酰腙配体双氧基钼(Ⅵ)配合物的合成、晶体结构和催化氧化性能
2016-12-15熊航行
熊航行
(荆楚理工学院化工与药学院,荆门448000)
酰腙配体双氧基钼(Ⅵ)配合物的合成、晶体结构和催化氧化性能
熊航行
(荆楚理工学院化工与药学院,荆门448000)
合成了2个新的单核双氧基钼(Ⅵ)配合物,其分子式通式为[MoO2L(MeOH)],其中L1和L2分别是N′-(3-溴-2-羟基苯亚甲基) -3,5-二甲氧基苯甲酰肼和N′-(5-溴-2-羟基苯亚甲基)异烟肼的二价阴离子,通过理化手段、光谱方法以及单晶X射线衍射表征了它们的结构。配合物1以单斜晶系P21/n空间群结晶,其晶体学参数a=0.848 98(3)nm,b=2.275 4(1)nm,c=1.069 82(5)nm,β= 109.108(1)°,V=1.952 8(1)nm3,Z=4,R1=0.036 7,wR2=0.084 0,S=1.027。配合物2以三斜晶系P1空间群结晶,其晶体学参数a= 0.655 05(9)nm,b=1.076 63(7)nm,c=1.303 3(1)nm,α=67.383(2)°,β=84.264(1)°,γ=76.195(2)°,V=0.823 9(1)nm3,Z=2,R1=0.071 3,wR2=0.151 0,S=1.004。X射线分析表明2个配合物中的Mo原子都采取八面体配位构型,配位原子分别来自酰腙配体的NO2给体基团和甲醇氧原子。催化实验表明2个配合物都是有效的烯烃氧化催化剂。
酰腙;钼配合物;晶体结构;催化性质
0 Introduction
The mechanism of molybdenum oxotransferase has been extensively investigated for a long time. Molybdenum complexes are known for considerable use in organic chemistry,in particular for the various oxidations of organic compounds[1-5].Dioxomolybdenum complexes have been intensively investigated as oxidation catalysts for variety of organic substrates, particularly for sulfoxidation and epoxidation of olefins[6-9].The synthesis,characterization and reactivity studies of a number of dioxomolybdenum complexes with Schiff bases have been reported[10-13].Some of the complexes have shown to possess oxygen atom transfer properties as they were found to oxidize thiols, hydrazine,polyketones and tertiary phosphines[14-15]. The catalytic ability of dioxomolybdenum(Ⅵ)complexes with benzohydrazone ligands toward the oxidation of sulfides have received satisfactory results[16-17].However, the number of documented dioxomolybdenum(Ⅵ) complexes catalyzing the peroxidic oxidation ofsulfides isstillvery limited.The rarely reportson such complexes are the preparation and structural characterization[18-21]. In the present paper,two new dioxomolybdenum(Ⅵ) complexes,[MoO2L1(MeOH)](1)and[MoO2L2(MeOH)] (2)(Scheme 1;H2L1=N′-(3-bromo-2-hydroxybenzylidene)-3,5-dimethoxybenzohydrazide for complex 1, H2L2=N′-(5-bromo-2-hydroxybenzylidene)isonicotinohydrazide for complex 2),were prepared and studied for their structures and catalytic properties.
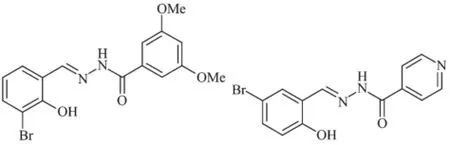
Scheme 1 Ligands H2L1(left)and H2L2(right)
1 Experimental
1.1 Materials and methods
All chemicals and solvents were of analytical reagent grade,and purchased from Beijing Chemical Reagent Company.The ligands H2L1and H2L2were prepared according to the literature method[22].Microanalyses(C,H,N)were performed using a Perkin-Elmer 2400 elemental analyzer.Infrared spectra were carried out using the JASCO FT-IR model 420 spectrophotometer with KBr disk in the region of 4 000~400 cm-1.GC analyses were performed on a Shimadzu GC-2014 gas chromatograph.
1.2 Syntheses of the complexes
A hot methanol solution(15 mL)of MoO2(acac)2(0.33 g,1 mmol)was added to a hotmethanol solution (15 mL)of H2L1(0.38 g,1 mmol)or H2L2(0.31 g,1 mmol).The mixture was refluxed for 30 minutes,and then cooled to room temperature,to give an orange solution.Single crystals suitable for X-ray diffraction were formed by slow evaporation of the solution containing the complex in air for a few days.
[MoO2L1(MeOH)](1).Yield:71%.Anal.Calcd.for C17H17BrMoN2O7(%):C,38.0;H,3.2;N,5.2.Found(%): C,37.8;H,3.1;N,5.3.IR data(KBr,cm-1):3 453w, 1 601m,1 441s,1 358m,1 164s,1 073s,949m,859s, 740w,540s,463w.UV-Vis data(acetonitrile,λ/nm): 300(15 510 L·mol-1·cm-1),395(3 020 L·mol-1·cm-1). ΛM(10-3mol·L-1in acetonitrile):23Ω-1·cm2·mol-1.
[MoO2L2(MeOH)](2).Yield:65%.Anal.Calcd. for C14H12BrMoN3O5(%):C,35.2;H,2.5;N,8.8.Found (%):C,35.3;H,2.6;N,8.7.IR data(KBr,cm-1):3 455w, 1 629m,1 434s,1 150s,1 081s,956m,851s,533s. UV-Visdata(acetonitrile,λ/nm):275(14 320 L·mol-1· cm-1),405(3 900 L·mol-1·cm-1).ΛM(10-3mol·L-1in acetonitrile):35Ω-1·cm2·mol-1.
1.3 X-ray structure determination
Single crystal X-ray diffraction measurement was performed using a Bruker Smart 1000 CCD diffractometer with graphite monochromated Mo Kαradiation (λ=0.071 073 nm)using theφ-ωscan technique.The positions of non-hydrogen atoms were located with direct methods.Subsequent Fourier syntheses were used to locate the remaining non-hydrogen atoms.All non-hydrogen atoms were refined anisotropically.The methanolhydrogen atoms were located from difference Fourier maps and refined isotropically,with O-H distances restrained to 0.085(1)nm.The remaining hydrogen atoms were placed in calculated positions and constrained to ride on their parent atoms.Theanalysis was performed with the SHELXS-97 and SHELXL-97 program[23-24].The crystallographic data for the complex are summarized in Table 1.Selected bond lengths and angles are given in Table 2.
CCDC:1476621,1;1476622,2.

Table 1 Crystal data for the complexes
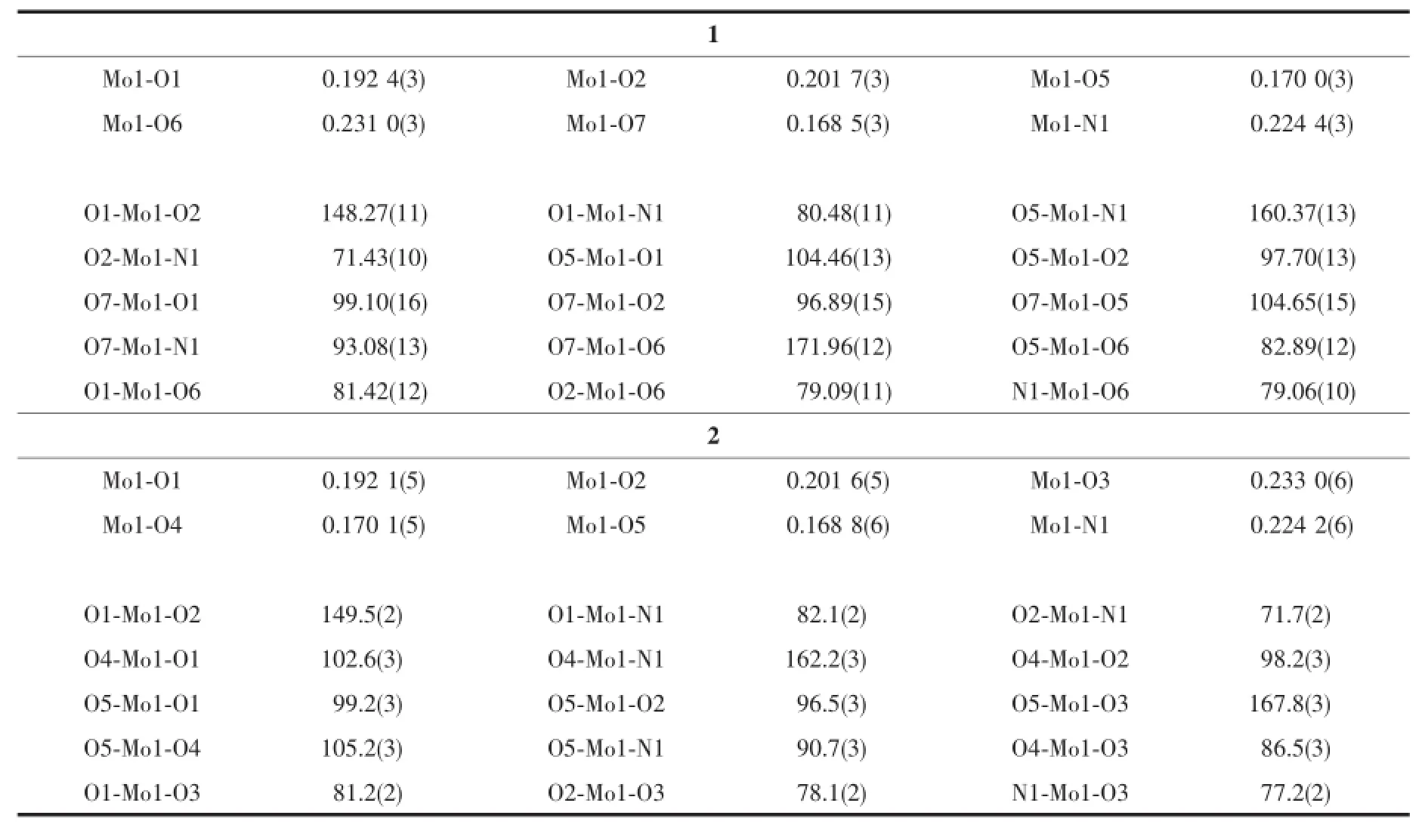
Table 2 Selected bond lengths(nm)and angles(°)for the complexes
2 Results and discussion
2.1 Synthesis
The hydrazone ligands H2L1and H2L2were readily prepared by the condensation reactions of 3-bromosalicylaldehyde with 3,5-dimethoxybenzohydrazide,and 5-bromosalicylaldehyde with isonicotinohydrazide,respectively,in methanol.The dioxomolybdenum(Ⅵ)complexes were synthesized by refluxing hydrazone ligands and MoO2(acac)2in methanolin a 1∶1 molar ratio.The reaction progress(Scheme 2)is accompanied by a color change of the solution from colorless to orange.We have attempted to prepare and grow diffraction quality crystals from various solvents, however,good quality crystals were finally obtained from methanol.The chemicalformulae ofthe complexes have been confirmed by elemental analyses,IR spectra, and X-ray single crystal structure determination.

Scheme 2 Preparation of the complexes
2.2 Structure description of the complexes
The molecular structures of complexes 1 and 2 are shown in Fig.1 and 2,respectively.The coordination geometry around the Mo atom can be described as a slightly distorted octahedral geometry, with the phenolic O,imino N,and enolic O atoms of the hydrazone ligand,one oxo O atom defining the equatorial plane,and with one methanol O atom and the other oxo O atom occupying the axial positions. The hydrazone ligand coordinates to the Mo atom in a meridional fashion forming five-and six-membered chelate rings with bite angles of 71.43(10)°and 80.48(11)°for 1 and 71.7(2)°and 82.1(2)°for 2.The dihedral angles between the aromatic rings of the hydrazone ligands are 11.2(3)°for 1 and 14.1(3)°for 2.The displacements of the Mo atoms in complexes 1 and 2 from the equatorial mean planes toward the apicaloxo atoms are 0.030 5(1)nm and 0.029 2(1)nm, respectively.The hydrazone ligands are coordinated in their dianionic form,which is evident from the N2-C8 and O2-C8 bond lengths in the hydrazone ligands. The abnormal bond values indicate the presence of the enolate form of the ligand amide groups.The Mo-O,Mo-N,and Mo=O bonds(Table 2)are within normal ranges and are similar to those observed in similar dioxomolybdenum(Ⅵ)complexes[18-21,25-28].

Fig.1 Molecular structure of 1 with 30%probability thermal ellipsoids

Fig.2 Molecular structure of 2 with 30%probability thermal ellipsoids
In the crystal structure of complex 1,adjacent two[MoO2L1]units are linked through two methanol ligands via hydrogen bonds(O6-H6 0.085(1)nm,H6…N2i0.192(2)nm,O6…N2i0.275 4(4)nm,O6-H6…N2i170(6)°,Symmetry codes:i-x,-y,1-z),to form a dimer.The dimers are further linked through weak Br…O interactions,to form a two-dimensional sheet(Fig.3).In the crystal structure of complex 2, adjacent two[MoO2L2]units are linked through two methanol ligands via hydrogen bonds(O3-H3 0.085(1) nm,H3…N3ii0.196(7)nm,O3…N3ii0.267 7(9)nm, O3-H3…N3ii141(10)°,Symmetry codes:ii1-x,1-y, -z),to form a dimer(Fig.4).
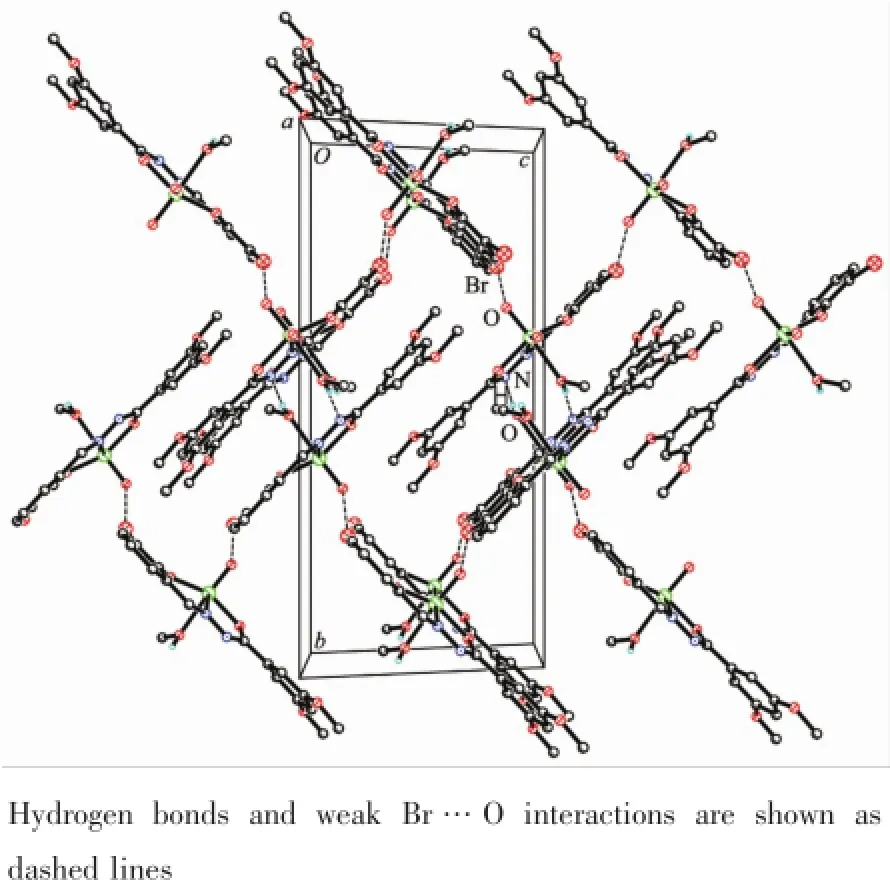
Fig.3 Molecular packing of 1 viewed along the a axis
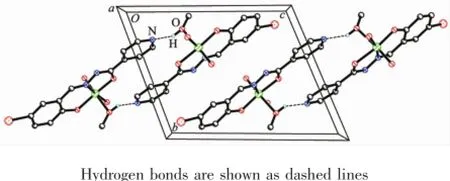
Fig.4 Molecular packing of 2 viewed along the a axis
2.3 IR spectra
The hydrazone ligands show stretching bands attributed to C=O,C=N,C-OH and NH at about 1 650, 1 610~1 620,1 180,and 3 250 cm-1,respectively[29]. For the complexes,absence ofthe bands characteristic of the N-H and C=O groups indicates enolization of the hydrazone ligands and coordination through the deprotonated enolic-oxygen atom.The Mo=O stretching mode occur as single and medium bands at 949 cm-1for 1 and 956 cm-1for 2,assigned to the asymmetric stretching mode of the MoO2moieties[29-30].The strong bands indicative of the C=N groups of the complexes are located at1 601 cm-1for1 and 1 629 cm-1for2[31].
Electronic spectra of the complexes recorded in acetonitrile solution display strong and medium absorption bands in the regions of 380~420 and 270~300 nm.These peaks are assigned as charge transfer transitionsofthe type N(pπ)-Mo(dπ)LMCT and O(pπ) -Mo(dπ)LMCT,respectively[22-23],as the ligand based orbitals are either N or O donor types.The slight changes ofλmaxvalues within each setofpeaks may be due to the difference of electron donating capacity of the hydrazone ligands.

Scheme 3 Proposed catalytic oxidation mechanism
2.4 Catalytic oxidation
The catalytic experiment was carried out according to the literature method by using tert-butyl hydrogen peroxide(TBHP)as oxidant[9].The results are listed in Table 3.Generally,excellent epoxide yields and selectivities were observed for all aliphatic and aromatic substrates.Oxidation of aromatic substrates gave the corresponding epoxides in 100% yields,while in the oxidation of aliphatic substrates, the conversion is less than 100%.Based on this consideration,it can be observed that the isolated double bonds are less reactive than the conjugated ones.The phenomenon is in good agreement withthose reported previously[9].There is no distinct difference for the catalytic property between the two complexes.The proposed catalytic mechanism of the catalytic reactions is shown in Scheme 3.First,TBHP was activated by coordination to the Mo atom and formation of hepta-coordinated molybdenum intermediate.Then a molecule of an olefin as a nucleophile attacks the electrophilic oxygen atom of the coordinated TBHP.As a result,the corresponding epoxides were formed,and TBHP was reduced to tertbutyl alcohol.

Table 3 Details of the catalytic oxidation of olefins catalyzed by the complexesa
3 Conclusions
Two new dioxomolydenum(Ⅵ)complexes with similar hydrazone ligands,N′-(3-bromo-2-hydroxybenzylidene)-3,5-dimethoxybenzohydrazide and N′-(5-bromo-2-hydroxybenzylidene)isonicotinohydrazide,have been prepared and structurally characterized by single crystal X-ray structure determination and infrared and electronic spectra.The hydrazone ligandscoordinate to the Mo atoms through the phenolic O, imino N and ethanolic O atoms.Both complexes have efficient catalytic properties on the oxidation of various olefins to their corresponding epoxides.
[1]Feng L S,Maass J S,Luck R L.Inorg.Chim.Acta,2011,373: 85-92
[2]Gamelas C A,Gomes A C,Bruno S M,et al.Dalton Trans., 2012,41:3474-3484
[3]Bagherzadeh M,Ghazali-Esfahani S.New J.Chem.,2012,36: 971-976
[4]Amarante T R,Neves P,Tome C,etal.Inorg.Chem.,2012,51: 3666-3676
[5]Neuenschwander J,Meier E,Hermans I.Chem.Eur.J.,2012, 18:6776-6780
[6]Krackl S,Company A,Enthaler S,etal.ChemCatChem,2011, 3:1186-1192
[7]Grover N,Kuhn F E.Curr.Org.Chem.,2012,16:16-32
[8]Jin N Y.J.Coord.Chem.,2012,65:4013-4022
[9]Rayati S,Rafiee N,Wojtczak A.Inorg.Chim.Acta,2012,386: 27-35
[10]Gao S P.J.Coord.Chem.,2011,64:2869-2877
[11]Tangestaninejad S,Moghadam M,Mirkhani V,et al.Catal. Commun.,2009,10:853-858
[12]El-Tabl A S,Issa R M,Morsi M A.Transition Met.Chem., 2004,29:543-549
[13]Grivani G,Tangestaninejad S,Halili A.Inorg.Chem. Commun.,2007,10:914-917
[14]Sheikhsoaie I,Rezaeffard A,Monadi N,et al.Polyhedron, 2009,28:733-738
[15]Rao S N,Kathale N,Rao N N,et al.Inorg.Chim.Acta, 2007,360:4010-4016
[16]Debel R,Buchholz A,Plass W.Z.Anorg.Allg.Chem., 2008,634:2291-2298
[17]Mancka M,Plass W.Inorg.Chem.Commun.,2007,10:677-680
[18]Vrdoljak V,Prugovecki B,Matkovic-Calogovic D,et al. Cryst.Growth Des.,2011,11:1244-1252
[19]Zhai Y L,Xu X X,Wang X.Polyhedron,1992,11:415-418
[20]Sergienko V S,Abramenko V L,Minacheva L K,et al. Koord.Khim.,1993,19:28-37
[21]LeiY,Fu C.Synth.React.Inorg.Met.-Org.Nano-Met.Chem., 2011,41:704-709
[22]Xu W X,Li W H.Synth.React.Inorg.Met.-Org.Nano-Met. Chem.,2012,42:160-164
[23]Sheldrick G M.SHELX-97,Program for Crystal Structure Solution and Refinement,University of Göttingen,Germany, 1997.
[24]Sheldrick G M.SHELXTL Version 5,Siemens Industrial Automation Inc.,Madison,1995.
[25]Dinda R,Ghosh S,Falvello L R,et al.Polyhedron,2006,25: 2375-2382
[26]Dinda R,Sengupta S,Ghosh S,et al.J.Chem.Soc.Dalton Trans.,2002,23:4434-4439
[27]Gupta S,Barik A K,Pal S,et al.Polyhedron,2007,26:133-141
[28]Bansse W,Ludwig E,Schilde U,etal.Z.Anorg.Allg.Chem., 1995,621:1275-1281
[29]Rao S N,Munshi K N,Rao N N,et al.Polyhedron,1999, 18:2491-2497
[30]El-Medani S M,Aboaly M M,Abdalla H H,et al.Spectrosc. Lett.,2004,37:619-632
[31]Ngan N K,Lo K M,Wong C S R.Polyhedron,2011,30: 2922-2932
Syntheses,Crystal Structures and Catalytic Oxidation of Dioxomolybdenum(Ⅵ) Complexes with Hydrazone Ligands
XIONG Hang-Xing
(College of Chemical Engineering and Pharmacy,Jingchu University of Technology,Jingmen,Hubei 448000,China)
Two new mononuclear dioxomolybdenum(Ⅵ)complexes with general formula[Mo O2L(Me OH)],where L1 and L2are the dianionic form of N′-(3-bromo-2-hydroxybenzylidene)-3,5-dimethoxybenzohydrazide and N′-(5-bromo-2-hydroxybenzylidene)isonicotinohydrazide,respectively,were prepared and structurally characterized by physico-chemical,spectroscopic methods and single crystal X-ray determination.The crystal of complex 1 crystallizes in monoclinic space group P21/n,with a=0.848 98(3)nm,b=2.275 4(1)nm,c=1.069 82(5)nm,β= 109.108(1)°,V=1.952 8(1)nm3,Z=4,R1=0.036 7,wR2=0.084 0,S=1.027.The crystal ofcomplex 2 crystallizes in triclinic space group P1,with a=0.655 05(9)nm,b=1.076 63(7)nm,c=1.303 3(1)nm,α=67.383(2)°,β=84.264(1)°, γ=76.195(2)°,V=0.823 9(1)nm3,Z=2,R1=0.071 3,wR2=0.151 0,S=1.004.X-ray analysis indicates that the Mo atom in each complex is in an octahedralcoordination environment,constructed by two oxo groups and NO2donor set of the hydrazone ligand,as well as a methanol O atom.The catalytic property of the complexes indicates that they are efficientcatalysts for the oxidation ofolefins.CCDC:1476621,1;1476622,2.
hydrazone;molybdenum complex;crystal structure;catalytic property
O614.61+2
A
1001-4861(2016)09-1596-07
10.11862/CJIC.2016.180
2016-05-01。收修改稿日期:2016-06-20。
E-mail:xionghx168@163.com
