硒源与硒水平对军曹鱼幼鱼生长性能、肝脏和血清抗氧化指标及组织硒含量的影响
2016-12-19杨原志聂家全谭北平董晓慧杨奇慧迟淑艳
杨原志 聂家全* 谭北平,2 董晓慧,2** 杨奇慧 迟淑艳
(1.广东海洋大学水产学院水产动物营养与饲料实验室,湛江524088;2.南海生物资源开发与利用协同创新中心,广州510006)
硒源与硒水平对军曹鱼幼鱼生长性能、肝脏和血清抗氧化指标及组织硒含量的影响
杨原志1聂家全1*谭北平1,2董晓慧1,2**杨奇慧1迟淑艳1
(1.广东海洋大学水产学院水产动物营养与饲料实验室,湛江524088;2.南海生物资源开发与利用协同创新中心,广州510006)
本试验旨在研究硒源和硒水平对军曹鱼幼鱼生长性能、肝脏和血清抗氧化指标及组织硒含量的影响,以确定军曹鱼幼鱼对不同硒源的最适需要量。在基础饲料中分别添加0(对照)、0.3、0.6、0.9和1.2 mg/kg(以硒计)的亚硒酸钠(Se-S)或蛋氨酸硒(Se-Met),配制9种试验饲料(共用对照饲料),饱食投喂初始体重为(22.18±0.35) g的军曹鱼幼鱼10周。每种试验饲料投喂3个网箱(重复),每个网箱放养30尾试验鱼。结果表明:1)硒水平对特定生长率(SGR)和增重率(WGR)有极显著影响(P<0.01),但对成活率(SR)和饲料系数(FCR)无显著影响(P>0.05);硒源及硒源与硒水平的交互作用对SGR、WGR、FCR和SR均无显著影响(P>0.05)。SGR和WGR随着硒水平的升高先升高后降低。2)硒水平极显著影响肝脏谷胱甘肽过氧化物酶(GSH-Px)、谷胱甘肽还原酶(GR)活性和丙二醛(MDA)含量及血清GSH-Px活性(P<0.01),并显著影响血清GR活性(P<0.05);硒源极显著影响肝脏GR、总超氧化物歧化酶(T-SOD)、过氧化氢酶(CAT)活性和MDA含量及血清T-SOD活性(P<0.01);硒源和硒水平的交互作用显著影响肝脏CAT活性和MDA含量及血清T-SOD活性(P<0.05)。肝脏和血清GSH-Px活性随着硒水平的升高呈先升高后稳定趋势,GR活性呈先下降后稳定趋势。2种硒源均在添加量为0.9 mg/kg时有最高的肝脏GSH-Px活性,最低的肝脏GR活性。肝脏CAT活性随着硒水平的升高逐渐升高。3)脊椎骨、全鱼和肝脏硒含量随硒水平的升高而增加。硒源极显著影响脊椎骨硒含量(P<0.01),硒源和硒水平的交互作用极显著影响肝脏和脊椎骨硒含量(P<0.01)。以蛋氨酸硒和亚硒酸钠为硒源,通过二次回归曲线分析得出饲料硒水平分别为1.29和1.46 mg/kg时军曹鱼幼鱼可以获得最大SGR。以SGR和全鱼硒含量为判据,军曹鱼幼鱼对蛋氨酸硒的生物利用率分别相当于亚硒酸钠的1.20和2.90倍。
军曹鱼幼鱼;硒;生长性能;抗氧化酶;硒沉积
硒在自然界中以无机硒或有机硒的形式存在,长期被误认为是一种有毒物质。1957年,Schwartz等[1]证实硒能预防因硒和/或维生素E缺乏导致的大白鼠的肝坏死。随后人们相继发现硒是参与构成谷胱甘肽过氧化物酶(glutathione peroxidase,GSH-Px)的微量元素,其活性中心是硒半胱氨酸,该酶能够把过氧化氢和脂质过氧化产物分别转换成水和脂醇,保护细胞膜和亚细胞膜的多不饱和磷脂免受过氧化损伤[2];同时,硒还能促进动物生长、提高繁殖性能、增强机体免疫力。

军曹鱼(Rachycentroncanadum),隶属鲈形目(Perciformes),军曹鱼科(Rachycentridae),军曹鱼属(Rachycentron),亦称海鲡、海龙、海竹鱼等,是大型肉食性鱼类,具有生长速度快、易于驯化摄食人工饲料、肉质鲜美等特点,是我国南方沿海海水网箱养殖的重要品种之一。
目前,仅见军曹鱼对蛋氨酸硒需要量的研究报道,如Liu等[7]指出军曹鱼幼鱼对蛋氨酸硒的需要量是0.788~0.811 mg/kg,未见硒源及硒水平对军曹鱼营养生理影响的研究报道。因此,本试验拟研究饲料中不同硒源和硒水平对军曹鱼幼鱼营养生理作用的影响,旨在为军曹鱼幼鱼营养需要参数数据库的完善及军曹鱼高效配合饲料的研制提供基础数据。
1 材料与方法
1.1 试验设计与试验饲料
以脱维酪蛋白为主要蛋白质源,玉米淀粉为糖源,玉米油、鱼油为主要脂肪源,配制基础饲料,基础饲料组成及营养水平见表1。在基础饲料中分别添加源于亚硒酸钠(购自国药集团化学试剂有限公司)或蛋氨酸硒(购自长沙兴嘉生物工程股份有限公司)的硒,添加水平分别为0(对照)、0.3、0.6、0.9和1.2 mg/kg,配制成9种试验饲料(共用对照饲料),对照饲料中硒水平实测值为0.35 mg/kg,命名为C-0.35;添加亚硒酸钠的4种饲料中硒水平实测值分别为0.68、1.09、1.26、1.65 mg/kg,并分别命名为Se-S-0.68、Se-S-1.09、Se-S-1.26、Se-S-1.65;添加蛋氨酸硒的4种饲料中硒水平实测值分别为0.67、1.02、1.33、1.69 mg/kg,并分别命名为Se-Met-0.67、Se-Met-1.02、Se-Met-1.33、Se-Met-1.69。

表1 基础饲料组成及营养水平(干物质基础)
1)脱维酪蛋白(粗蛋白质含量为94%)购自美国Sigma公司。Vitamin-free casein (CP content was 94%) obtained from Sigma Chemical Co., USA.
2)维生素预混料为每千克饲料提供The vitamin premix provided the following per kg of the diet: VB125 mg,VB245 mg,泛酸钙 calcium pantothenate 60 mg,烟酸 nicotinic acid 200 mg,吡哆醇 pyridoxine 20 mg,生物素 biotin 1.20 mg,VB120.1 mg,叶酸 folic acid 20 mg,肌醇 inositol 800 mg,VA 32 mg,VD35 mg,VK310 mg,VE 120 mg。
3)无硒矿物质预混料为每千克饲料提供The Se-free mineral premix provided the following per kg of the diet: CH3CHOHCOO2Ca·5H2O 6 100 mg,Na2HPO46 200 mg,NaF 2 mg,Ca(IO3)20.94 mg,CoCl2·6H2O (1%) 50 mg,CuSO4·5H2O 10 mg,MnSO4·H2O 60 mg,FeSO4·H2O 240 mg,ZnSO4·7H2O 180 mg,MgSO4·7H2O 1 200 mg,NaCl 100 mg。
4)实测值Measured values。
1.2 试验用鱼与饲养管理
养殖试验在广东省湛江市南三岛海上浮式鱼排中进行,试验用军曹鱼幼鱼购于广东湛江市英利镇海尾村养殖户。军曹鱼幼鱼暂养2周,期间投喂商品饲料。分组前停食24 h,随机挑选规格一致、健康无病、初始体重为(22.18±0.35) g的试验鱼进行分组,根据试验设计,共分为9个组,每组3个重复,每个重复1个2.5 m×1.2 m×1.4 m的网箱,共27个网箱,每个网箱放养30尾鱼。为减少试验误差,所有网箱均随机摆放。每种试验饲料投喂3个网箱,每天投喂2次(07:00、18:00),日投喂量为试验鱼体重的6%~9%。养殖期间水体温度28~33 ℃,pH 7.6~7.8,盐度29~31,溶氧浓度>6.0 mg/L,试验期10周。养殖海水中硒未检出。
1.3 样品采集和指标测定
试验结束禁食24 h后,将各网箱试验鱼全部捞出,丁香酚(1∶10 000)麻醉后称重计数。每个网箱随机抽取5尾鱼,用2.5 mL的注射器从鱼体围心腔刺入,在心脏动脉球处抽血,置于1.5 mL的Eppendorf管中,4 ℃、4 500 r/min离心10 min,吸取血清保存于-80 ℃冰箱中备用。每个网箱随机抽取5尾鱼解剖后迅速取出肝脏放入防冻管,立即放入液氮罐保存,后转存于-80 ℃冰箱中备用。每个网箱随机抽取8尾鱼,去除内脏后放入沸水中3 min,剥离肌肉,取出脊椎骨,用超纯水冲洗去掉附着的肌肉。处理后的脊椎骨105 ℃烘干,粉碎过80目筛,乙醚抽提12 h去除脂肪,并再次105 ℃烘干[8]。
饲料成分分析:水分含量采用105 ℃常压干燥法测定;粗蛋白质含量采用凯氏定氮法(总氮×6.25)测定;粗脂肪含量采用索氏抽提法测定;粗灰分含量采用550 ℃马弗炉灼烧法测定。
肝脏和血清抗氧化指标测定:GSH-Px、谷胱甘肽还原酶(glutathione reductase,GR)、总超氧化物歧化酶(total superoxide dismutase,T-SOD)、过氧化氢酶(catalase,CAT)活性和丙二醛(malondialdehyde,MDA)含量采用南京建成生物工程研究所提供的试剂盒测定,样品前处理、试剂配制及测定步骤均严格按照操作说明书执行。
饲料、全鱼、脊椎骨和肝脏中硒水平/含量测定:用硝酸和双氧水充分消解定量样品,用电感耦合等离子体-质谱仪测定硒含量。
1.4 计算公式
特定生长率(specific growth rate,SGR,%/d)=
100×(ln试验期末均重-ln试验期初均重)/试验天数;
增重率(weight gain rate,WGR,%)=
100×(试验期末均重-试验期初均重)/试验期初均重;
成活率(survival rate,SR,%)=
100×试验期末鱼尾数/试验期初鱼尾数;
饲料系数(feed conversion rate,FCR)=
总摄食量/(试验期末总重+死亡鱼总重-试验期初总重)。
1.5 统计分析
试验结果用平均值±标准误表示,采用SPSS 16.0的一般线性模型(GLM)进行双因素方差分析,模型的主效应分析包括硒源、硒水平及两者之间的交互作用。P<0.05表示差异显著,P<0.01表示差异极显著。
2 结 果
2.1 硒源和硒水平对军曹鱼幼鱼生长性能的影响
由表2可知,硒源对SGR、WGR、FCR和SR均无显著影响(P>0.05);硒水平对SGR和WGR有极显著影响(P<0.01),但对SR和FCR无显著影响(P>0.05)。硒源和硒水平的交互作用对SGR、WGR、FCR和SR均无显著影响(P>0.05)。SGR和WGR随着硒水平的升高先升高后逐渐降低。分别以蛋氨酸硒和亚硒酸钠为硒源,通过二次回归曲线分析得出饲料硒水平分别为1.29和1.46 mg/kg时军曹鱼幼鱼可以获得最大SGR(图1);以SGR为判据时,军曹鱼幼鱼对蛋氨酸硒的生物利用率相当于亚硒酸钠的1.20倍(蛋氨酸硒:y=0.209x+2.111,R2=0.98;亚硒酸钠:y=0.175x+2.218,R2=0.99;x为饲料硒水平,y为SGR)。
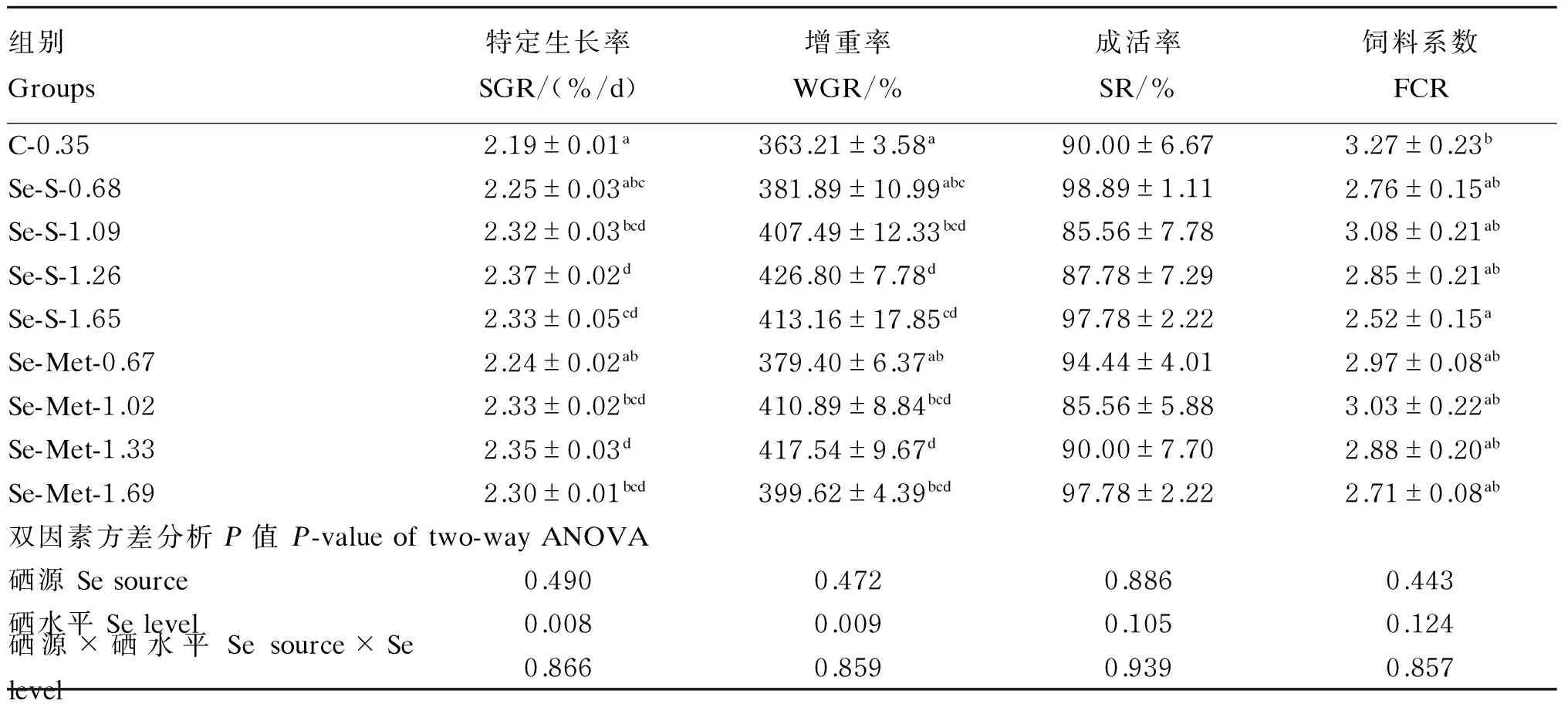
表2 硒源和硒水平对军曹鱼幼鱼生长性能的影响
同列数据肩标无字母或相同字母表示差异不显著(P>0.05),不同小写字母表示差异显著(P<0.05)。下表同。
In the same column, values with no letter or the same letter superscripts mean no significant difference (P>0.05), while with different small letter superscripts mean significant difference (P<0.05). The same as below.

图1 饲料硒水平与军曹鱼幼鱼特定生长率的关系(2种硒源)
2.2 硒源和硒水平对军曹鱼幼鱼肝脏和血清抗氧化指标的影响
由表3可知,硒源极显著影响肝脏GR、T-SOD、CAT活性和MDA含量及血清T-SOD活性(P<0.01),但对肝脏GSH-Px活性及血清GSH-Px、GR、CAT活性和MDA含量无显著影响(P>0.05)。硒水平极显著影响肝脏GSH-Px、GR活性和MDA含量及血清GSH-Px活性(P<0.01),显著影响血清GR活性(P<0.05),但对肝脏T-SOD、CAT活性及血清T-SOD、CAT活性和MDA含量无显著影响(P>0.05);硒源和硒水平的交互作用对肝脏CAT活性、MDA含量和血清T-SOD活性有显著影响(P<0.05),但对肝脏GSH-Px、GR、T-SOD活性及血清GSH-Px、GR、CAT活性和MDA含量无显著影响(P>0.05)。肝脏和血清GSH-Px活性随着硒水平的升高表现为先升高后稳定的趋势,GR活性则表现为先下降后稳定的趋势。2种硒源中,分别以Se-S-1.26组和Se-Met-1.33组的肝脏GSH-Px活性最高,并均显著高于对照组(C-0.35组)(P<0.05);同时这2组的肝脏GR活性最低,并均显著低于对照组(P<0.05);肝脏CAT活性随着硒水平的升高逐渐升高,在以亚硒酸钠为硒源的组中,Se-S-1.65组肝脏CAT活性显著高于除Se-S-1.26组外的其他3组(P<0.05);在以蛋氨酸硒为硒源的组中,Se-Met-1.69组肝脏CAT活性显著高于其他各组(P<0.05)。各组间血清CAT活性无显著差异(P>0.05)。
2.3 硒源和硒水平对军曹鱼幼鱼组织硒含量的影响
由表4可知,硒源极显著的影响脊椎骨硒含量(P<0.01),但对全鱼和肝脏硒含量无显著影响(P>0.05)。硒水平对脊椎骨、全鱼和肝脏硒含量有极显著影响(P<0.01),脊椎骨、全鱼和肝脏硒含量随着饲料硒水平的升高而升高。硒源和硒水平的交互作用对全鱼硒含量没有显著影响(P>0.05),但对肝脏和脊椎骨硒含量有极显著影响(P<0.01)。以全鱼硒含量为判据时,军曹鱼幼鱼对蛋氨酸硒的生物利用率相当于亚硒酸钠的2.90
倍(蛋氨酸硒:y=0.938x+0.052,R2=0.99;亚硒酸钠:y=0.323x+2.252,R2=0.99;x为饲料硒水平,y为全鱼硒含量)。

表3 硒源和硒水平对军曹鱼幼鱼肝脏和血清抗氧化指标的影响
3 讨 论
3.1 硒源和硒水平对军曹鱼幼鱼生长性能的影响


表4 硒源和硒水平对军曹鱼幼鱼组织硒含量的影响

3.2 硒源和硒水平对军曹鱼幼鱼肝脏和血清抗氧化指标的影响

MDA是脂质氧化产物,经常用作氧化胁迫指标之一[33]。Zhu等[22]对大口黑鲈的研究发现饲料中硒水平对肝脏MDA含量没有显著影响,并认为这是由于饲料中有较多的抗氧化剂(维生素E:400 mg/kg,维生素C:1 000 mg/kg)与活性氧起反应从而掩盖掉硒在体内的抗氧化作用所致。在本试验中,硒水平和硒源的交互作用对肝脏MDA含量有显著影响,对血清MDA含量没有显著影响。亚硒酸钠组和蛋氨酸硒组的肝脏MDA含量均随着饲料中硒水平的升高先下降后升高。这可能与本试验中抗氧化剂(维生素:120 mg/kg,维生素C:350 mg/kg)含量相对较低有关。当饲料中硒达到较高水平时,可能超出了鱼体的需要量,且对鱼体有一定的毒害作用,因此造成鱼体氧自由基代谢紊乱,T-SOD和GSH-Px活性下降,自由基含量升高,MDA含量升高。这与人类硒营养研究中硒生物效应与硒水平的关系相似,即当硒水平在适宜范围内时能够有效地清除生物体内的活性自由基,而当硒水平较高时则会催化产生活性自由基[34]。中华绒螯蟹[19]血清、肝脏中MDA含量及鲈鱼[21]血清中MDA含量随饲料硒水平的升高呈先降低后升高趋势,与本试验结果类似;而凡纳滨对虾血清MDA含量则随饲料硒水平的升高呈逐渐下降趋势[26]。
在鱼体内GSH-Px、T-SOD和CAT共同起作用,清除超氧阴离子、羟自由基和过氧化氢,减少脂质过氧化物的损害。在本试验中,肝脏CAT活性随着硒水平的升高逐渐升高,Se-S-1.65组和Se-Met-1.69组的肝脏CAT活性高于同一硒源的其他组;肝脏T-SOD活性随硒水平的升高先升高后降低,2种硒源中分别以Se-S-1.26组和Se-Met-1.33组肝脏T-SOD活性最高,这表明饲料中添加硒能够提高军曹鱼幼鱼体内CAT和T-SOD的活性。Monteiro等[35]发现饲料补充硒能够提高缺帘鱼(Bryconcephalus)肝脏CAT和T-SOD活性,共同抵抗甲基对硫磷毒性的氧化胁迫,未添加硒组肝脏T-SOD和CAT活性降低。在异育银鲫饲料中添加硒至1 mg/kg时,血清T-SOD活性达到最大值,添加至5 mg/kg时血清T-SOD活性显著降低;饲料中添加硒对异育银鲫血清CAT活性没有产生显著影响,但血清CAT活性有随饲料硒水平的升高而下降的趋势,表明饲料中过量的硒降低了血清CAT和T-SOD活性[10]。鲈鱼血清和肝脏超氧化物歧化酶(SOD)活性随饲料硒水平的升高呈先上升后下降趋势[21]。饲料中添加硒提高了吉富罗非鱼(Oreochromisniloticus)血清中CAT和T-SOD的活性[36]。
3.3 硒源和硒水平对军曹鱼幼鱼组织硒含量的影响

4 结 论
① 以亚硒酸钠和蛋氨酸硒为硒源,通过二次回归曲线分析得出饲料硒水平分别为1.46和1.29 mg/kg时军曹鱼幼鱼可以获得最大SGR。
② 以SGR为判据时,军曹鱼幼鱼对蛋氨酸硒的生物利用率相当于亚硒酸钠的1.20倍;以全鱼硒含量为判据时,军曹鱼幼鱼对蛋氨酸硒的生物利用率相当于亚硒酸钠的2.90倍。
[1] SCHWARZ K,FOLTZ C M.Selenium as an integral part of factor 3 against dietary liver degeneration[J].Journal of the American Chemical Society,1957,79(12):3292-3293.
[2] GATLIN Ⅲ D M,WILSON R P.Dietary selenium requirement of fingerling channel catfish.[J].The Journal of Nutrition,1984,114(3):627-633.
[3] LORENTZEN M,MAAGE A,JULSHAMN K.Effects of dietary selenite or selenomethionine on tissue selenium levels of Atlantic salmon (Salmosalar)[J].Aquaculture,1994,121(4):359-367.
[4] WANG C,LOVELL R T.Organic selenium sources,selenomethionine and selenoyeast,have higher bioavailability than an inorganic selenium source,sodium selenite,in diets for channel catfish (Ictaluruspunctatus)[J].Aquaculture,1997,152(1/2/3/4):223-234.
[5] RIDER S A,DAVIES S J,JHA A N,et al.Supra-nutritional dietary intake of selenite and selenium yeast in normal and stressed rainbow trout (Oncorhynchusmykiss):implications on selenium status and health responses[J].Aquaculture,2009,295(3/4):282-291.
[6] JARAMILLO F,Jr,PENG L,GATLIN Ⅲ D M.Selenium nutrition of hybrid striped bass (Moronechrysops×M.saxatilis) bioavailability,toxicity and interaction with vitamin E[J].Aquaculture Nutrition,2009,15(2):160-165.
[7] LIU K,WANG X J,AI Q H,et al.Dietary selenium requirement for juvenile cobia,RachycentroncanadumL.[J].Aquaculture Research,2010,41(10):e594-e601.
[8] AOAC.Official methods of analysis of the Association of Official Analytical Chemists[S].16th ed.Arlington,VA:AOAC,1995.
[9] LE K T,FOTEDAR R.Dietary selenium requirement of yellowtail kingfish (Seriolalalandi)[J].Agricultural Sciences,2013,4(6A):68-75.
[10] HAN D,XIE S,LIU M,et al.The effects of dietary selenium on growth performances,oxidative stress and tissue selenium concentration of gibel carp (Carassiusauratusgibelio)[J].Aquaculture Nutrition,2011,17(3):e741-e749.
[11] LIN Y H.Effects of dietary organic and inorganic selenium on the growth,selenium concentration and meat quality of juvenile grouperEpinephelusmalabaricus[J].Aquaculture,2014,430:114-119.
[12] 曹娟娟,张文兵,徐玮,等.大黄鱼幼鱼对饲料硒的需求量[J].水生生物学报,2015,39(2):241-249.
[13] ASHOURI S,KEYVANSHOKOOH S,SALATI A P,et al.Effects of different levels of dietary selenium nanoparticles on growth performance,muscle composition,blood biochemical profiles and antioxidant status of common carp (Cyprinuscarpio)[J].Aquaculture,2015,446:25-29.
[14] HILTON J W,HODSON P V,SLINGER S J.The requirement and toxicity of selenium in rainbow trout (Salmogairdneri)[J].The Journal of Nutrition,1980,110(12):2527-2535.
[15] WATANABE T,KIRON V,SATOH S.Trace minerals in fish nutrition[J].Aquaculture,1997,151(1/2/3/4):185-207.
[16] DEFOREST D K,BRIX K V,ADAMS W J.Critical review of proposed residue-based selenium toxicity thresholds for freshwater fish[J].Human and Ecological Risk Assessment:An International Journal,1999,5(6):1187-1228.
[17] HAMILTON S J.Review of selenium toxicity in the aquatic food chain[J].Science of the Total Environment,2004,326(1/2/3):1-31.
[18] LIN Y H,SHIAU S Y.Dietary selenium requirements of juvenile grouper,Epinephelusmalabaricus[J].Aquaculture,2005,250(1/2):356-363.
[19] 田文静,李二超,陈立侨,等.酵母硒对中华绒螯蟹幼蟹生长、体组成分及抗氧化能力的影响[J].中国水产科学,2014,21(1):92-100.
[20] COTTER P A,CRAIG S R,MCLEAN E.Hyperaccumulation of selenium in hybrid striped bass:a functional food for aquaculture?[J].Aquaculture Nutrition,2008,14(3):215-222.
[21] 谈枫,梁萌青,郑珂珂,等.鲈鱼(Lateolabraxjaponicus)养殖中期对饲料硒的需求量[J].渔业科学进展,2015,36(3):93-100.
[22] ZHU Y,CHEN Y J,LIU Y J,et al.Effect of dietary selenium level on growth performance,body composition and hepatic glutathione peroxidase activities of largemouth bassMicropterussalmoide[J].Aquaculture Research,2012,43(11):1660-1668.
[23] HARDY R W,ORAM L L,MÖLLER G.Effects of dietary selenomethionine on cutthroat trout (Oncorhynchusclarkibouvieri) growth and reproductive performance over a life cycle[J].Archives of Environmental Contamination and Toxicology,2010,58(1):237-245.
[24] ARSHAD U,TAKAMI G A,SADEGHI M,et al.Influence of dietaryL-selenomethionine exposure on growth and survival of juvenileHusohuso[J].Journal of Applied Ichthyology,2011,27(2):761-765.
[25] LE K T,FOTEDAR R.Bioavailability of selenium from different dietary sources in yellowtail kingfish (Seriolalalandi)[J].Aquaculture,2014,420-421:57-62.
[26] 李小霞,陈锋,潘庆,等.硒源对凡纳滨对虾生长、体组成和抗氧化能力的影响[J].水产科学,2016,35(3):199-203.
[27] LIN Y H,SHIAU S Y.Mutual sparing of dietary requirements for alpha-tocopherol and selenium in grouper,Epinephelusmalabaricus[J].Aquaculture,2009,294(3/4):242-245.
[28] BELL J G,COWEY C B.Digestibility and bioavailability of dietary selenium from fishmeal,selenite,selenomethionine and selenocystine in Atlantic salmon (Salmosalar)[J].Aquaculture,1989,81(1):61-68.
[29] ILHAM,FOTEDAR R,MUNILKUMAR S.Effects of organic selenium supplementation on growth,glutathione peroxidase activity and histopathology in juvenile barramundi (LatescalcariferBloch 1970) fed high lupin meal-based diets[J].Aquaculture,2016,457:15-23.
[30] 梁萌青,王家林,常青,等.饲料中硒的添加水平对鲈鱼生长性能及相关酶活性的影响[J].中国水产科学,2006,13(6):1017-1022.
[31] JOVANOVIC A,GROUBOR-LAJSIC G,DJUKIC N,et al.The effect of selenium on antioxidant system in erythrocytes and liver of the carp (CyprinuscarpioL.)[J].Critical Reviews in Food Science and Nutrition,1997,37(5):443-448.
[32] RIDER S A,DAVIES S J,JHA A N,et al.Bioavailability of co-supplemented organic and inorganic zinc and selenium sources in a white fishmeal-based rainbow trout (Oncorhynchusmykiss) diet[J].Journal of Animal Physiology and Animal Nutrition,2010,94(1):99-110.
[33] DOTAN Y,LICHTENBERG D,PINCHUK I.Lipid peroxidation cannot be used as a universal criterion of oxidative stress[J].Progress in Lipid Research,2004,43(3):200-227.
[34] LEVANDER O A.A global view of human selenium nutrition[J].Annual Review of Nutrition,1987,7(1):227-250.

[36] 覃希,黄凯,刘康,等.维生素E和硒对吉富罗非鱼(Oreochromisniloticus)幼鱼生长及血清抗氧化酶活性的影响[J].渔业科学进展,2014,35(4):77-84.
[37] 许明珠,张琴,童潼,等.饲料中硒含量对方格星虫稚虫生长、体成分、组织硒含量及相关酶活性的影响[J].动物营养学报,2015,27(6):1733-1739.
[38] LE K T,FOTEDAR R.Toxic effects of excessive levels of dietary selenium in juvenile yellowtail kingfish (Seriolalalandi)[J].Aquaculture,2014,433:229-234.
[39] BHANDARI B.Trace elements in human health and disease[J].Quarterly Medical Review,1983,34(4):1-33.
[40] SCHRAUZER G N.Selenomethionine:a review of its nutritional significance,metabolism and toxicity[J].The Journal of Nutrition,2000,130(7):1653-1656.
*Contributed equally
**Corresponding author, professor, E-mail: dongxiaohui2003@163.com
(责任编辑 菅景颖)
Effects of Selenium Source and Selenium Level on Growth Performance, Liver and Serum Antioxidant Indices and Selenium Content in Tissues of Juvenile Cobia (Rachycentroncanadum)
YANG Yuanzhi1NIE Jiaquan1*TAN Beiping1,2DONG Xiaohui1,2**YANG Qihui1CHI Shuyan1
(1.LaboratoryofAquaticAnimalNutritionandFeed,FisheriesCollege,GuangdongOceanUniversity,Zhanjiang524088,China; 2.SouthChinaSeaBio-ResourceExploitationandUtilizationCollaborativeInnovationCenter,Guangzhou510006,China)
A feeding trial was carried out to investigate the effects of selenium (Se) source and Se level on growth performance, liver and serum antioxidant indices and Se content in tissues of juvenile cobia (Rachycentroncanadum), in order to determine the Se requirement from different Se sources of juvenile cobia. Nine experimental diets (control diet was shared) were prepared by supplementing 0 (control), 0.3, 0.6, 0.9 and 1.2 mg/kg Se from sodium selenite (Se-S) or selenium methionine (Se-Met), respectively. Juvenile cobia with an initial body weight of (22.18±0.35) g were fed to satiation for 10 weeks. Fish in three cages (replicates) were fed a kind of experimental diet and 30 fish in a cage. The results showed as follows: 1) Se level extremely significantly affected the weight gain rate (WGR) and specific growth rate (SGR) (P<0.01), but did not significantly affected the feed conversion ratio (FCR) and survival rate (SR) (P>0.05). The SGR, WGR, FCR and SR were not affected by Se source and the interaction of Se source and Se level (P>0.05). With the Se level increasing, the SGR and WGR were increased firstly and then decreased. 2) The glutathione peroxidase (GSH-Px), glutathione reductase (GR) activities and malondialdehyde (MDA) content in liver and GSH-Px activity in serum were extremely significantly affected by Se level (P<0.01), and GR activity in serum was significantly affected by Se level (P<0.05). The GR, total superoxide dismutase (T-SOD), catalase (CAT) activities and MDA content in liver and T-SOD activity in serum were extremely significantly affected by Se source (P<0.01). The interaction of Se level and Se source had significant effects on liver CAT activity, MDA content and serum T-SOD activity (P<0.05). With the Se level increasing, the activity of GSH-Px in liver and serum was increased firstly and then stabilized, and the GR activity in liver and serum was firstly decreased and then stabilized. The highest GSH-Px activity and the lowest GR activity in liver were obtained at the 0.9 mg/kg added level from two Se sources. The CAT activity in liver was increased with the Se level increasing. 3) Se content in vertebrae, liver and whole body was increased with Se level increasing. Se content in vertebrae was extremely significantly affected by Se source (P<0.01). Se content in liver and vertebrae was extremely significantly affected by the interaction of Se level and Se source (P<0.01). In conclusion, using Se-Met and Se-S as Se sources, the highest SGR of juvenile cobia can be obtained when dietary Se level is 1.29 and 1.46 mg/kg, respectivly, by quadratic polynomial regression equations. With SGR and Se content in whole body as dependent variables, the biological utilization of Se-Met is 1.20 and 2.90 times that of Se-S, respectivly.[ChineseJournalofAnimalNutrition, 2016, 28(12):3894-3904]
juvenile cobia (Rachycentroncanadum); selenium; growth performance; antioxidant enzyme; selenium accumulation
10.3969/j.issn.1006-267x.2016.12.022
2016-06-12
公益性行业(农业)科研专项(201003020);广东省农业攻关(2012B020307005);广东省教育厅引进人才项目(粤财教[2013]246号)
杨原志(1974—),男,山东郓城人,助理研究员,硕士,从事水产动物营养与饲料研究。E-mial: hdyyz@163.com
*同等贡献作者
**通信作者:董晓慧,教授,博士生导师,E-mail: dongxiaohui2003@163.com
S963
A
1006-267X(2016)12-3894-11
