected astrocytes [J]. Glia, 2015, 63(11): 1915-1932.
[35] Verma S, Kumar M, Gurjav U, Lum S, Nerurkar VR. Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor [J]. Virology, 2010, 397(1): 130-138.
[36] Wang P, Dai J, Bai F, Kong KF, Wong SJ, Montgomery RR, Madri JA, Fikrig E. Matrix metalloproteinase 9 facilitates West Nile virus entry into the brain [J]. J Virol 2008, 82(18): 8978-8985.
[37] Sierralta J, Mendoza C. PDZ-containing proteins: alternative splicing as a source of functional diversity [J]. Brain Res Rev, 2004, 47(1-3): 105-115.
[38] Rousset R, Fabre S, Desbois C, Bantignies F, Jalinot P. The C-terminus of the HTLV-1 Tax oncoprotein mediates interaction with the PDZ domain of cellular proteins [J]. Oncogene, 1998, 16(5): 643-654.
[39] Kehn K, de la Fuente C, Strouss K, Berro R, Jiang H, Brady J, Mahieux R, Pumfery A, Bottazzi ME, Kashanchi F. The HTLV-I Tax oncoprotein targets the retinoblastoma protein for proteasomal degradation [J]. Oncogene, 2005, 24(4): 525-540.
[40] Afonso PV, Ozden S, Prevost MC, Schmitt C, Seilhean D, Weksler B, Couraud PO, Gessain A, Romero IA, Ceccaldi PE. Human blood-brain barrier disruption by retroviral-infected lymphocytes: role of myosin light chain kinase in endothelial tight-junction disorganization [J]. J Immunol, 2007, 179(4): 2576-2583.
[41] Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, Achim CL. Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis [J]. Am J Pathol, 1999, 155(6): 1915-1927.
[42] Kanmogne GD, Primeaux C, Grammas P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia [J]. J Neuropathol Exp Neurol, 2005, 64(6): 498-505.
[43] Ivey NS, Renner NA, Moroney-Rasmussen T, Mohan M, Redmann RK, Didier PJ, Alvarez X, Lackner AA, Maclean AG. Association of FAK activation with lentivirus-induced disruption of blood-brain barrier tight junction-associated ZO-1 protein organization [J]. J Neurovirol, 2009, 15(4): 312-323.
[44] Louboutin JP, Agrawal L, Reyes BA, van Bockstaele EJ, Strayer DS. HIV-1 gp120-induced injury to the blood-brain barrier: role of metalloproteinases 2 and 9 and relationship to oxidative stress [J]. J Neuropathol Exp Neurol, 2010, 69(8): 801-816.
[45] Pu H, Hayashi K, Andras IE, Eum SY, Hennig B, Toborek M. Limited role of COX-2 in HIV Tat-induced alterations of tight junction protein expression and disruption of the blood-brain barrier[J]. Brain research, 2007, 1184: 333-344.
[46] Mishra R, Singh SK. HIV-1 Tat C phosphorylates VE-cadherin complex and increases human brain microvascular endothelial cell permeability [J]. BMC Neurosci, 2014, 15: 80. doi: 10.1186/1471-2202-15-80.
[47] Raymond AD, Diaz P, Chevelon S, Agudelo M, Yndart-Arias A, Ding H, Kaushik A, Jayant RD, Nikkhah-Moshaie R, Roy U, Pilakka-Kanthikeel S, Nair MP. Microglia-derived HIV Nef+exosome impairment of the blood-brain barrier is treatable by nanomedicine-based delivery of Nef peptides [J]. J Neurovirol, 2016, 22(2): 129-139.
[48] Miner JJ, Daniels BP, Shrestha B, Proenca-Modena JL, Lew ED, Lazear HM, Gorman MJ, Lemke G, Klein RS, Diamond MS. The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity [J]. Nat Med, 2015, 21(12): 1464-1472.
[49] Alexaki A, Wigdahl B. HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination [J]. PLoS Pathog, 2008, 4(12): e1000215.
[50] Clay CC, Rodrigues DS, Ho YS, Fallert BA, Janatpour K, Reinhart TA, Esser U. Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection [J]. J Virol, 2007, 81(21): 12040-12048.
[51] Ancuta P, Wang J, Gabuzda D. CD16+monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells [J]. J Leukoc Biol, 2006, 80(5): 1156-1164.
[52] Roberts TK, Buckner CM, Berman JW. Leukocyte transmigration across the blood-brain barrier: perspectives on neuroAIDS [J]. Front Biosc, 2010, 15: 478-536.
[53] Wu DT, Woodman SE, Weiss JM, McManus CM, D’Aversa TG, Hesselgesser J, Major EO, Nath A, Berman JW. Mechanisms of leukocyte trafficking into the CNS [J]. J Neurovirol, 2000, 6(Suppl 1): S82-S85.
[54] Monaco MC, Atwood WJ, Gravell M, Tornatore CS, Major EO. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency [J]. J Virol, 1996, 70(10): 7004-7012.
[55] Garcia-Tapia D, Loiacono CM, Kleiboeker SB. Replication of West Nile virus in equine peripheral blood mononuclear cells [J]. Vet Immunol Immunopathol, 2006, 110(3-4): 229-244.
[56] Dai J, Wang P, Bai F, Town T, Fikrig E. ICAM-1 participates in the entry of West Nile virus into the central nervous system [J]. J Virol, 2008, 82(8): 4164-4168.
[57] Wang S, Welte T, McGargill M, Town T, Thompson J, Anderson JF, Flavell RA, Fikrig E, Hedrick SM, Wang T. Drak2 contributes to West Nile virus entry into the brain and lethal encephalitis [J]. J Immunol, 2008, 181(3): 2084-2091.
[58] Liu H, Liu Y, Wang S, Zhang Y, Zu X, Zhou Z, Zhang B, Xiao G. Structure-based mutational analysis of several sites in the E protein: implications for understanding the entry mechanism of Japanese encephalitis virus [J]. J Virol, 2015, 89(10): 5668-5686.
[59] Luca VC, Abimansour J, Nelson CA, Fremont DH. Crystal structure of the Japanese encephalitis virus envelope protein [J]. J Virol, 2012, 86(4): 2337-2346.
[60] Fan YC, Chiu HC, Chen LK, Chang GJ, Chiou SS. Formalin inactivation of Japanese encephalitis virus vaccine alters the antigenicity and immunogenicity of a neutralization epitope in envelope protein domain III [J]. PLoS Negl Trop Dis, 2015, 9(10): e0004167.
[61] Li C, Zhang LY, Sun MX, Li PP, Wei JC, Yao YL, Isahg H, Chen PY, Mao X. Inhibition of Japanese encephalitis virus entry into the cells by the envelope glycoprotein domain III (EDIII) and the loop3 peptide derived from EDIII [J]. Antiviral Res, 2012, 94(2): 179-183.
[62] Yun SI, Song BH, Kim JK, Yun GN, Lee EY, Li L, Kuhn RJ, Rossmann MG, Morrey JD, Lee YM. A molecularly cloned, live-attenuated Japanese encephalitis vaccine SA14-14-2 virus: a conserved single amino acid in the ij hairpin of the viral E glycoprotein determines neurovirulence in mice [J]. PLoS Pathog, 2014, 10(7): e1004290.
[63] Ferguson MC, Saul S, Fragkoudis R, Weisheit S, Cox J, Patabendige A, Sherwood K, Watson M, Merits A, Fazakerley JK. Ability of the encephalitic arbovirus Semliki Forest virus to cross the blood-brain barrier is determined by the charge of the E2 glycoprotein [J]. J Virol, 2015, 89(15): 7536-7549.
. QI Zhongtian, E-mail: qizt@smmu.edu.cn
Two ways and possible mechanisms of virus crossing the blood-brain barrier
ZHU Naiwei, ZHU Yongzhe, QI Zhongtian
DepartmentofMicrobiology,ShanghaiKeyLaboratoryofMedicalBiodefense,TheSecondMilitaryMedicalUniversity,Shanghai200433,China
The blood-brain barrier (BBB) is an important protective structure to maintain the homeostasis of the central nervous system, limiting the invasion of most pathogens in the blood. Some viruses can cross the BBB to invade the central nervous system by cellular and (or) paracellular ways, causing neurological dysfunction. The cellular ways consist of direct infection of the brain microvascular endothelial cells and transcellular pathway. The paracellular ways are composed of breaching of the tight junction between endothelial cells and the “Trojan horse” pathway. In this review, the current research progress on viral impacts on the BBB is summarized.
Virus; Blood-brain barrier; Mechanism
国家自然科学基金(81273557)
戚中田
2016-09-30)
