Hydro-geochemical simulation of the mixing balance of exploitation and reinjection of geothermal fluid
2016-12-12LIUQiJIANGSiminPUYefengZHANGWei
LIU Qi, JIANG Si-min, PU Ye-feng, ZHANG Wei
1Department of Geotechnical Engineering, College of Civil Engineering, Tongji University, Shanghai 200092, China.
2Department of Hydraulic Engineering, College of Civil Engineering, Tongji University, Shanghai 200092, China.
3Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences, Shijiazhuang 050061, China.
Abstract: This paper targets its research at the exploitation-reinjection well of geothermal fluid of one geothermal heating project in Tianjin, China, examines such factors as ground temperature, CO2 partial pressure and stratum lithology, and simulates the changes in the main component contents of geothermal fluids mixed at different proportions in the exploitation and reinjection well. The research findings show that the mixed fluids are increasingly similar in nature to the reinjected water as the reinjection process goes on. It's suggested that the manual method should be used to ensure the reinjected water has the similar mineralization as the exploited ground water in the process of reinjection and some acceptable adjustments should be made according to the specific component and water temperature. The study on water-rock balance calculation shows that PHREEQC can simulate the complicated chemical reactions related to water when the transfer of solute happens, so the necessary technological supports are given for the reasonable development and protection of geothermal resources.
Keywords: Geothermal; Exploitation-reinjection; Groundwater; Hydro-geochemical simulation
Introduction
Currently, the reinjection of geothermal fluids can be used as an effective measure to guarantee the sustainable development and utilization of resources and have developed into one important component of present geo-thermal resource management work. The geothermal fluid components mainly come from geothermal reservoir and enclosing rock (WU Kong-jun et al. 2010). The sectional difference of hydro-chemical characteristics can often be used to identify the nature of faults (ZHANG Bao-jian et al. 2010). The research shows that the dynamic changes in hydro-chemical fields, temperature fields and hydrodynamic fields inside geothermal reservoir are closely related(ZHANG Bao-jian et al. 2010). During the re-injection of geothermal fluids, because the re-injected water has a lower temperature than the geothermal reservoir, the concentration of hydrochemical components may also differ from the fluids in original geothermal reservoir. Besides the changes in hydro-dynamic field and temperature field, the reinjection of geothermal reservoir may cause the changes in hydro-chemical field.Because it's hard to predict the chemical reactions caused by the mixture of water with different qualities, the actual conditions can hardly be identified by simulating the mixture of geothermal fluids through indoor test method, but the fully developed geo-chemical simulation method can be used to solve this problem (LI Jun-feng et al. 2013;WANG Wei-xing et al. 2010; ZHANG Xiao-lun et al. 2007; BI Er-ping et al. 1998; CHEN Zong-yu et al. 1998; LI Yi-lian et al. 2002).
With the exploitation and reinjection of geothermal fluid of one geothermal heating project in Tianjin as an example, this paper adopts the hydro-geochemical numeric simulation method,simulates the mixture of exploitation water and re-injected water at different proportions, considers the temperature and pressure of underground enclosing rock, performs the water-rock balance reaction when the stratum lithology is taken into consideration, forecasts the geo-chemical evolution process in the re-injection project, and offers the basis for proper development, exploitation and protection of geothermal resources from the hydro-geochemical perspective.
1 Hydro-geochemical simulation
1.1 Ionic dissociation theory
In general, any chemical balance or non-balance reactions, if reversible, can be described through the law of mass action.
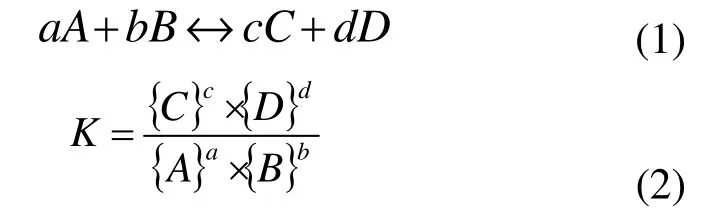
where, a, b, c and d are the mole numbers of reactants A and B, and reaction products C and D separately, and K is the thermodynamic balance constant in this reaction.
With Debye-Hüchel equation as a basis, ionic strength of solution can be used to calculate and produce the activity coefficient through regression equation.
In the Debye-Hüchel equation (Debye and Hüchel, 1923),

where, F is activity coefficient, z is ionic charge numbers, I is ionic strength, and A is temperature-related parameter. For the temperature of 25 ℃ and water density of 1 g/cm3, A=0.51.
The effective scope of ionic dissociation theory is within 1 mol/kg. Some researchers even think that the scope is no more than 0.7 mol/kg (sea water). When ionic strength is proved considerable(like the highly concentrated brine), Pitzer equation can be used (Merkel B J and Planer-Friedrich B, 2005). This semi-empirical model is also based on the Debye-Hüchel equation,and also involves Virial equation which describes the ionic reaction (intermolecular force). Unlike the ionic dissociation theory, this calculation actually proves more complex and needs more parameters. In fact, more complex solution components often lack these parameters. Furthermore,when ionic strength is more than 6 mol/L, Pitzer equation becomes less than practical (Merkel B J and Planer-Friedrich B, 2005).
1.2 PHREEQC model
The geo-chemical model is a conceptual model to describe geochemical functions through chemical reaction equation and mathematical calculation formula and one important approach to geochemical simulation and quantitative research into geochemical functions. PHREEQC is currently the well-developed hydro-geochemical simulation software and the calculation program created with C-language and based on the principles of thermodynamics and the theory of conservation of mass. It can almost solve the balance thermodynamics and chemical kinetics of all the water-gar-rock interaction systems including the mixture, dissolution and precipitation of water, soluble support, oxidation and reduction,and absorption and desorption.
In the deep geothermal reservoir, the geothermal fluids stay in an environment of high temperature and pressure. If the external action is absent, chemical components and surrounding rocks may reach or come close to the balance.When the new geothermal fluids are involved, the mixed water and the enclosing rock minerals will reach a new balance. So PHREEQC will be used to calculate the water-rock balance in mixed water at different proportions.
1.3 Basic setting
The geo-strata characteristics in the study area are dominated by dolomite, which mainly includes ions like Ca2+, Mg2+and. When the basic setting is simulated, this paper mainly considers three environmental factors like temperature,pressure and geo-strata conditions. In the geothermal fluids of geothermal reservoir, CO2partial pressure is usually 10-3kpa, and the sample of geothermal fluids is extracted from the mouth of pumping pipe of centrifugal pump. Due to the mechanical operation of centrifugal pump, the violent impact and vibration as well as the decreasing pressure can cause CO2to undergo the rapid volatilization. As a result, the following response equation will move rightwards.

The equation (4) shows that carbonate tends to precipitate and separate out as2COP decreases, and to change into the soluble ions like Ca2+andas2COP increases. Therefore, under the normal temperature and pressure, the conclusions drawn from the indoor water sample mixing experiments cannot reflect the actual situations in the environment of high temperature and pressure(LI Jun-feng et al. 2013).
In the temperature setting process, because different temperatures may cause some changes in geothermal fluid components, the mixing temperature of exploited geothermal fluid (93 ℃) and ground water in reinjection well (15.3 ℃) can be set as 15-93 ℃. Let's assume that there's one reaction system whose reaction temperature gradually increases from 15 ℃ to 93 ℃. We can observe how the contents of major components change at the possible temperature when the exploited geothermal fluid and the ground water in reinjection well are mixed at different proportions.This paper uses one geothermal heating project in Tianjin for the hydro-geochemical simulation of the mixing balance of exploitation and reinjection of geothermal fluid.
1.4 Mixing simulation of geothermal fluid and reinjection well water
When exploitation well and reinjection well are completed, their respective geothermal fluid's main ionic contents are indicated in Table 1. We can find that the geothermal fluids in exploitation well and the main water components of reinjection well differ in some ions. This simulation involves four steps: (1) Such data as the main ionic contents of ground water when the reinjection well is completed to define the water quality of this reinjection well; (2) Such data as the main ionic contents of geothermal fluids in exploitation well are used to define the geothermal fluids of exploitation well; (3) Given underground environment of high temperature (the mixing temperature 15-93 ℃), the geothermal fluids of exploitation well and the ground water of reinjection well are mixed at the volume ratio of 0.1:1-1:1 to calculate the distribution of main component contents of geothermal fluids at different exploitationreinjection mixing proportion, and the data at the temperature of 93 ℃ are used as an example for comparison; (4) Use the simulation results and the data of main component contents at different mixing proportion, consider the underground environment of high temperature (the mixing temperature 15-93 ℃) and the geo-strata conditions (mainly dolomite), compare the saturations of dolomite at each temperature and calculate and compare the mineralization of groundwater.

Table 1 Chemical analysis result of geothermal water (Unit: mg/L) (Sampling date:November, 2011)
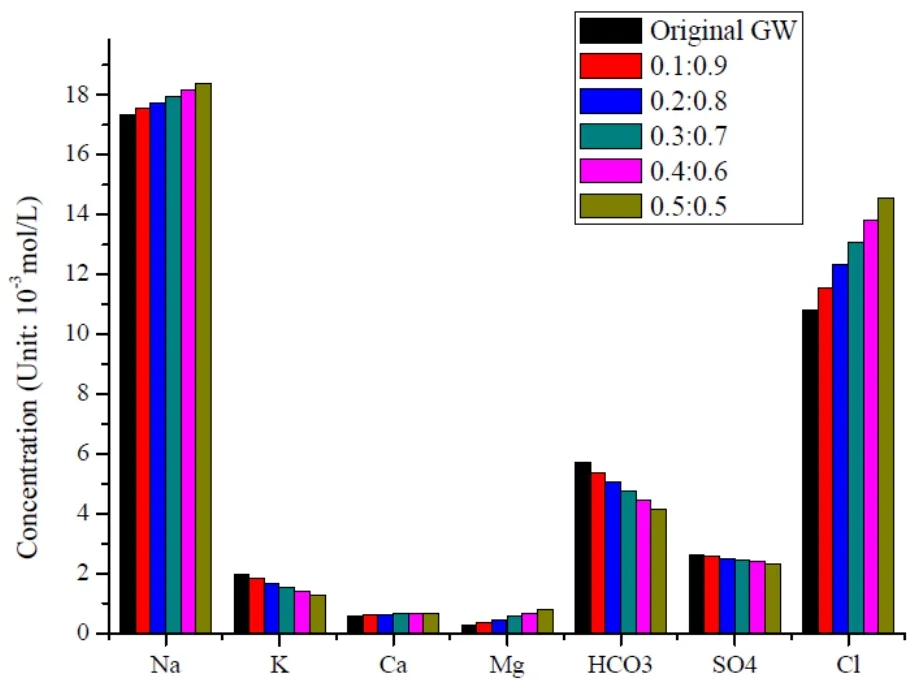
Fig. 1 Simulation results at different mixing proportion (93 ℃)
2 Analysis of simulation results
2.1 Changes of major ions
The simulation results show that, if the stratum lithology is not considered, the component contents of Na+and Cl-will gradually decrease but the component contents of other ions remain steady as the mixing proportion increases (Table 2).
As indicated in Fig. 1, as the mixing proportion increases, the component contents of Na+, Cl-, Ca2+and Mg2+all experience an upward trend, but the component contents of K+,andall go down. Coupled by the increasing reinjection proportion, the changes of main components are in consistency with the trend in which the mixed liquid gradually comes close to the component contents of reinjected water.

Table 2 Simulation results at different exploitation-reinjection mixing proportion (93 ℃) (Unit: mol/L)

Fig. 2 Change of Ca2+ at different mixing proportion (asterisk as measured value, the same as below)

Fig. 3 Change of Mg2+ at different mixing proportion

Fig. 4 Change of at different mixing proportion
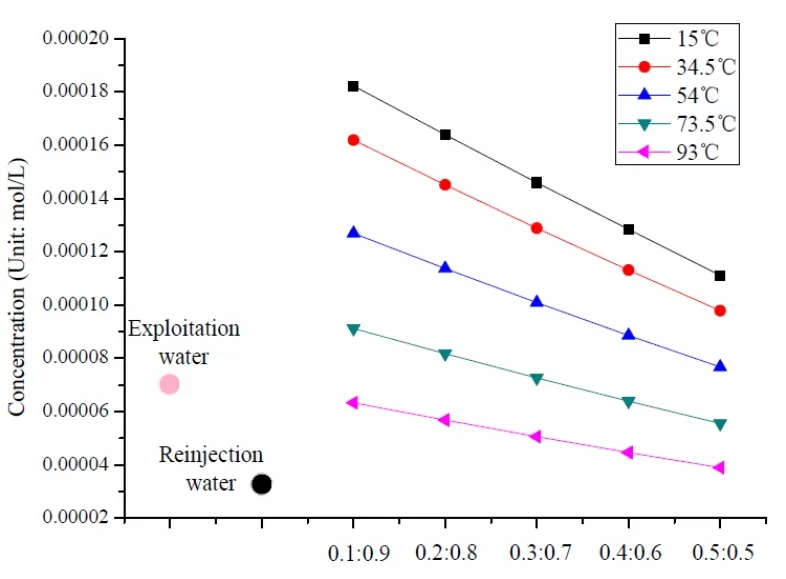
Fig. 5 Change of at different mixing proportion
The mixing of geothermal fluid in exploitation well (93 ℃) and ground water in reinjection well will cause the changes in the contents of main contents (15.3 ℃) when water temperature varies.The changes mainly happen to Ca2+, Mg2+,and(as indicated in Fig. 2-Fig. 5).
The content changes of Ca2+, Mg2+,andat different temperatures show that, within the scope of 15-93 ℃, the higher temperature of mixed fluid will lead to the lower concentration of four ions; in other words, the mixture reaction moves along the direction of precipitation; at the same temperature, the increase in mixing proportion will drive up the concentration of Ca2+and Mg2+, but drive down the concentration ofand.
In the mixed fluid, the content of Ca2+and Mg2+will decrease as temperature increases mainly because clathrate like,andcan be produced. What's more, the temperature increase will also contribute to the decomposition ofand then generate the precipitation of CaCO3anddecreases when temperature rises mainly because the temperature increase will cause its decomposition:

Since the above reaction moves along the positive direction, the content ofwill decrease; the content ofwill decrease when temperature increases because the temperature increase causes the decomposition of someand the possible production of carbonate, and the content ofwill decrease as temperature increases.
The content of Ca2+and Mg2+will increase when the reinjection proportion rises. It's true that some CaCO3and MgCO3may be produced in the reinjection process, but since the contents of Ca2+and Mg2+in reinjection water is noticeably higher than those in exploited ground water, the component of mixed fluid will gradually move into the reinjection water. As a result, the contents of Ca2+and Mg2+will increase when the reinjection proportion increases. The content ofwill decrease as the reinjection proportion increases.This can be attributed not only to its instability and possible decomposition, but to its low presence in reinjection water. So it will gradually decrease as the reinjection process moves on. Moreover, the production of clathrate likewill also cause the decrease of. Because the content ofin reinjection water is far lower than that in exploitation water, the overall trend is that the content ofwill gradually decrease as the reinjection process moves on.
2.2 Mineralization
Because the stratum lithology within the study area can influence the geothermal fluid, it's necessary to consider this factor in the simulated calculation of mixing process. Dolomite is the main composition of rock mass in the deep geothermal reservoir. According to Fig. 6, the temperature increase may causeto decompose and precipitate in reaction with some ions like Ca2+and Mg2+. So the mineralization of mixed fluid tends to decrease. Then, because the reinjection water itself has higher mineralization than exploited underground water, mineralization will gradually increase as the reinjection process moves on. Overall, all the ion concentration and mineralization will come close to the value of geothermal value in reinjection process.

Fig. 6 Change of mineralization at different mixing proportion
2.3 Saturation index of dolomite (SI)
The contents of Ca2+and Mg2+in the exploited water are lower than those in reinjection water, but the content ofin the exploited water is higher than that in reinjection water. As temperature increases,will decompose and precipitate in reaction with Ca2+and Mg2+.Because of the high contents ofin the exploited water, H+as the product of decomposition process will dissolve some precipitations following the precipitation reaction. So the saturation of dolomite in the exploited ground water will rise and then fall when temperature increases. Specifically, the mixed fluid will dissolve dolomite and then produce its precipitation as temperature moves up (Fig. 7).
The measured materials in Table 3 indicate that the mixed fluid almost has the same water quality as the exploited ground water at the early reinjection stage. The contents of Ca2+and Mg2+are lower, but the content ofis higher, so Ca2+and Mg2+will be used to produce more of clathrate instead of precipitation. So dissolution dominates the early reinjection stage. Then, as the reinjection process moves on, the reinjection proportion will constantly increase and the components of mixed fluid will gradually come close to those in reinjection water, so dolomite's SI will gradually decline.

Fig. 7 Change of dolomite's SI at different mixing proportions

Table 3 Measured data of groundwater
The simulation result shows that the dissolution of mixed fluid will become a less prominent trend at the high temperature, so the reinjection water can be used for reinjection after its temperature is raised a right level (advisably about 35 ℃).Because dolomite saturation at this temperature is the closest to the exploited water, the ground water and rock mass will confront the minimum influence. It is advisable to involve reinjection water and residual geothermal water in the reinjection process. For one thing, the component of residual geothermal water is roughly the same as the fluid component of underground geothermal reservoir, and can be mixed and neutralized with the component of reinjection water for the sake of reinjection. In this way, there will be a smaller influence on ground water and rock mass, and the processing cost and time of reinjection water can be saved. For another, the residual geothermal water usually stays at about 45 ℃, and once mixed with reinjection water, it will have a controlled temperature of 35 ℃, thus saving the hearting cost of reinjection water.
3 Conclusions
(1) In this simulation, the exploited water and the reinjection water are mixed at different proportions to simulate the reinjection process of groundwater. The simulation results show that the mixed fluid will has a more similar water quality as the reinjection water when the reinjection process moves on (the reinjection proportion increases). It is true that the original ground water(exploited water) has a different component from the reinjection water, but the overall trend remains steady and there's no undesirable reaction. It's feasible to use such reinjection water for the reinjection of geothermal fluid.
(2) The reinjection water and the exploited ground water differ in their components. The simulation results show that the mixed fluid will tend more to dissolve than to precipitate when the reinjection process moves on (the reinjection proportion increases). In other words, the mineralization of mixed fluid will increase but the saturation of dolomite will decrease. When temperature increases, all the ionic components will witness a downward trend and the dolomite's SI will show an upward and then downward trend.This means that the reaction in the mixed fluid shows a precipitation and then dissolution trend in the process of temperature increase.
(3) In the reinjection process, after the manual methods are used to make sure the similar mineralization between reinjection water and exploited water, it is also necessary to make some adjustments to the specific components. This simulation result indicates that the mixed fluid at a high temperature will have a less noticeable dissolution trend, so the reinjection water can be used for another reinjection after the temperature is raised to a right level (for instance, because the dolomite saturation is almost the same at 34.5 ℃and 93 ℃, and proves more economical when temperature rises to 34.5 ℃). In this way, an economical and safe result can be achieved.
Acknowledgements
This study is supported by geological survey projects of China Geological Survey(No.12120113077500), and National Basic Research Program of China (973 Program)(No.2013CB036001), National Natural Science Foundation of China (No.41302220).
杂志排行
地下水科学与工程(英文版)的其它文章
- Responses of groundwater system to water development in northern China
- Column test-based features analysis of clogging in artificial recharge of groundwater in Beijing
- Geological environment impact analysis of a landfill by the Yangtze River
- Research on Pisha-sandstone's anti-erodibility based on grey multi-level comprehensive evaluation method
- Study on ecological and economic effects of land and water resources allocation in Sanjiang Plain
- Evaluation of the water resources carrying capacity of Shandong peninsula, China
