Rosiglitazone ameliorates diffuse axonal injury by reducing loss of tau and up-regulating caveolin-1 expression
2016-12-02YonglinZhaoJinningSongXudongMaBinfeiZhangDandongLiHonggangPang
Yong-lin Zhao, Jin-ning Song, Xu-dong Ma, Bin-fei Zhang, Dan-dong Li, Hong-gang Pang
Department of Neurosurgery, the First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, Shaanxi Province, China
RESEARCH ARTICLE
Rosiglitazone ameliorates diffuse axonal injury by reducing loss of tau and up-regulating caveolin-1 expression
Yong-lin Zhao, Jin-ning Song*, Xu-dong Ma, Bin-fei Zhang, Dan-dong Li, Hong-gang Pang
Department of Neurosurgery, the First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, Shaanxi Province, China
Graphical Abstract

orcid: 0000-0002-0620-8983 (Jin-ning Song)
Accepted: 2015-12-22
Rosiglitazone up-regulates caveolin-1 levels and has neuroprotective effects in both chronic and acute brain injury. Therefore, we postulated that rosiglitazone may ameliorate diffuse axonal injury via its ability to up-regulate caveolin-1, inhibit expression of amyloid-beta precursor protein, and reduce the loss and abnormal phosphorylation of tau. In the present study, intraperitoneal injection of rosiglitazone significantly reduced the levels of amyloid-beta precursor protein and hyperphosphorylated tau (phosphorylated at Ser404(p-tau (S404)), and it increased the expression of total tau and caveolin-1 in the rat cortex. Our results show that rosiglitazone inhibits the expression of amyloid-beta precursor protein and lowers p-tau (S404) levels, and it reduces the loss of total tau, possibly by up-regulating caveolin-1. These actions of rosiglitazone may underlie its neuroprotective effects in the treatment of diffuse axonal injury.
nerve regeneration; diffuse axonal injury; rosiglitazone; hyperphosphorylated tau; total tau; caveolin-1; rats; amyloid precursor protein; ser404; cortex; immunocytochemistry; western blot assay; neural regeneration
Introduction
There is an urgent need for more effective treatments for traumatic brain injury, including diffuse axonal injury (DAI). In this study, we examined whether rosiglitazone (RSG), a peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist, may have therapeutic potential for DAI. DAI initiates a series of pathophysiological changes including inflammation and glutamate excitotoxicity, resulting in neurodegeneration, neuronal death and neurological dysfunction (Chelly et al., 2011). During axonal degeneration, hyperphosphorylated microtubule-associated protein tau dissociates from microtubules, leading to total tau loss, microtubule destabilization and morphological changes in axons (Dong et al., 2014; Lv et al., 2014). As a consequence, amyloid-beta precursor protein (β-APP) accumulates rapidly and massively, serving as a sensitive biomarker for diagnosis of DAI (Li et al., 2010). Although there are many promising drug and cell-based therapeutic approaches for reducing brain injury and enhancing functional outcome after DAI, the clinical effectiveness of these approaches is limited (Xiong et al., 2009). Consequently, further research is needed to explore new therapeutic strategies for DAI.
RSG is a Food and Drug Administration-approved drug with few short-term side effects, and is an excellent candidatefor rapid translation to clinical trials. RSG is currently in Phase III clinical trials for Alzheimer's disease, based on its ability to reduce β-amyloid pathology and inflammation (Liu et al., 2015). RSG has been reported to reduce neuroinflammation, oxidative stress, apoptotic markers and lesion volume in mouse models of traumatic brain injury (Yonutas et al., 2013; Yao et al., 2015). However, few studies have investigated whether RSG affects tau phosphorylation or tau loss, which are pathological features of both Alzheimer's disease and DAI (Xu et al., 2014). Cheng et al. (2014) reported that RSG suppresses the proliferation of vascular smooth muscle cells by up-regulating caveolin-1, and that it attenuates cerebral vasospasm following experimental subarachnoid hemorrhage. The up-regulation of caveolin-1 by RSG may also impact β-APP levels and tau hyperphosphorylation (Hattori et al., 2006; Head et al., 2010). However, it remains unknown whether RSG has therapeutic efficacy for DAI. In the present study, we investigated whether intraperitoneal injection of RSG rescues the axonal pathology in rats with DAI, and we examined the underlying mechanisms.
Materials and Methods
Animals
A total of 108 male 8—10-week-old Sprague-Dawley rats weighing 280—320 g were purchased from the Experimental Animal Center of Xi'an Jiaotong University of China (license No. SCXK (Shaanxi) 2006-001). Animals were housed and fed in a temperature- and humidity-controlled environment with a standardized 12-hour reversed light-dark cycle for 1 week. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Biomedical Ethics Committee of Medical College of Xi'an Jiaotong University of China (2014-124).
Establishment of a rat model of DAI and RSG administration
To investigate changes in levels of β-APP, hyperphosphorylated tau (phosphorylated at serine 404 (p-tau (S404)) and total tau after DAI, and to evaluate the effect of RSG, 84 rats were randomly assigned to 7 groups (n = 12 per group) as follows: control group, DAI 6 hour (h) group, DAI 1 day (d) group, DAI 3 d group, DAI 7 d group, DAI 3 d + saline group (the same as the DAI 3 d group, but given intraperitoneal injection of saline at 5 minutes, 24 h and 48 h post DAI), and DAI 3 d + RSG group (the same as the DAI 3 d + saline group, but administered RSG (Cayman Chemical Co., Ann Arbor, MI, USA), 10 mg/kg intraperitoneal injection, diluted with saline to a final concentration of 2 mg/mL prior to injection). Rats were euthanized at the indicated time points post injury.
The DAI model was established using a lateral head rotation device, which was created by our team (Li et al., 2013). After anesthesia with 30 g/L pentobarbital sodium (intraperitoneal injection, 30 mg/kg), the rat's head was horizontally secured to the lateral head rotation device by two lateral ear bars, a head clip and an anterior teeth hole, with its body at a 30° angle with respect to the top of the laboratory table. When the trigger was pushed, the rat head was rapidly rotated 90°, involving sudden acceleration and deceleration. All injured rats were in a coma for at least 30 minutes. The control group only underwent anesthesia and fixation to the device. Rats that died because of injury were excluded and later replaced by new rats.
Hematoxylin-eosin staining
To ensure that the animal model of DAI used in this study was successful, hematoxylin-eosin staining was performed. Rats in each group were deeply anesthetized and perfused with 250 mL of normal saline followed by 400 mL of 40 g/L paraformaldehyde. The whole brain was removed and postfixed in paraformaldehyde. All tissues were desiccated, embedded in paraffin, and sectioned into 5-µm-thick sections. Three sections per animal were processed for hematoxylin-eosin staining. Hematoxylin-eosin-stained sections were observed at 400× magnification (BX-40; Olympus, Tokyo, Japan).
Semi-quantitative immunohistochemical staining
Brain sections were de-paraffinized in xylene and rehydrated in a decreasing graded alcohol series and distilled water. Endogenous peroxidase activity was quenched with 3% H2O2for 15 minutes, followed by a wash in phosphate-buffered saline (PBS). Sections were placed in 0.01 M citrate buffer and heated in a microwave oven at 95°C for 30 minutes. Sections were cooled at room temperature for 40 minutes and rinsed in PBS. Non-specific protein binding was blocked with normal goat serum at room temperature for 30 minutes, followed by incubation with primary antibodies—rabbit anti-caveolin-1 monoclonal antibody (3267P, 1:1,000; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-tau (phospho S404) polyclonal antibody (ab131338, 1:200; Abcam, Cambridge, UK) or rabbit anti-β-APP monoclonal antibody (ab32136, 1:100; Abcam)—for 24 hours at 4°C, followed by a 15-minute wash in PBS. Sections were then incubated with goat anti-rabbit IgG-biotin for 30 minutes (sp9001, 1:200; ZSGB-BIO Co., Beijing, China) followed by streptavidin-horseradish peroxidase for 30 minutes at 37°C. Sections were washed with PBS for 15 minutes after each step. Diaminobenzidine was used as the chromogen, and hematoxylin was used as the counterstain.
Microscopic observation of the immunohistochemically-stained sections was performed by an experienced pathologist blinded to the experimental conditions. Six animals in each group and five sections per animal were chosen for quantitative analysis. Under a light microscope (Olympus), each section was scored in five random visual fields at 400× magnification. Immunoreactivity was scored based on the number of positive cells and staining intensity using Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA). The immunohistochemical score was obtained by multiplying the staining quantity and intensity scores(Soslow et al., 2000). The staining quantity was rated on a scale of 0—4 as follows: 0, no staining; 1, 1—10% cells stained; 2, 11—50%; 3, 51—80%; and 4, 81—100%. Staining intensity was rated on a scale of 0—3 as follows: 0, negative; 1, weak; 2, moderate; and 3, strong. Theoretically, the scores can range from 0 to 12. An immunohistochemical score of 9—12 was considered to indicate strong immunoreactivity; 5—8, moderate; 1—4, weak; and 0, negative.

Figure 1 Photomicrographs of the cerebral cortex in the control and DAI groups (hematoxylin-eosin staining).
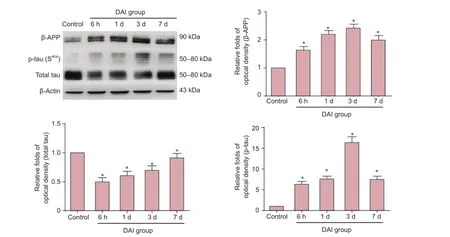
Figure 2 Dynamic expression of β-APP, p-tau (S404) and total tau protein in the cortex of control and DAI groups assessed by western blot assay.
Western blot assay
Rats were anesthetized, and the cortex was immediately removed and stored in liquid nitrogen until processing. Total protein was purified using radioimmunoprecipitation assay buffer (Sigma, St. Louis, MO, USA). Protein samples (20 µg) were analyzed with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred onto polyvinylidene fluoride membranes (Merck Millipore, Darmstadt, Germany). The membranes were blocked with 5% skimmed milk powder in Tris-buffered saline/Tween 20 buffer (TBST) for 2 hours at room temperature, and incubated overnight with rabbit anti-tau (phospho S404) polyclonal antibody (ab131338, 1:1,000; Abcam), rabbit anti-β-APP monoclonal antibody (ab32136, 1:1,000; Abcam), mouse anti-tau (tau46) monoclonal antibody (4019P, 1:1,000; Cell Signaling Technology) or monoclonal rabbit anti-caveolin-1 antibody (3267P, 1:1,000; Cell Signaling Technology). Membranes were washed three times with TBST for 10 minutes each, and then incubated with goat anti-rabbit IgG-horseradish peroxidase secondary antibody (ab6721, 1:3,000; Abcam) or goat anti-mouse IgG-horseradish peroxidase secondary antibody (ab97023, 1:3,000; Abcam) for 1 hour at room temperature, with subsequent washing in TBST. β-Actin (ab8227, 1:5,000; Abcam, Cambridge, UK) was used as an internal control for protein loading. The membranes were visualized using the ChemiDoc MP System (Bio-Rad, Hercules, CA, USA) with enhanced chemiluminescence substrate (Millipore). Densitometric quantification of the bands was performed usingImage J software (version 1.29x, NIH, Bethesda, MD, USA). First, the ratio of the optical density of the protein to that of β-actin was determined. These values in the experimental groups were then normalized to the values in the control group to obtain relative expression levels.
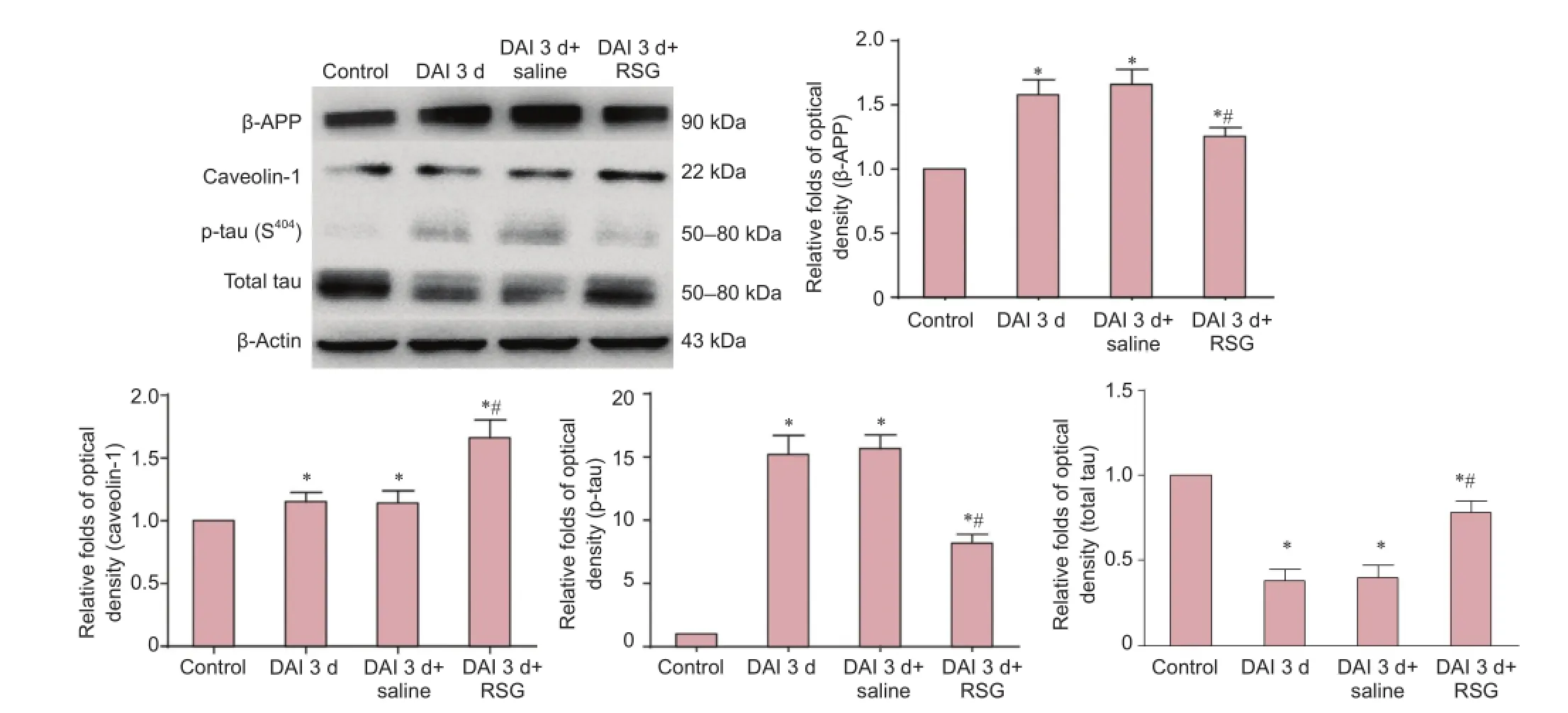
Figure 3 Treatment with RSG blocks the increases in β-APP and p-tau (S404) levels and up-regulates the expression of total tau and caveolin-1 in the cortex 3 d after DAI.
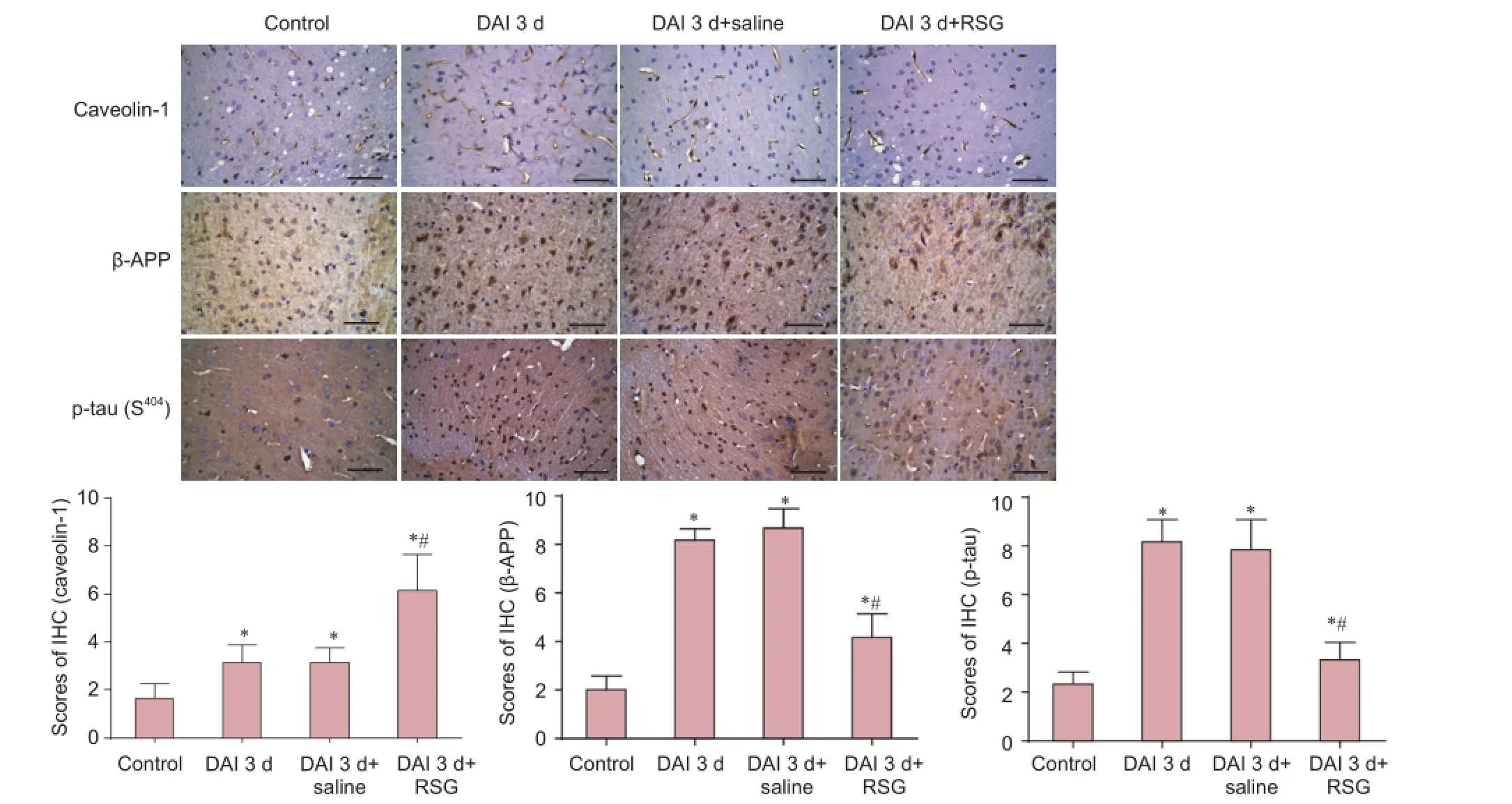
Figure 4 RSG administration inhibits the increases in β-APP and p-tau (S404) and up-regulates caveolin-1 expression in the rat cortex 3 d afterDAI (immunohistochemical staining).
Statistical analysis
SPSS 17.0 software (SPSS, Chicago, IL, USA) was used for statistical analyses. All data were expressed as the mean ± SD. Comparisons among multiple groups were performed using one-way analysis of variance, followed by Tukey's post hoc test. A P-value of less than 0.05 was considered statistically significant.
Results
Histopathological changes in the rat model of DAI
Hematoxylin-eosin-stained sections showed the presence of vacuoles around neurons in the cerebral cortex of rats with DAI. Pyknosis was observed in the DAI 6 h, 1 d, 3 d and 7 d groups (Figure 1B—E). Swollen neurons and distorted axons were visible in the cerebral cortex of the DAI 6 h and 1 d groups (Figure 1B, C). Pyknotic, swollen and tangled neurons were clearly visible in the DAI 1 d group (Figure 1C). In contrast, in the cortex of rats in the control group, these features were absent (Figure 1A).
Dynamic changes in expression of β-APP and in p-tau (S404) and total tau levels
Compared with the control group, the expression of β-APP was increased 1.6-fold, 2.1-fold, 2.4-fold and 1.9-fold in the DAI 6 h, 1 d, 3 d and 7 d groups, respectively (P < 0.05). Compared with the control group, the levels of p-tau (S404) were increased 6.2-fold, 7.6-fold, 16.7-fold and 7.4-fold, respectively. Levels of total tau were decreased to 0.5, 0.6, 0.7 and 0.9× the levels in the control group in the DAI 6 h, 1 d, 3 d and 7 d groups, respectively (all P < 0.05) (Figure 2).
Effects of RSG on β-APP, p-tau (S404), total tau and caveolin-1 levels 3 days after DAI
Western blot assay
Compared with the control group, β-APP expression was increased 1.6-fold, 1.8-fold and 1.3-fold in the DAI 3 d, DAI 3 d + saline and DAI 3 d + RSG groups, respectively (all P < 0.05). p-tau (S404) levels were increased 15.1-fold, 16.2-fold and 7.9-fold in the DAI 3 d, DAI 3 d + saline and DAI 3 d + RSG groups, respectively (all P < 0.05). Caveolin-1 expression was increased 1.1-fold, 1.2-fold and 1.6-fold in the DAI 3 d, DAI 3 d + saline and DAI 3 d + RSG groups, respectively (all P < 0.05). Total tau levels were decreased to 0.4, 0.4 and 0.8× the level in the control group in the DAI 3 d, DAI 3 d + saline and DAI 3 d + RSG groups, respectively (all P < 0.05). There were no significant differences between the DAI 3 d and DAI 3 d + saline groups (all P > 0.05). Compared with the DAI 3 d or DAI 3 d + saline groups, the DAI 3 d + RSG group showed increased caveolin-1 expression and decreased p-tau (S404) and β-APP levels (P < 0.05) (Figure 3).
Immunohistochemical staining
p-tau (S404) levels were significantly higher in the DAI 3 d and DAI 3 d + saline groups, with scores of 8.5 and 7.9, respectively, compared with the control group, which had a score of 2.6 (P < 0.05). The DAI 3 d + RSG group showed decreased p-tau (S404) staining with a score of 4.3 (P < 0.05, vs. DAI 3 d or DAI 3 d + saline groups). Similarly, β-APP expression was significantly higher in the DAI 3 d and DAI 3 d + saline groups, with scores of 7.6 and 8.2, respectively, compared with the control group, which had a score of 1.9 (P< 0.05). β-APP expression in the DAI 3 d + RSG group was decreased, with a score of 3.6 (P < 0.05, vs. DAI 3 d or DAI 3 d + saline groups). Caveolin-1 was mainly expressed in the cell membrane in the cerebral cortex. Caveolin-1 expression was significantly higher in the DAI 3 d and DAI 3 d + saline groups, with scores of 3.1 and 3.3, respectively, compared with the control group, which had a score of 1.5 (P < 0.05). RSG treatment increased caveolin-1 expression (score of 6.0; P < 0.05, vs. DAI 3 d or DAI 3 d + saline groups) (Figure 4).
Discussion
Hyperphosphorylation and loss of tau have been implicated in the pathogenesis of DAI. β-APP accumulates rapidly and massively in axonal bulbs when the axon is damaged (Li et al., 2010). Therefore, drugs that simultaneously attenuate tau hyperphosphorylation, lower β-APP levels and inhibit the decrease in total tau may have therapeutic potential in the treatment of DAI in patients. In this study, we investigated the pathology and dynamic changes in the levels of β-APP, p-tau (S404) and total tau at different time points in a rat model of DAI. The effects of RSG on β-APP, p-tau (S404), total tau and caveolin-1 at 3 d after DAI were also assessed. The major findings of our study are as follows: (1) the levels of p-tau (S404) and β-APP peaked 3 d post DAI, while total tau levels decreased after DAI; (2) RSG treatment attenuated DAI-induced increases in β-APP and p-tau (S404) levels; (3) RSG prevented the decrease in total tau levels 3 d after DAI; (4) RSG treatment increased caveolin-1 expression.
β-APP can be detected at the site of axonal injury. Mu et al. (2015) reported that following impact acceleration traumatic brain injury, β-APP is detectable in injured axons as early as 2 to 6 h after trauma and continues to accumulate 1—3 d post injury. Hyperphosphorylation of tau results in microtubule destabilization. Shultz et al. (2015) showed that in the rat DAI model created by lateral fluid percussion, there is increased phosphorylation of tau (p-tau (S198/S262)) in the cortex 24 h and 3 d post injury, although total tau levels did not significantly differ from the control group (Shultz et al., 2015). In the present study, β-APP expression and hyperphosphorylation of tau, induced by rapid lateral head rotation, had similar temporal trends, with significantly increased expression from 6 h to 3 d, in accordance with the previous study. However, p-tau (S404) was detected soon after DAI, while total tau was significantly decreased at 6 h, 1 d and 3 d post DAI in this study. Moreover, the histopathological study revealed the presence of axonal injury 6 h post injury, which greatly worsened 1 to 3 h post injury. These findings suggest thatsecondary processes contribute to further axonal damage and degeneration after the initial injury.
RSG is a commonly prescribed insulin-sensitizing drug that is a selective agonist of PPAR-γ. It has been shown that RSG provides neuroprotection in animal models of focal ischemia, spinal cord injury, and Alzheimer's disease (Madeira et al., 2015). RSG also confers neuroprotection after traumatic brain injury via anti-inflammatory, anti-apoptotic, anti-oxidative mechanisms (Yi et al., 2008).
Researches on the effects of RSG on tau hyperphosphorylation have mainly focused on Alzheimer's disease. RSG alleviates spatial learning deficits in APP/PS1/tau transgenic mice by reducing tau hyperphosphorylation in the brain (Mazanetz and Fischer, 2007; Escribano et al., 2010; Yoon et al., 2010; Tokutake et al., 2012; Song et al., 2014; Yu et al., 2014). In addition, there are a few reports on the effects of RSG on β-APP and total tau.
The present study is the first to demonstrate that RSG treatment attenuates the dramatic rise in β-APP expression and p-tau (S404) levels after DAI. Furthermore, RSG inhibited the decrease in total tau following DAI, thereby producing a neuroprotective effect. However, the mechanisms underlying these effects of RSG after DAI are still unknown.
In this study, the down-regulation of β-APP and p-tau (S404) induced by RSG was accompanied by increased expression of caveolin-1, a scaffolding protein in caveolae that physically interacts with membrane-associated signaling molecules. RSG dose and time-dependently increases caveolin-1 mRNA and protein levels by activating PPAR-γ and/or epidermal growth factor receptor (Burgermeister et al., 2003; Llaverias et al., 2004; Seda et al., 2008; Tencer et al., 2008). Up-regulation of caveolin-1 by RSG may mediate some of the phenotypic changes in cancer cells, suppress tumor growth and inhibit proliferation of vascular smooth muscle cells after subarachnoid hemorrhage (Burgermeister et al., 2003; Chintharlapalli et al., 2004; Cheng et al., 2014). When caveolin-1 is overexpressed, β-APP and β-secretase localize to caveolae, resulting in decreased β-amyloid production, suggesting a protective role of caveolin-1 (Hattori et al., 2006). Hyperphosphorylation of tau is observed in hippocampal homogenates from caveolin-1 knockout mice. Knockout of caveolin-1 accelerates neurodegeneration and aging (Head et al., 2010). However, caveolin-1 deletion reduces early brain injury after experimental intracerebral hemorrhage (Chang et al., 2011). Our current findings suggest that the up-regulation of caveolin-1 produced by RSG is neuroprotective, and may lower β-APP and p-tau (S404) levels and inhibit the reduction in total tau. Further studies are required to clarify the underlying mechanisms of action.
There are multiple phosphorylation sites on tau, including Ser404, Ser198and Thr205. The phosphorylation status of these sites differ in different pathological conditions. In this study, only one site (Ser404) was assessed. Additional phosphorylation sites should be examined, and the effects of RSG on neurological deficits should also be examined in future studies. Furthermore, additional studies are needed to clarify how the RSG-induced up-regulation of caveolin-1 provides neuroprotection. Interestingly, the inhibition of PPAR-γ results in a decrease in proliferation and loss of the undifferentiated phenotype in neural precursor cells (Bernal et al., 2015). Therefore, RSG treatment may also promote nerve regeneration.
In summary, RSG exerts a significant neuroprotective effect by suppressing excessive expression of β-APP, by lowering p-tau (S404) levels, and by preventing the decrease in total tau in a rat model of DAI. Caveolin-1 may be involved in the neuroprotection provided by RSG. Our novel findings suggest that RSG may have therapeutic potential for the treatment of DAI. Further studies are required to elucidate the molecular mechanisms underlying the neuroprotective effects of RSG.
Author contributions: JNS obtained funding, participated in the definition of intellectual content of this topic and paper review. YLZ and XDM did literature searching, designed the experiment and collected data. YLZ, XDM, and BFZ wrote the paper and provided critical revision of the paper. DDL and HGP made the model and contributed to statistical analysis. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Bernal C, Araya C, Palma V, Bronfman M (2015) PPARβ/δ and PPARγ maintain undifferentiated phenotypes of mouse adult neural precursor cells from the subventricular zone. Front Cell Neurosci 9:78-86.
Burgermeister E, Tencer L, Liscovitch M (2003) Peroxisome proliferator-activated receptor-gamma upregulates caveolin-1 and caveolin-2 expression in human carcinoma cells. Oncogene 22:3888-3900.
Chang CF, Chen SF, Lee TS, Lee HF, Chen SF, Shyue SK (2011) Caveolin-1 deletion reduces early brain injury after experimental intracerebral hemorrhage. Am J Pathol 178:1749-1761.
Chelly H, Chaari A, Daoud E, Dammak H, Medhioub F, Mnif J, Hamida CB, Bahloul M, Bouaziz M (2011) Diffuse axonal injury in patients with head injuries: an epidemiologic and prognosis study of 124 cases. J Trauma 71:838-846.
Cheng MF, Song JN, Li DD, Zhao YL, An JY, Sun P, Luo XH (2014) The role of rosiglitazone in the proliferation of vascular smooth muscle cells after experimental subarachnoid hemorrhage. Acta Neurochir 156:2103-2109.
Chintharlapalli S, Smith R, Samudio I, Zhang W, Safe S (2004) 1, 1-Bis (3'-indolyl)-1-(p-substitutedphenyl) methanes induce peroxisome proliferator-activated receptor gamma-mediated growth inhibition, transactivation, and differentiation markers in colon cancer cells. Cancer Res 64:5994-6001.
Dong DW, Zhang YS, Yang WY, Wang-Qin RQ, Xu AD, Ruan YW (2014) Hyperphosphorylation of tau protein in the ipsilateral thalamus after focal cortical infarction in rats. Brain Res 1543:280-289.
Escribano L, Simon AM, Gimeno E, Cuadrado-Tejedor M, Lopez de Maturana R, Garcia-Osta A, Ricobaraza A, Perez-Mediavilla A, Del Rio J, Frechilla D (2010) Rosiglitazone rescues memory impairment in Alzheimer's transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology 35:1593-1604.
Hattori C, Asai M, Onishi H, Sasagawa N, Hashimoto Y, Saido TC, Maruyama K, Mizutani S, Ishiura S (2006) BACE1 interacts with lipid raft proteins. J Neurosci Res 84:912-917.
Head BP, Peart JN, Panneerselvam M, Yokoyama T, Pearn ML, Niesman IR, Bonds JA, Schilling JM, Miyanohara A, Headrick J, Ali SS, Roth DM, Patel PM, Patel HH (2010) Loss of caveolin-1 accelerates neurodegeneration and aging. PLoS One 5:e15697.
Li J, Li XY, Feng DF, Pan DC (2010) Biomarkers associated with diffuse traumatic axonal injury: exploring pathogenesis, early diagnosis, and prognosis. J Trauma 69:1610-1618.
Li Y, Song J, Liu X, Zhang M, An J, Sun P, Li D, Jin T, Wang J (2013) High expression of STIM1 in the early stages of diffuse axonal injury. Brain Res 1495:95-102.
Liu J, Wang LN, Jia JP (2015) Peroxisome proliferator-activated receptor-gamma agonists for Alzheimer's disease and amnestic mild cognitive impairment: a systematic review and meta-analysis. Drugs Aging 32:57-65.
Llaverias G, Vazquez-Carrera M, Sanchez RM, Noe V, Ciudad CJ, Laguna JC, Alegret M (2004) Rosiglitazone upregulates caveolin-1 expression in THP-1 cells through a PPAR-dependent mechanism. J Lipid Res 45:2015-2024.
Lv Q, Lan W, Sun W, Ye R, Fan X, Ma M, Yin Q, Jiang Y, Xu G, Dai J, Guo R, Liu X (2014) Intranasal nerve growth factor attenuates tau phosphorylation in brain after traumatic brain injury in rats. J Neurol Sci 345:48-55.
Madeira JM, Schindler SM, Klegeris A (2015) A new look at auranofin, dextromethorphan and rosiglitazone for reduction of glia-mediated inflammation in neurodegenerative diseases. Neural Regen Res 10:391-393.
Mazanetz MP, Fischer PM (2007) Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov 6:464-479.
Mu J, Song Y, Zhang J, Lin W, Dong H (2015) Calcium signaling is implicated in the diffuse axonal injury of brain stem. Int J Clin Exp Pathol 8:4388-4397.
Shultz SR, Wright DK, Zheng P, Stuchbery R, Liu SJ, Sashindranath M, Medcalf RL, Johnston LA, Hovens CM, Jones NC, O'Brien TJ (2015) Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Brain 138:1297-1313.
Seda O, Sedova L, Oliyarnyk O, Kazdova L, Krenova D, Corbeil G, Hamet P, Tremblay J, Kren V (2008) Pharmacogenomics of metabolic effects of rosiglitazone. Pharmacogenomics 9:141-155.
Song JZ, Sun J, Jin DC, Deng YQ (2014) Rosiglitazone improves learning and memory impairment of 3 x Tg mice. Yao Xue Xue Bao 49:807-812.
Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT (2000) COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 89:2637-2645.
Tencer L, Burgermeister E, Ebert MP, Liscovitch M (2008) Rosiglitazone induces caveolin-1 by PPARγ-dependent and PPRE-independent mechanisms: the role of EGF receptor signaling and its effect on cancer cell drug resistance. Anticancer Res 28:895-906.
Tokutake T, Kasuga K, Yajima R, Sekine Y, Tezuka T, Nishizawa M, Ikeuchi T (2012) Hyperphosphorylation of Tau induced by naturally secreted amyloid-beta at nanomolar concentrations is modulated by insulin-dependent Akt-GSK3beta signaling pathway. J Biol Chem 287:35222-35233.
Xiong Y, Mahmood A, Chopp M (2009) Emerging treatments for traumatic brain injury. Expert Opin Emerg Drugs 14:67-84.
Xu S, Guan Q, Wang C, Wei X, Chen X, Zheng B, An P, Zhang J, Chang L, Zhou W, Mody I, Wang Q (2014) Rosiglitazone prevents the memory deficits induced by amyloid-beta oligomers via inhibition of inflammatory responses. Neurosci Lett 578:7-11.
Yao J, Zheng K, Zhang X (2015) Rosiglitazone exerts neuroprotective effects via the suppression of neuronal autophagy and apoptosis in the cortex following traumatic brain injury. Mol Med Rep 12: 6591-6597.
Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R (2008) PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res 1244:164-172.
Yonutas HM, Sullivan PG (2013) Targeting PPAR isoforms following CNS injury. Curr Drug Targets 14:733-742.
Yoon SY, Park JS, Choi JE, Choi JM, Lee WJ, Kim SW, Kim DH (2010) Rosiglitazone reduces tau phosphorylation via JNK inhibition in the hippocampus of rats with type 2 diabetes and tau transfected SHSY5Y cells. Neurobiol Dis 40:449-455.
Yu Y, Li X, Blanchard J, Li Y, Iqbal K, Liu F, Gong CX (2014) Insulin sensitizers improve learning and attenuate tau hyperphosphorylation and neuroinflammation in 3xTg-AD mice. J Neural Transm 122:593-606.
Copyedited by Patel B, Norman C, Wang J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.184493
How to cite this article: Zhao YL, Song JN, Ma XD, Zhang BF, Li DD, Pang HG (2016) Rosiglitazone ameliorates diffuse axonal injury by reducing loss of tau and up-regulating caveolin-1 expression. Neural Regen Res 11(6)∶944-950.
Funding: This study was funded by the New Century Supporting Programs to Excellent Talents in China, No. NCET-05-0831.
*Correspondence to: Jin-ning Song, M.D., jinningsong@126.com.
杂志排行
中国神经再生研究(英文版)的其它文章
- Bone marrow mesenchymal stem cell therapy in ischemic stroke: mechanisms of action and treatment optimization strategies
- Optic radiation injury in a patient with intraventricular hemorrhage: a diffusion tensor tractography study
- Synergetic effects of ciliary neurotrophic factor and olfactory ensheathing cells on optic nerve reparation (complete translation)
- miR-148b-3p promotes migration of Schwann cells by targeting cullin-associated and neddylationdissociated 1
- Transplantation of human adipose tissue-derived stem cells for repair of injured spiral ganglion neurons in deaf guinea pigs
- Indirubin-3′-monoxime suppresses amyloid-betainduced apoptosis by inhibiting tau hyperphosphorylation
