Graphene platform for neural regenerative medicine
2016-12-02TasneemBouzid,AlexanderSinitskii,JungYulLim
PERSPECTIVE
Graphene platform for neural regenerative medicine
Graphene is a material composed of a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice. The unique electrical, optical, thermal, and mechanical properties of graphene are extensively exploited for various applications in electronics, energy, and sensors. Studies also proposed the potential of graphene for biomedical applications. The intrinsic characteristics of graphene and its availability for chemical and physical modifications make graphene a promising vehicle for various biomedical applications including drug delivery, bioimaging, disease diagnostics, etc. The chemical structure of graphene and, in turn, its functionality, can be altered by attaching functional groups, which not only modify the properties of graphene but also allow its conjugation with antibodies, peptides, ligands, contrast agents, drugs, and genes for various biomedical applications (John et al., 2015).
Graphene can also be used as a cell-contacting biomaterial for tissue engineering and regenerative medicine. Recent studies have shown that graphene substrates can support the adhesion, proliferation, and differentiation of mesenchymal stem cells (MSCs), induced pluripotent stem cells, and other mammalian cells. Specifically for neural regenerative medicine, graphene has demonstrated that it can perform as an effective culture platform compatible with neural cells and their precursors. Hippocampal cells and neural stem cells (NSCs) cultured on graphene substrates showed significantly enhanced neurogenesis, as assessed by neurite sprouting and neural network formation (Li et al., 2011; Tang et al., 2013). Human MSC growth and its neural differentiation were also supported by graphene culture (Kim et al., 2015). Further, the capability of graphene to electrically stimulate differentiated neuronal cells was demonstrated (Park et al., 2011). The current status of knowledge is that graphene can be conductive/inductive for cellular neurogenesis and may be a promising candidate scaffold material for neural tissue engineering. In this perspective, our group's series of projects on neural regenerative medicine that exploit unconventional materials, including graphene, and mechanical stimuli will be introduced. Then, potential future directions in the study of graphenebased neural regenerative medicine will be discussed.
Much of our group's effort has been centered on regulating cell function and fate via the integration of biomaterial and mechanical cues. While conventional neural bioscience researches have mostly depended on soluble neurogenesis triggering factors such as nerve growth factor, we have been exploring the potential of mechanophysical cues to induce neurogenesis (see our review: Stoll et al., 2014). First, we made an attempt to utilize the capacity of geometric cell function control via micropatterning. Cell patterning can geometrically organize cells, enabling precise regulation of the cell size, shape, and interconnectivity. By investigating geometry-dependent intra- and intercellular signaling, the mechanism of morphology-directed cellular function change can be systematically studied. For neural regenerative medicine, we tested the hypothesis that micropatterning of neuronal cells within narrow lane shapes may increase neurogenesis (Poudel et al., 2013). This was to simply mimic the anisotropic architecture of the axons. Further, by testing the interplay between geometric and biochemical signals, the microenvironments could be optimized for enhanced neuronal regeneration. We observed that SH-SY5Y human neuroblastoma cells seeded on collagen-I micropatterned lanes responded to the micropatterning dimension synergistically with the treatment of retinoic acid (RA), a soluble neurogenic factor for neuroblastoma cells. Cells confined within narrow (5- and 10-µm-wide) lanes exhibited greater neurite extension and preferred nucleus orientation along the anisotropic lane direction, relative to unpatterned control, which effect was strengthened under RA. Neurite extension was significantly greater for microlane-patterned but RA-untreated cells compared with RA-treated but unpatterned cells, indicating that the geometric cell confinement alone may induce neurogenesis to a greater degree than the conventional soluble induction.
As another attempt exploiting mechanophysical cues, we tested the effect of mechanical loading. This was to test whether neuronal cell function could be altered by dynamic mechanical environments, although neurons have not been normally recognized as mechanoresponsive. If mechanical loading results in improved neurogenesis, even though it is not physiologically relevant, the method can be practically considered as useful for in vitro neural tissue engineering. This prompted a study to test the effect of dynamic loading on neurogenesis. We applied cyclic stretch to human neuronal cells seeded on collagen-coated elastic membranes without or with RA (Higgins et al., 2013). For cells exposed to sinusoidal equiaxial stretching at 10% strain and 0.25 Hz frequency for 120 min per day for 7 days, both neurite extension and outburst number were significantly increased in comparison with unstretched control. Echoing the result on the static micropatterning study described above, dynamic mechanical stretch was also found to induce neurite outgrowth even in the absence of soluble neurogenesis signal from RA. Further, specific neuronal markers, microtubule-associated protein 2 (MAP2) and neurofilament light chain (NFL), were observed to be responsive to mechanical stretch. Combined data strongly suggest that extracellular mechanophysical signal can be a possible route for neuronal regenerative medicine.
Our group recently explored the effect of graphene substrate culture on the induction of cellular neurogenesis. While several studies have tested cellular behaviors on graphene-covered surfaces, some studies did not have a good control over the quality of the graphene substrate with respect to the number of graphene layers or substrate coverage. For instance, cell cultures on partly graphene-covered substrate with inconsistent graphene layer numbers were used. In our recent study (Lee et al., 2015), we could successfully fabricate a monolayer film of high-quality graphene on 15 × 15 mm2glass substrate, which was sufficiently large to assess various cell behaviors. Graphene was grown using chemical vapor deposition (CVD) on a copper foil and then transferred on glass substrates. The formation of a high-quality graphene monolayer was confirmed by Raman and UV-vis-NIR spectra. It was observed that neurite outgrowth in human neuroblastoma cells was significantly increased when seeded on graphene compared with control glass substrate, both in neurite extension length and number (Figure 1). The graphene triggering of neurite outgrowth was, interestingly, detected even without RA soluble factor, similarly to the mechanical stimulation cases described above. The NFL expression was accordingly upregulated on graphene culture. Combined with the above mechanical stretch study, NFL may be a molecular sensor governing neuronal cell sensing and response to extracellular material and mechanical milieus.
The mechanisms of graphene induction of cellular neurogenesis have not been revealed, but our data (Lee et al., 2015) may suggest some clues. In cell-material interaction, various material characteristics (chemistry, topography, wettability, etc.) can direct cell behaviors. Hydrophilic surfaces, rather than hydrophobic, are known to better support cell adhesion, spreading, and growth. Since graphene was more hydrophobic (water contact angle of 77°) than glass (34°), increased neural cytocompatibility on graphene may not be caused by the surface wettability. Also, since the two surfaces showed no predetermined textures and had surface roughness parameters of similar order of magnitude (3.9 nm and 0.74 nm for graphene and glass, respectively), differences in topography might not affect graphene's cytocompatibility. Excluding these parameters, graphene's superior capability to support cellular neurogenesis could be attributed to its inherent chemical composition and architecture, e.g., hexagonal arrangement of carbon atoms in a single layer, but more justification is required.
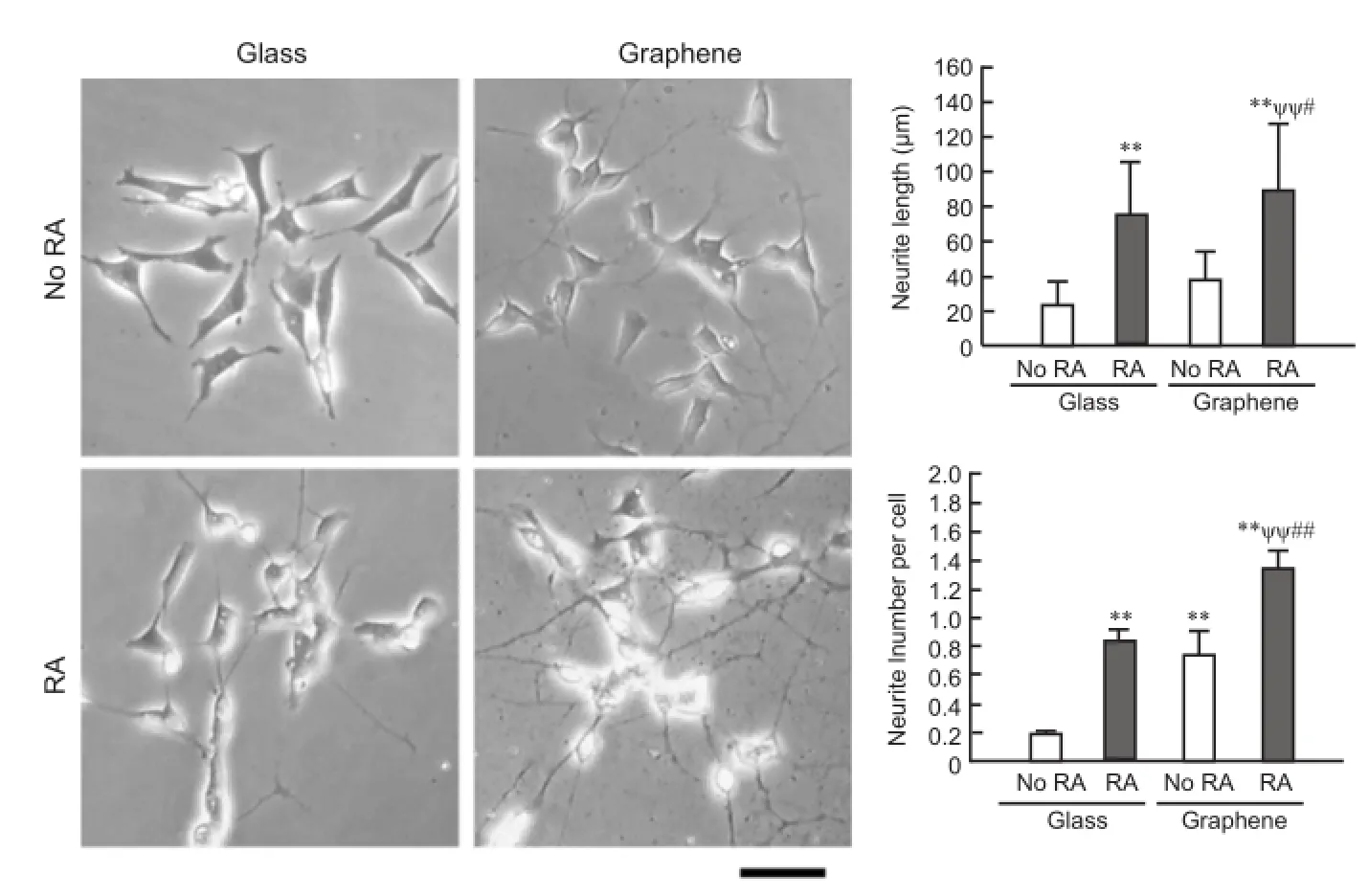
Figure 1 Graphene culture induces cellular neurogenesis.
In regard to molecular mechanism of cell-graphene interaction, our data for the first time revealed that focal adhesion kinase (FAK) and p38 mitogen-activated protein kinase (MAPK) may play mediatory roles in the graphene triggering of neurogenesis (Lee et al., 2015). In the presence of FAK and p38 inhibitors, graphene triggering of neurite extension was significantly impaired, suggesting that focal adhesion formation on graphene and environmental stimulation from graphene culture may be mainly responsible for the enhanced neurite development on graphene. It was found that the other important sensors, such as extracellular signal regulated kinase (ERK) or RhoA kinase (ROCK), may not majorly participate in the graphene control of neuronal cell functioning.
Despite promising early results on the graphene induction of cell neurogenesis, there remains much more to improve for accomplishing graphene-based neural regenerative medicine. First, the studies on neuronal cell-graphene interaction have only dealt with plain cell culture using mono- or multilayered graphene films, and there has been very little effort to incorporate other vital cell-stimulatory cues such as geometric, topographic, etc. Considering our data on the geometric (micropatterning) stimulation of neurogenesis, an incorporation of the geometric cue with the graphene control of neuronal cells may provide potential synergistic effects. Also, there has been relatively little effort to actively take advantage of the unique properties of graphene to stimulate neural cells, specifically, its excellent electrical conductivity. Keeping in mind that electrical stimuli can be beneficial for neurogenesis, more systematic attempts to exploit the electrical stimulation capability of graphene are recommended. Fundamentally, while studies reported positive neuronal cell-stimulatory effects from graphene, very little has been determined about the molecular mechanisms on how neuronal cells adapt to graphene. Our data suggested that focal adhesion (FAK) and environmental stress (p38) signaling may play governing roles in neural cell-graphene interaction. FAK, a linker protein that connects transmembrane integrin to cytoskeleton at the focal adhesion site, mediates a range of cell behaviors including adhesion, growth, differentiation, and mechanical and substrate sensitivity. p38 MAPK responds to environmental stimulatory signals, such as substrate and soluble factors, and may also be activated by an electrical signal. A more in-depth signaling pathway studies including the up- and downstream effectors of FAK and p38 are needed to fully understand the neural cell-graphene interaction. Practically, sensitizing FAK and p38 in neural precursor cells may enhance the graphene stimulatory effects on neurogenesis. Taken all together, a combinatorial approach that simultaneously utilizes identified stimulatory factors (geometric, electrical, sensitized FAK and p38, etc.) may maximize the effect of graphene induction of neurogenesis. The combined approach may help develop novel graphenebased neural regenerative medicine protocols.
This work was supported by the National Science Foundation (NSF) Graduate Research Fellowship 1610400 (to TB); NSF through the Nebraska Materials Research Science and Engineering Center (MRSEC) and DMR-1420645 (all to AS); Nebraska Research Initiative (to AS and JYL); NSF CAREER Award 1351570, Nebraska Department of Health and Human Services Stem Cell Research Project 2015-06, and Nebraska Tobacco Settlement Biomedical Research Seed Grant (all to JYL).
Tasneem Bouzid, Alexander Sinitskii, Jung Yul Lim*
Department of Mechanical and Materials Engineering, University of Nebraska-Lincoln, Lincoln, NE, USA (Bouzid T, Lim JY)
Department of Chemistry, University of Nebraska-Lincoln, Lincoln, NE, USA (Sinitskii A)
Graduate School of Dentistry, Kyung Hee University, Seoul, Korea
(Lim JY)
*Correspondence to: Jung Yul Lim, Ph.D., jlim4@unl.edu.
Accepted: 2016-05-17
orcid: 0000-0002-9409-7522 (Jung Yul Lim)
How to cite this article: Bouzid T, Sinitskii A, Lim JY (2016) Graphene platform for neural regenerative medicine. Neural Regen Res 11(6):894-895.
References
Higgins S, Lee JS, Ha L, Lim JY (2013) Inducing neurite outgrowth by mechanical cell stretch. Biores Open Access 2:212-216.
John AA, Subramanian AP, Vellayappan MV, Balaji A, Mohandas H, Jaganathan SK (2015) Carbon nanotubes and graphene as emerging candidates in neuroregeneration and neurodrug delivery. Int J Nanomedicine 10:4267-4277.
Kim J, Park S, Kim YJ, Jeon CS, Lim KT, Seonwoo H, Cho SP, Chung TD, Choung PH, Choung YH, Hong BH, Chung JH (2015) Monolayer graphene-directed growth and neuronal differentiation of mesenchymal stem cells. J Biomed Nanotechnol 11:2024-2033.
Lee JS, Lipatov A, Ha L, Shekhirev M, Andalib MN, Sinitskii A, Lim JY (2015) Graphene substrate for inducing neurite outgrowth. Biochem Biophys Res Commun 460:267-273.
Li N, Zhang X, Song Q, Su R, Zhang Q, Kong T, Liu L, Jin G, Tang M, Cheng G (2011) The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials 32:9374-9382.
Park SY, Park J, Sim SH, Sung MG, Kim KS, Hong BH, Hong S (2011) Enhanced differentiation of human neural stem cells into neurons on graphene. Adv Mater 23:H263-H267.
Poudel I, Lee JS, Tan L, Lim JY (2013) Micropatterning-retinoic acid co-control of neuronal cell morphology and neurite outgrowth. Acta Biomater 9:4592-4598.
Stoll H, Kwon IK, Lim JY (2014) Material and mechanical factors: new strategy in cellular neurogenesis. Neural Regen Res 9:1810-1813.
Tang M, Song Q, Li N, Jiang Z, Huang R, Cheng G (2013) Enhancement of electrical signaling in neural networks on graphene films. Biomaterials 34:6402-6411.
10.4103/1673-5374.184454
杂志排行
中国神经再生研究(英文版)的其它文章
- Bone marrow mesenchymal stem cell therapy in ischemic stroke: mechanisms of action and treatment optimization strategies
- Optic radiation injury in a patient with intraventricular hemorrhage: a diffusion tensor tractography study
- Synergetic effects of ciliary neurotrophic factor and olfactory ensheathing cells on optic nerve reparation (complete translation)
- miR-148b-3p promotes migration of Schwann cells by targeting cullin-associated and neddylationdissociated 1
- Transplantation of human adipose tissue-derived stem cells for repair of injured spiral ganglion neurons in deaf guinea pigs
- Indirubin-3′-monoxime suppresses amyloid-betainduced apoptosis by inhibiting tau hyperphosphorylation
