Developmental process of the arcuate fasciculus from infancy to adolescence: a diffusion tensor imaging study
2016-12-02HyeongJunTakJinHyunKimSuMinSon
Hyeong Jun Tak, Jin Hyun Kim, Su Min Son
Department of Physical Medicine and Rehabilitation, School of Medicine, Yeungnam University, Daemyungdong, Namku, Daegu, Republic of Korea
RESEARCH ARTICLE
Developmental process of the arcuate fasciculus from infancy to adolescence: a diffusion tensor imaging study
Hyeong Jun Tak, Jin Hyun Kim, Su Min Son*
Department of Physical Medicine and Rehabilitation, School of Medicine, Yeungnam University, Daemyungdong, Namku, Daegu, Republic of Korea
We investigated the radiologic developmental process of the arcuate fasciculus (AF) using subcomponent diffusion tensor imaging (DTI) analysis in typically developing volunteers. DTI data were acquired from 96 consecutive typically developing children, aged 0—14 years. AF subcomponents, including the posterior, anterior, and direct AF tracts were analyzed. Success rates of analysis (AR) and fractional anisotropy (FA) values of each subcomponent tract were measured and compared. AR of all subcomponent tracts, except the posterior, showed a significant increase with aging (P < 0.05). Subcomponent tracts had a specific developmental sequence: First, the posterior AF tract, second, the anterior AF tract, and last, the direct AF tract in identical hemispheres. FA values of all subcomponent tracts, except right direct AF tract, showed correlation with subject's age (P < 0.05). Increased AR and FA values were observed in female subjects in young age (0—2 years) group compared with males (P < 0.05). The direct AF tract showed leftward hemispheric asymmetry and this tendency showed greater consolidation in older age (3—14 years) groups (P <0.05). These findings demonstrated the radiologic developmental patterns of the AF from infancy to adolescence using subcomponent DTI analysis. The AF showed a specific developmental sequence, sex difference in younger age, and hemispheric asymmetry in older age.
orcid: 0000-0003-2818-3884 (Su Min Son)
Accepted: 2015-12-22
nerve regeneration; development; arcuate fasciculus; fractional anisotropy; infants; adolescents; diffusion tensor imaging; neural regeneration
Introduction
Language is one of the highest cognitive functions of the human brain (Colom et al., 2010). The linguistic function distinguishes humans from other species, and it can influence the process of learning and cognitive development in the immature brain (Colom et al., 2010; Schoon et al., 2010). This linguistic processing requires multifocal brain activation and widespread networks that interconnect specific areas of the brain (Vigneau et al., 2006; Friederici, 2011). For example, people have to receive clues from environmental stimuli and determine how those stimuli convey meanings. Then, in order to express their emotions and thoughts, they are required to select proper vocabularies using their memory function and combine these words correctly in accordance with grammar (Rescorla and Mirak, 1997).
In the past, a number of studies investigated the linguistic developmental process using behavioral assessments (Feldman, 2007; Simms, 2007). Radiologic evaluation techniques were also used in several previous studies of language development (Holland et al., 2001; Lidzba et al., 2011). However, there were few studies to reveal the detailed developmental process of connecting tracts at the subcortical level where the arcuate fasciculus (AF) is located.
Diffusion tensor tractography (DTT), derived from diffusion tensor imaging (DTI), is a powerful modality for investigation of neural tracts in vivo. It can also provide quantitative, directional information on neural tracts at the subcortical level (Assaf and Pasternak, 2008; Neil, 2008). Therefore, DTI has frequently been used for assessment of neural tracts such as AF, which is regarded as an important neural tract for language ability, connecting Wernicke's area and Broca's area (Catani et al., 2005; Catani and Mesulam, 2008; Glasser and Rilling, 2008; Kim and Jang, 2013). Using DTI, Catani et al. (2005) reported an analytical method for dividing the AF. Wakana et al. (2007) provided protocols for analysis of the direct AF tract connecting the inferior frontal and superior temporal cortices (Wakana et al., 2007). These suggestions have been used in several other studies using DTI (Eluvathingal et al., 2007; Catani and Mesulam, 2008; Lawes et al., 2008; Hasan et al., 2010). However, no quantitative assessment of developmental changes of AF subcomponents in tensor metrics from birth through adolescence has been reported. Therefore, using subcomponent DTI analysis, we investigated the radiologic developmental process of the AF in typically developing volunteers from infants to adolescents.
Subjects and Methods
Subjects
A total of 96 typically developing subjects were recruited via advertisement (mean age 6.79 ± 4.23 years; range 0 to 14 years; 51 males/45 females) according to the following criteria: (1) subjects delivered after 37 weeks, and with an uncomplicated birth; (2) subjects diagnosed with a normal neurologic developmental state by pediatric neurologists using several developmental tests according to their age, such as the Denver Developmental Delay Screening Test (DDST) or Korean-Ages and Stages Questionnaires (K-ASQ) (Frankenburg et al., 1992; Ga and Kwon, 2011); (3) no specific history of brain trauma or surgery; (4) no specific lesions on conventional brain MRI; (5) absence of any diagnosed genetic syndrome or epilepsy; (6) right handedness diagnosed by pediatric neurologists, however, young infants who did not show definite handedness were also included in this study.
A total of 138 children, who ranged in age from 0 to 14 years, were originally selected. However, 12 children born before 37 weeks of gestation were excluded, and another 11 children were excluded for a history of head trauma or distocia. Of these 118 subjects, six were excluded for abnormal results on the developmental screening test and 13 subjects who showed left handedness or ambidexterity were also excluded. The remaining 96 subjects were enrolled in the study (Figure 1).
Results of DTI and neurologic assessments were provided free to the parents of subjects. Written informed consent was obtained from the parents of all children. The study was approved by the institutional review board at Yeungnam University hospital (PCR-10-31). For statistical analysis of the AF developmental process, subjects were divided into five subgroups according to age: group 1, 0—2 years; group 2, 3—5 years; group 3, 6—8 years; group 4, 9—11 years; group 5, 12—14 years. A prior pilot study, used to determine the minimum sample size for this study, was analyzed using G★Power3.1 (Heinrich-Heine-UniversitätDüsseldorf, available from: http:// www.gpower.hhu.de/). The number of each group was found sufficient when α error was 0.05 with power of 0.8.
Developmental screening test
The DDST or K-ASQ was used for evaluation of neurologic developmental state. The DDST and K-ASQ are reliable and valid methods for assessment of neurologic developmental state in children, and have been widely used in the clinic (Frankenburg et al., 1992; Ga and Kwon, 2011). The K-ASQ, which was published in 1995, is a developmental screening test that is widely used in Korea (Ga and Kwon, 2011). All examinations were performed by pediatric psychologists and six children showing abnormal development state were excluded.
MRI and diffusion tensor imaging (DTI) data acquisition DTI data were acquired using the 1.5-T Philips Gyroscan Intera system equipped with a synergy-L Sensitivity Encoding (SENSE) head coil, utilizing a single-shot, spin-echo planar imaging pulse sequence. For each of the 32 noncollinear and noncoplanar diffusion-sensitizing gradients, 67 contiguous slices parallel to the anterior commissure — posterior commissure line were acquired. Imaging parameters were as follows: acquisition matrix = 96 × 96, reconstructed to matrix = 128 × 128 matrix, field of view (FOV) = 221 × 221 mm2, repetition time (TR) = 10,726 ms, echo time (TE) = 76 ms, parallel imaging reduction factor (SENSE factor) = 2, echo-planar imaging (EPI) factor = 49, b = 1,000 s/mm2, number of excitation (NEX) = 1, and slice thickness of 2.3 mm (acquired isotropic voxel size 2.3 × 2.3 × 2.3 mm3).
Removal of eddy current-induced image distortions was performed using affine multi-scale two-dimensional registration at the Oxford Centre for Functional Magnetic Resonance Imaging of Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl) (Smith et al., 2004). DTI-Studio software (CMRM, Johns Hopkins Medical Institute, Baltimore, MD, USA) (Jiang et al., 2006) was used for analysis of AF subcomponents of both sides, which was based on the fiber assignment continuous tracking (FACT) algorithm and the multiple ROIs approach.
Modified Wakana and Catani's protocols were used in subcomponent analysis of the AF. For direct AF tract analysis, we used specified analytical criteria of the protocol reported by Wakana et al. (2007), and Catani's protocol was applied for analysis of the anterior and posterior AF tracts (Catani et al., 2005; Vandermosten et al., 2012). For the first ROI of posterior AF tract, the lateral location of the sagittal stratum was delineated in the axial slice corresponding with the anterior commissure level. The second ROI of the posterior AF tract was placed to 6—7 upper slices of the first ROI of posterior AF tract. Fibers passing through the body of the superior longitudinal fasciculus in the coronal slice were then excluded. For analysis of anterior AF tract, the first ROI was placed at the body of the superior longitudinal fasciculus in the coronal slice, which was identified as green fiber with a triangular shape. For the second ROI of the anterior AF tract, a coronal slice was selected at the middle of the splenium of the corpus callosum. Fibers passing through the first ROI of posterior AF tract were then excluded. For analysis of the direct AF tract, the first ROI was placed at the same locations of the anterior AF tract. For the second ROI, the lateral location of the sagittal stratum was delineated in the axial slice (Figure 2).
Termination criteria used for fiber tracking were fractional anisotropy (FA) < 0.2 and angle < 60° (Kim and Jang, 2013). When the tract was not analyzed according to modified Wakana and Catani's protocols, we confirmed non-analysis of each tract by re-analysis using the lowered FA threshold of 0.1. Values of each AF subcomponent in the left and right hemispheres were measured. Success rate of analysis (AR) for each AF subcomponent in the respective age groups was also measured. AF subcomponent tracking was performed by two investigators (HJT & SMS). Inter-rater and intra-rater reproducibility was examined by repeating all measurements for the AF subcomponents of both sides. To measure the inter-observer variation, the data were randomly analyzed by two authors (HJT & SMS), who were blinded to the other's analysis data. The AR made by the two analyzers was the same for all subjects. Inter-observer reproducibility calculated using FA values of the two analyzers showed high agreement (allinterclass correlation (ICC) ≥ 0.90). This finding indicates a high rate of reproducibility. Therefore, this technique is reliable for evaluation of AFs in pediatric subjects.
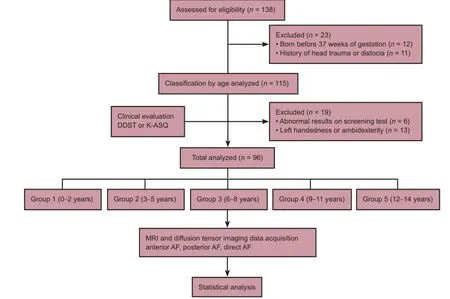
Figure 1 Study flow chart.
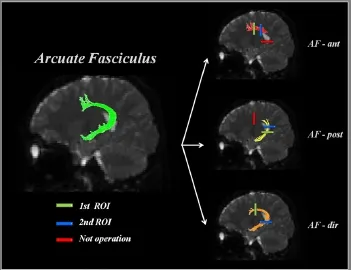
Figure 2 Diffuse tensor imaging tracking of arcuate fasciculus (AF) subcomponents.
All examinations were supervised by a pediatrician experienced in MR imaging procedures. Temperature, pulse oximetry, and electrocardiograph monitoring were applied throughout the MR imaging examination. Each child's ears were protected by placement of earplugs in the external ear or neonatal earmuffs.
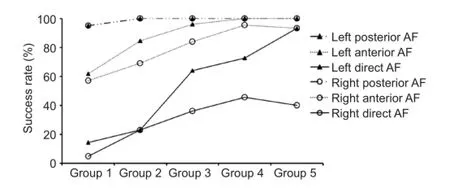
Figure 3 Success rate of analysis (AR) for the AF subcomponents in different age groups.
Statistical analysis
Statistical analysis was performed in three steps. The first step was the statistical analysis for determination of the effect of age on AF development. Chi-square test was used forcomparison of AR in the respective age groups. Pearson's correlation analysis was performed for comparison of FA values of AF subcomponents in both hemispheres. In addition, chisquare test and one-way analysis of variance were used for determination of the most critical period in which significant changes occurred between adjacent age groups during the entire developmental process. In the second step, sex difference upon AF maturation was assessed using the chi-square test for comparison of AR. Independent t-test was used for comparison of the FA value between male and female subjects in the respective age groups. The third step was to investigate hemispheric asymmetry on AF maturation. AR subcomponents were examined using the chi-square test and FA values between right and left hemispheres were compared using the paired t-test. SPSS 18.0 software (SPSS, Chicago, IL, USA) was used for data analysis. A level of P < 0.05 was considered statistically significant.
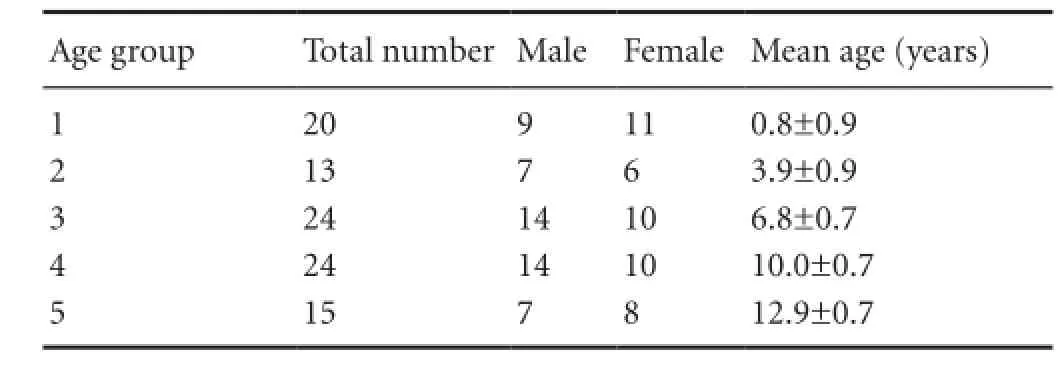
Table 1 Characteristics of all included subjects

Table 3 Sex difference and hemispheric asymmetry of the AF subcomponents
Results
Baseline characteristics of subjects
A total of 96 subjects were classified into five groups accord-ing to age. There were no significant differences in subject number and sex distribution between age groups (P > 0.05; Table 1).
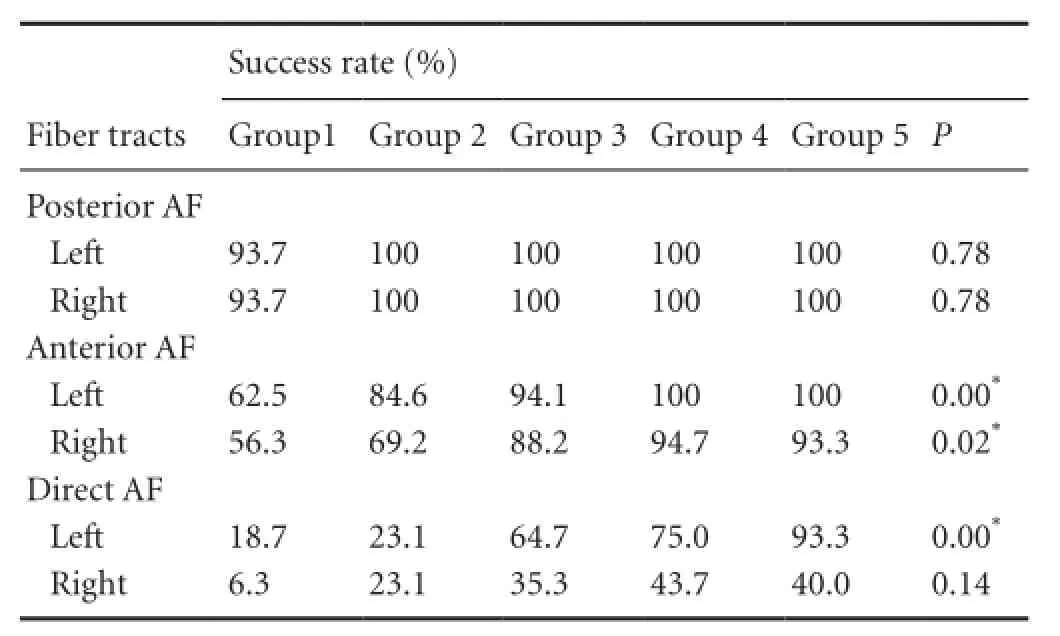
Table 2 Success rate of analysis of the arcuate fasciculus (AF) subcomponents in different age groups
Effects of age
AR of AF subcomponents in the left and right hemispheres are shown in Table 2 and Figure 3. Except for posterior AF tract, which showed a nearly completed development level, even in the early age of group 1, all other tracts showed significantly increased AR with age (P < 0.05). Figure 3 also shows the identical pattern of the AF developmental process applied to all age groups, which showed highest AR in the posterior AF tract, followed by the anterior AF tract, with the lowest in the direct AF tract. This pattern implied the specific sequence for the developmental order, revealing that the posterior AF tract developed first, followed by anterior AF tract, and, finally the direct AF tract in each hemisphere. Figure 4 shows the correlation between FA values in each subcomponent and subject's age. All tracts showed significant correlation with age, except right direct AF tract, which did not reach the completed development level, even in the group 5.
Sex differences
In the current study, in general, no definite significant result was observed for sex effect on AF development (Table 31). However, comparative analysis within respective age groups revealed significant differences between male and female subjects. In group 1, female subjects showed a significantly increased AR of left anterior AF tract compared with male subjects (P < 0.05). FA value of the left posterior AF tract also showed a significant increase in female subjects (P <0.05). However, in other age groups, no significant differences were observed between male and female subjects.
Hemispheric asymmetry
Results for hemispheric asymmetry are shown in Table 3. There was significant difference in the AR of the direct AFtract between the left and right hemispheres. Significant difference in the FA values of the direct AF tract was also found between the left and right hemispheres (P < 0.05). Within respective age groups, the AR of the left direct AF tract was significantly increased with age in groups 3 and 5 (P < 0.05) and the FA value of the left direct AF tract showed a significant increase in groups 4 and 5 (P < 0.05). Anterior and posterior AF tracts showed no definite hemispheric asymmetry.
Discussion
In the current study, using subcomponent DTI analysis, we investigated the radiologic developmental process of the AF. Our results can be summarized as follows: (1) AR and FA values of most subcomponent tracts of the AF showed a significant increase with age. (2) Subcomponent tracts showed a specific order of developmental process: First, posterior AF tract, second, anterior AF tract, and, last, direct AF tract in identical hemispheres. (3) In general, no significant difference was observed between male and female subjects. However, significantly increased AR and mean FA values were observed in female subjects than in male subjects in group 1. (4) Direct AF tract showed leftward hemispheric asymmetry and this tendency became conspicuous in older age groups.
Effects of age
A large number of typically developing subjects aged from 0.6 months old (infants) to 177 months old (adolescents) were included in this study. Therefore, we could investigate the developmental process of the AF from a very early age, when detailed clinical language evaluation was impossible, to older, linguistically developed age. Our results showing significant correlation of AF development with aging were in consistent with those of previous studies (Paus et al., 1999; Schmithorst et al., 2002; Eluvathingal et al., 2007; Lebel et al., 2008; Hasan et al., 2010). Our results also demonstrated the specific developmental sequence of the AF upon the subcomponent tracts. This might suggest that each AF subcomponent has different developmental patterns and critical periods upon maturation. A few previous studies have reported that, frequently, the right AF tract could not be analyzed in healthy volunteers (Nucifora et al., 2005; Powell et al., 2006; Hasan et al., 2010). Other previous studies using DTI have reported on the poor identifiability of the AF in pediatric patients (Gopal et al., 2012). We presume that these previous results are related to specific characteristics of the developmental process of the AF associated with aging, as shown in our study. That is, in the current study, complete development of anterior and direct AF tracts did not occur at an early age; therefore, the AR could have decreased normally. In addition, a difference of AR was observed between the subcomponent tracts, even at the same age.
Previous behavioral studies on language development have also reported on these sequential developmental patterns. That is, acquisition of comprehensive language ability precedes expressive language ability (Benedict, 1979; Tsao et al., 2004; Simms, 2007). Recent radiologic studies have demonstrated that the language process was related to several regional activations of various brain areas (Holland et al., 2001; Vigneau et al., 2006; Friederici, 2011; Lidzba et al., 2011). A study using fMRI reported by Vigneau et al. (2006) showed activation of the inferior frontal gyrus and lower precentral gyrus with phonological tasks, and activation of temporal areas with speech listening. Another study using DTI reported by Catani et al. (2005) suggested that impairment of left anterior AF tract can lead to failure of vocalizing and injuries of left posterior AF tract would result in failure of auditory comprehension. In the current study, the posterior AF tract was found to be located in the posterior portion of the entire AF tract, representing Wernicke's area, as described in previous studies. Anterior AF is located in the anterior portion of the whole AF tract, representing Broca's area. Our results regarding sequential developmental pattern of anterior and posterior AF and AF tracts were in agreement with those of previous behavioral and radiologic studies. We supposed that last maturation order of direct AF tract would be related to higher cognitive functional processes of language, such as connecting the comprehension center of Wernicke's area with the expression center of Broca's area.
Sex differences
No significant effect of sex on AF development was observed in overall subjects. This result was consistent with those of several previous studies using DTI, which did not show significant differences between males and females (Eluvathingal et al., 2007; Lebel et al., 2008; Hasan et al., 2010). In a quantitative study of 31 healthy children using DTI, Eluvathingal et al. (2007) reported no difference associated with sex. Hasan et al. (2010) investigated the microstructural change of white matter in healthy subjects and found no difference between sexes. In this study, AR of sex effect within respective age groups revealed significant differences in age group 1. These results would be related to previous behavioral studies of sex differences in language development. Johnson and Meade (1987) reported that females had precocity in language skills at an early age. Another study by Zambrana et al. (2012) reported that at 18 to 36 months of age, girls were superior to boys in language comprehension. These findings coincide with the results of our study, and we supposed that the sex difference becomes diminished as children growing up.
Hemispheric asymmetry
The analysis for hemispheric asymmetry showed significantly higher AR and FA values for left direct AF tract, compared with right direct AF tract. In a previous study, using postmortem dissection investigation, Geschwind and Levitsky (1968) reported that the temporal region of the adult brain had leftward hemispheric asymmetries. Recent radiologic studies using various MRI techniques have reported leftward hemispheric asymmetry in the AF (Holland et al., 2001; Nucifora et al., 2005; Powell et al., 2006; Lebel and Beaulieu, 2009; Hasan et al., 2010). Paus et al. (1999) analyzed structural MRIs obtained from 111 children who ranged in age from 4 to 17 years and reported that maturation of fronto-temporal pathways was predominant in theleft hemisphere. Another study using fMRI involving 7-to-18-year-old children reported left hemisphere dominance for language task; this lateralization showed an increase with age (Holland et al., 2001). Other studies using DTI in healthy adult volunteers also reported that the right AF tract was not frequently visualized and had lower FA values than the left AF tract (Nucifora et al., 2005; Powell et al., 2006; Lebel and Beaulieu, 2009). Our results were in agreement with those of previous studies reporting on leftward hemispheric asymmetry. In addition, we found that this leftward hemispheric asymmetry was mainly attributed to the asymmetry of the direct AF tract and it became apparent in older age groups.

Figure 4 Correlation between FA values of the AF subcomponents and subject's age.
In conclusion, AF subcomponents have specific developmental sequences, sex difference in younger age, and hemispheric asymmetry in older age. These results could provide valuable clinical information for understanding of AF development, and have important implications in terms of setting the rehabilitative strategy in patients with developmental language delay. To the best of our knowledge, this is the first study to report on the radiologic developmental sequence of the AF from the infancy using subcomponent DTI analysis. However, limitations of this study should be considered. The main limitation of the current study is a lack of uniform clinical data due to various ranges of age. Therefore, there is limitation to demonstrate the interaction between AF maturation and functional development. The age grouping of subjects and uneven subject number of each group are other limitations. Comparison by age grouping method was attributed to the characteristics of the developmental process of the AF. We originally attempted to investigate the correlation of parameters and aging without grouping. However, some subcomponent tracts could not be analyzed, especially in the young age group, statistical comparison for overall subjects was impossible due to uneven distribution of the analyzed number of subjects. Crossing fibers can prevent full reflection of the underlying fiber architecture. A previous study showed that crossing fibers can be detected in over 90% of white matter voxels (Jeurissen et al., 2010), and regions with complex crossing fiber tend to have lower FA values, compared with predominantly unidirectional white matter (Parker and Alexander, 2005; Yamada, 2009). We suppose that there could be crossing fiber effect for some pathways including the right anterior AF or direct AF. Therefore, further analysis with constrained spherical constrained spherical deconvolution should be made to more closely delineate the development process of AF. In-depth studies with larger case numbers including older subjects and more detailed clinical parameters should be performed to show the detailed whole developmental process of AF and the interaction between AF maturation and functional development.
Author contributions: HJT and JHK performed the research. SMS designed the research and wrote the paper. All authors approved the final version of this paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Assaf Y, Pasternak O (2008) Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci 34:51-61.
Benedict H (1979) Early lexical development: comprehension and production. J Child Lang 6:183-200.
Catani M, Mesulam M (2008) The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex 44:953-961.
Catani M, Jones DK, ffytche DH (2005) Perisylvian language networks of the human brain. Ann Neurol 57:8-16.
Colom R, Karama S, Jung RE, Haier RJ (2010) Human intelligence and brain networks. Dialogues Clin Neurosci 12:489-501.
Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L (2007) Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex 17:2760-2768.
Feldman HM (2007) Using the language characteristics of clinical populations to understand normal language development. Pediatr Clin North Am 54:585-607.
Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B (1992) The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics 89:91-97.
Friederici AD (2011) The brain basis of language processing: from structure to function. Physiol Rev 91:1357-1392.
Ga HY, Kwon JY (2011) A comparison of the korean-ages and stages questionnaires and denver developmental delay screening test. Ann Rehabil Med 35:369-374.
Geschwind N, Levitsky W (1968) Human brain: left-right asymmetries in temporal speech region. Science 161:186-187.
Glasser MF, Rilling JK (2008) DTI tractography of the human brain's language pathways. Cereb Cortex 18:2471-2482.
Gopal SP, Tiwari VN, Veenstra AL, Kumar A, Behen M, Chugani HT, Sundaram SK (2012) Sensitive diffusion tensor imaging quantification method to identify language pathway abnormalities in children with developmental delay. J Pediatr 160:147-151.
Hasan KM, Kamali A, Abid H, Kramer LA, Fletcher JM, Ewing-Cobbs L (2010) Quantification of the spatiotemporal microstructural organization of the human brain association, projection and commissural pathways across the lifespan using diffusion tensor tractography. Brain Struct Funct 214:361-373.
Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS, Jr. (2001) Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage 14:837-843.
Jeurissen B, Leemans A., Tournier J., Jones D.K., and Sijbers J. (2010). Estimating the Number of Fiber Orientations in Diffusion MRI Voxels: A Constrained Spherical Deconvolution Study. Proc Intl Soc Mag Reson Med18, 573
Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S (2006) DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 81:106-116.
Johnson ES, Meade AC (1987) Developmental patterns of spatial ability: An early sex difference. Child Dev 58:725-740.
Kim SH, Jang SH (2013) Prediction of aphasia outcome using diffusion tensor tractography for arcuate fasciculus in stroke. AJNR Am J Neuroradiol 34:785-790.
Lawes INC, Barrick TR, Murugam V, Spierings N, Evans DR, Song M, Clark CA (2008) Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. Neuroimage 39:62-79.
Lebel C, Beaulieu C (2009) Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp 30:3563-3573.
Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C (2008) Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40:1044-1055.
Lidzba K, Schwilling E, Grodd W, Krageloh-Mann I, Wilke M (2011) Language comprehension vs. language production: age effects on fMRI activation. Brain Lang 119:6-15.
Neil JJ (2008) Diffusion imaging concepts for clinicians. J Magn Reson Imaging 27:1-7.
Nucifora PG, Verma R, Melhem ER, Gur RE, Gur RC (2005) Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport 16:791-794.
Parker GJ, Alexander DC (2005) Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci 360:893-902.
Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC (1999) Structural maturation of neural pathways in children and adolescents: in vivo study. Science 283:1908-1911.
Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, Barker GJ, Noppeney U, Koepp MJ, Duncan JS (2006) Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage 32:388-399.
Rescorla L, Mirak J (1997) Normal language acquisition. Semin Pediatr Neurol 4:70-76.
Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK (2002) Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology 222:212-218.
Schoon I, Parsons S, Rush R, Law J (2010) Children's language ability and psychosocial development: a 29-year follow-up study. Pediatrics 126:e73-80.
Simms MD (2007) Language disorders in children: classification and clinical syndromes. Pediatr Clin North Am 54:437-467, v.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1:S208-219.
Tsao FM, Liu HM, Kuhl PK (2004) Speech perception in infancy predicts language development in the second year of life: a longitudinal study. Child Dev 75:1067-1084.
Vandermosten M, Boets B, Poelmans H, Sunaert S, Wouters J, Ghesquiere P (2012) A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain 135:935-948.
Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N (2006) Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage 30:1414-1432.
Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S (2007) Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36:630-644.
Yamada K (2009). Diffusion tensor tractography should be used with caution. Proc Natl Acad Sci U S A 106:E14.
Zambrana IM, Ystrom E, Pons F (2012) Impact of gender, maternal education, and birth order on the development of language comprehension: a longitudinal study from 18 to 36 months of age. J Dev Behav Pediatr 33:146-155.
Copyedited by Bokde AL, Wang J, Li CH, Song LP, Zhao M
10.4103/1673-5374.184492
How to cite this article: Tak HJ, Kim JH, Son SM (2016) Developmental process of the arcuate fasciculus from infancy to adolescence∶ a diffusion tensor imaging study. Neural Regen Res 11(6)∶937-943.
Funding: This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, No. 2012-013997.
*Correspondence to: Su Min Son, M.D., Ph.D., sumin430@hanmail.net.
杂志排行
中国神经再生研究(英文版)的其它文章
- Bone marrow mesenchymal stem cell therapy in ischemic stroke: mechanisms of action and treatment optimization strategies
- Optic radiation injury in a patient with intraventricular hemorrhage: a diffusion tensor tractography study
- Synergetic effects of ciliary neurotrophic factor and olfactory ensheathing cells on optic nerve reparation (complete translation)
- miR-148b-3p promotes migration of Schwann cells by targeting cullin-associated and neddylationdissociated 1
- Transplantation of human adipose tissue-derived stem cells for repair of injured spiral ganglion neurons in deaf guinea pigs
- Indirubin-3′-monoxime suppresses amyloid-betainduced apoptosis by inhibiting tau hyperphosphorylation
