Protective effect of tetrahydroxy stilbene glucoside on learning and memory by regulating synaptic plasticity
2016-12-01HongboLuoYunLiZunjingLiuLiCaoZhiqiangZhangYongWangXiaoyanZhangZhaoLiuXiangqunShiDepartmentofNeurologyLanzhouGeneralHospitalLanzhouMilitaryAreaCommandLanzhouGansuProvinceChinaDepartmentofNeurologyChinaJapanFriendsh
Hong-bo Luo, Yun Li, Zun-jing Liu, Li Cao Zhi-qiang Zhang Yong Wang Xiao-yan Zhang Zhao Liu Xiang-qun Shi Department of Neurology, Lanzhou General Hospital, Lanzhou Military Area Command, Lanzhou, Gansu Province, China Department of Neurology, China-Japan Friendship Hospital, Beijing, China
Protective effect of tetrahydroxy stilbene glucoside on learning and memory by regulating synaptic plasticity
Hong-bo Luo1,#, Yun Li1,#, Zun-jing Liu2, Li Cao1, Zhi-qiang Zhang1, Yong Wang1, Xiao-yan Zhang1, Zhao Liu1, Xiang-qun Shi1,*
1 Department of Neurology, Lanzhou General Hospital, Lanzhou Military Area Command, Lanzhou, Gansu Province, China
2 Department of Neurology, China-Japan Friendship Hospital, Beijing, China
How to cite this article: Luo HB, Li Y, Liu ZJ, Cao L, Zhang ZQ, Wang Y, Zhang XY, Liu Z, Shi XQ (2016) Protective effect of tetrahydroxy stilbene glucoside on learning and memory by regulating synaptic plasticity. Neural Regen Res 11(9):1480-1486.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81303097, 81373794.
Xiang-qun Shi, Ph.D.,
shixq_2003@163.com.
#These authors contributed
equally to this study.
orcid:
0000-0002-4567-2244
(Xiang-qun Shi)
Accepted: 2016-08-22
Graphical Abstract
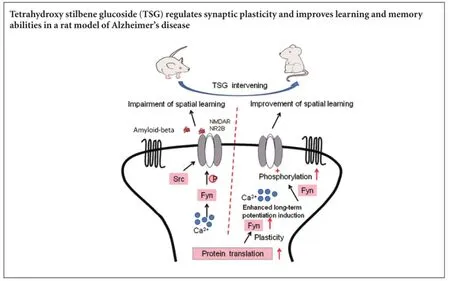
Damage to synaptic plasticity induced by neurotoxicity of amyloid-beta is regarded to be one of the pathological mechanisms of learning and memory disabilities in Alzheimer’s disease patients. This study assumed that the damage of amyloid-beta to learning and memory abilities was strongly associated with the changes in the Fyn/N-methyl-D-aspartate receptor 2B (NR2B) expression. An APP695V7171 transgenic mouse model of Alzheimer’s disease was used and treatment with tetrahydroxy-stilbene glucoside was administered intragastrically. Results showed that intragastric administration of tetrahydroxy-stilbene glucoside improved the learning and memory abilities of the transgenic mice through increasing NR2B receptors and Fyn expression. It also reversed parameters for synaptic interface structure of gray type I. These findings indicate that tetrahydroxy stilbene glucoside has protective effects on the brain, and has prospects for its clinical application to improve the learning and memory abilities and treat Alzheimer’s disease.
nerve regeneration; tetrahydroxy stilbene glucoside; Alzheimer’s disease; amyloid-beta; cognitive impairment; learning and memory; synaptic plasticity; Fyn/N-methyl-D-aspartate receptor 2B signaling pathway; neuroprotection; neural regeneration
Introduction
Alzheimer’s disease (AD) is a common neurological disorder in which the death of brain cells causes gradual memory loss and cognitive decline (Robakis, 2014). AD affects the ability to perform everyday activities. With an unfavorable prognosis and a life expectancy of approximately 8—10 years post diagnosis, AD is becoming one of the most costly diseases for families and society (Alzheimer’s Association, 2015; Santana et al., 2015). Some studies show that incidence rates of AD may have declined in recent years (Matthews et al., 2013; Qiu et al., 2013; Langa, 2015), possibly because of the improved life-style that reduces AD risk, such as diabetes and smoking (Langa, 2015; Satizabal et al., 2016). However, no effective method or medicine is available currently (Chiang and Koo,2014; Kumar et al., 2015). Therefore, there is a great need for an efficient treatment to be developed for this disease. It is currently held that the neurotoxicity of amyloid-beta (Aβ) is a key factor in the occurrence and progression of AD (Querfurth and LaFerla, 2010; Allen et al., 2014; Morris et al., 2014). Damage to synaptic plasticity induced by early extracellular aggregation of Aβ is regarded to be one of the pathological mechanisms of learning and memory disabilities of AD patients (Chin et al., 2005; Calabrese et al., 2007; Lacor et al., 2007; Shankar et al., 2007; Evans et al., 2008). Fyn is a major member in the non-receptor tyrosine kinase Src family that regulates the scaffolding proteins of the N-methyl-D-aspartate (NMDA) receptor 2B (NR2B) in the postsynaptic density of the cell membrane. It plays a significant role in inducing synaptic plasticity during the learning and memorizing processes (Mucke and Selkoe, 2012; Trepanier et al., 2012; Spires-Jones and Hyman, 2014).
Our previous research shows that tetrahydroxy stilbene glucoside (2,3,5,4-tetrahydroxy-stilbene-2-glucoside, TSG), the main active ingredient from Polygonum multiflorum, can help to improve the learning and memorizing abilities of AD mice, and to reduce the expression level of amyloid precursor protein (APP), Beclin-1 and LC3-II (Luo et al., 2009, 2015). Additionally, TSG has a wide range of biological functions, including anti-oxidant and anti-inflammatory properties (Zeng et al., 2011; Zhang et al., 2012; Zhang et al., 2013).
In this experiment, APP695 V717I transgenic mice were used as AD models. We planned to observe the effect of TSG on behaviors of the mice, synaptic plasticity and phosphorylation of Fyn and NR2B in the hippocampus. The results could help reveal the main mechanism of AD and TSG might be developed as a new drug of treatment for AD.
Materials and Methods
Ethics statement
All animal handling and procedures were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The study was approved by the Experimental Animal Ethics Committee of Lanzhou General Hospital, Lanzhou Military Area Command, China (approval No. 2014KYLL021). Precautions were taken to minimize suffering and the number of animals used in each experiment.
Experimental mice and group assignment
Forty clean specific-pathogen-free APP695V717I transgenic mice aged 10 months (20 females and 20 males), and 20 specific-pathogen-free C57BL/6J mice (10 females and 10 males) at the same age and with the same background as for the normal controls, were selected for this study. The mice were all purchased from Research Center for Laboratory Animal Science, Chinese Academy of Medical Sciences in China (certification No. SCKK 20080013). The transgenic mice were equally randomized into two groups by random number table method: TSG group (n = 20) and AD group (n = 20). The TSG group received intragastric administration of TSG (100 mg/kg), once a day for 30 days. TSG was dry powder extracted and separated from Polygonum Multiflorum Thunb., with a content of 68% (supplied by Hunan Academy of Chinese Medicine, China), and TSG was dissolved in water at a concentration of 100 mg/mL. The normal control and AD groups were intragastrically administered physiological saline for 30 days (5 mL/kg per day).
Y-maze test
Y-maze test was implemented before and after 30 days of TSG intervention. The Y-maze system was provided by TSE Systems China Ltd., Beijing, China. The number of electric shocks was a measure of the learning and memorizing abilities, and the number of each mouse escaping the electric-stimulus was recorded, with the maximum number of 30 (O’Keefe and Dostrovsky, 1971).
Morris water maze
The Morris water maze test was performed after 30 days of TSG intervention. The water maze system was provided by TSE Systems China Ltd.
In the navigation test, the mice were put into water to swim freely for 2 minutes on the day before the test. At a fixed time after the formal test, with the mouse facing the pool wall and without selecting the quadrant of the platform as the place of entry, the mice were put into water in a clockwise direction. The escape latency and the swimming distance from being put into water to finding and climbing onto the platform were observed and recorded (D’Hooge and De Deyn, 2001).
In the spatial probe test, the platform was removed after the last place navigation test for each group of mice. The mouse was put into water from the last place of entry, facing the pool tank, and kept swimming in the water maze. The number of times each mouse crossed the former platform within 120 seconds was recorded (D’Hooge and De Deyn, 2001).
Ultrastructure detection
After TSG intervention for 30 days and when the behavior tests were finished, each mouse was decapitated after intraperitoneal anesthesia with 2.5 mL/kg chloral hydrate. The brain tissue was taken out. Half of each mouse’s hippocampus was separated (—2 mm at the anterior/posterior axis, ± 1.8 mm at the lateral/medial axis and —1.5 mm at the dorsal/ ventral axis) (Paxinos and Franklin, 2001), fixed with 4% glutaraldehyde, and cut into pieces after cooling. Samples were then fixed with 2% osmium tetroxide, dehydrated, soaked and embedded through a graded acetone series, and finally dual stained with uranyl acetate-lead citrate.
Observation was made in a transmission electron microscope (Hitachi Ltd., Tokyo, Japan) with three copper screens for each case and then five random shot photos magnified to 40,000 times for each copper screen. The width of synaptic cleft, thickness of postsynaptic density, chord length and arc length of the postsynaptic membrane and length of the synaptic active zone were measured with an image analyzer (Hitachi Ltd.). The synapse number, length of active zone,curvature of synaptic interface and thickness of the postsynaptic density were also measured by a previously published method (Güldner and Ingham, 1980). The curvature of the synaptic interface was measured in accordance with an established method (Jones and Devon, 1978). A multi-point averaging method was used to measure the width of the synaptic cleft (Jones and Devon, 1978). A comparison of the percentage of concave synapses, flat synapses, convex synapses and perforated synapses between the three groups was analyzed statistically. The double-blind method was adopted in observation and measurement in the study.
Western blot assay
The sample was sequentially centrifuged for 10 minutes at 14,000 × g, and then centrifuged for 15 minutes at 12,000 × g. After the supernatant was taken, an additional centrifugation for 30 minutes at 12,000 × g was performed. Lysate and protease inhibitor were added, and protein concentration was measured with bicinchoninic acid protein assay kit (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The sample was mixed with buffer solution, boiled for denaturation, and separated with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Protein was transferred to nitrocellulose membrane. The membrane was blocked with 5% skim milk, and incubated with primary antibodies, including rat anti-mouse Fyn monoclonal antibody (1:1,000; Stressgen, Victoria, Canada), rat anti-NR2B polyclonal antibody (1:1,000; Stressgen), rat anti-phospho-NR2B polyclonal antibody (1:1,000, Thermo Fisher Scientific Inc., St. Louis, MO, USA) and β-actin (1:1,000; Abcam, Cambridge, UK). The proteins were detected using horseradish peroxidase conjugated goat anti-rat secondary antibodies (1:1,000; Abcam) and visualized using chemiluminescence reagents provided with the enhanced chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The intensity of blots was quantified using NIH image J software (NIH, Bethesda, MD, USA).
Statistical analysis
The data, expressed as the mean ± SEM, were analyzed using SPSS 12.0 software (SPSS, Chicago, IL, USA). All data were compared by one-way analysis of variance followed by post-hoc Scheffe’s test. A value of P < 0.05 was considered statistically significant.
Results
Effects of TSG on learning and memory abilities of AD model mice
Y-maze test
Compared with the normal control group, after 30 days, the number of electric-stimulus escapes was significantly greater in the AD and TSG groups (t = 10.0884, P < 0.01; t = 2.7781, P < 0.05). Compared with the AD group, the number of electric-stimulus escapes was significantly less in the TSG group (t = 11.1755, P < 0.01; Table 1).
Morris water maze
In the Morris water maze test, the mean swimming latency of mice was prolonged (t = 14.4648, P < 0.01), the distance increased (t = 5.6550, P < 0.01), the time of crossing the former platform significantly decreased (t = 15.5895, P < 0.01) in the AD group compared with the normal control group. The latency shortened (t = 5.5023, P < 0.01), the swimming distance decreased (t = 2.2341, P < 0.05), and the time of crossing the former platform increased (t = 4.8163, P < 0.01) in the TSG group compared with the AD group (Table 2).
Effect of TSG on synaptic ultrastructure in CA1 region of the hippocampus of AD model mice
In the normal control group, there were distinct and complete synaptic structures. The presynaptic mitochondria were normal and had distinct cristae without swelling. Typical asymmetric interfaces for synapses of gray type I (synaptic cleft approximately 20 nanometers) were visible with significantly thicker postsynaptic densities than those presynaptic. Small synaptic clefts, the existence of concave synapses, convex synapses, flat synapses and perforated synapses, with more concave synapses, were all observed.
In the AD group, unlike the normal control group, we observed unclear presynaptic mitochondrial cristae, significantly increased width of synaptic clefts, shorter synaptic active zones, thinner postsynaptic densities, significantly increased flat synapses, but significantly reduced concave synapses and perforated synapses.
The TSG group showed less damage than in the AD group. We observed distinct presynaptic mitochondrial crista, smaller width of synaptic cleft, increased postsynaptic densities, reduced flat synapses, but significantly increased concave synapses and perforated synapses. Compared with the normal control group, we observed larger synaptic cleft width, thinner postsynaptic densities, slightly increased flat synapses, but significantly reduced concave synapses and perforated synapses in the TSG group (Figure 1, Table 3).
Effects of TSG on protein expression of Fyn and phosphorylation of NR2B in CA1 region of the hippocampus of AD model mice
Results of western blot assay showed that, compared with the normal control group, Fyn and phosphorylation of NR2B subunit expression significantly decreased in the AD group (P < 0.05). Compared with the AD group, the Fyn and phosphorylation of NR2B subunit expression significantly increased in the TSG group (P < 0.05; Figure 2).
Discussion
Neurotoxicity of Aβ is currently considered to be a key factor in the progression of AD (Holtzman et al., 2011; Li et al., 2012; Bass et al., 2015). Therefore, many principal targets and clinical trials of the compounds have been aimed at reducing Aβ formation and plaques (Castello and Soriano, 2014; Chiang and Koo, 2014; Folch et al., 2016). However, no effective medicine has been found to decrease Aβ (Chiang and Koo, 2014; Kumar et al., 2015). In this study, we have shown that TSG can change the plasticity of synaptic structures and functions and Fyn-mediated NR2B phosphorylation pathway, byincreasing the expressions of NR2B receptors and Fyn and by reversing the changes associated with AD to the synaptic interface structure of type Gray I to some extent.
In this study, behavioral experiments show that mice in the AD group had significantly decreased learning, spatial orientation and working memory abilities. After TSG intervention, escape latency shortened, swimming distance decreased, and times of crossing the former platform increased in the transgenic mice. The above-described results showed that TSG relieved the impairment of learning memory, spatial memory and working memory abilities caused by Aβ, and had protective effects on the brain.
The pathogenesis of AD has been investigated extensively (Liang et al., 2015). Much research shows that expressions of synapse-related proteins were high in the molecular layer of the dentate gyrus of the hippocampus and low in other areas of the hippocampus and in the neocortex during early phases of AD, which will cause abnormal synaptic structure and transmission (Mota et al., 2014). Several studies have also revealed that soluble Aβ was highly correlated with memory-related synaptic dysfunctions (Parodi et al., 2010; Sivanesan et al., 2013). Some findings concluded that Aβ interacts with phosphorylated tau and calcium channel (Lau et al., 2009; Hermann et al., 2013), so that Aβ may damage neuronal structure and function, particularly synapses, leading to cognitive decline in AD (Manczak and Reddy, 2013). Therefore, abnormal synaptic transmission and synaptic damage at the early phase may be major causes for the progressive decline in learning and memory abilities of AD patients. The learning and memory process requires structural and functional plasticity of synaptic connections (Nabavi et al., 2014). Structural plasticity mainly includes changes in the form of the presynaptic terminal, the curvature of the synaptic interface and postsynaptic densities. Functional plasticity includes changes in synthesis and release of neurotransmitters, phosphorylation and dephosphorylation of the second messenger G protein and regulatory protein after activation of the receptor (Colón-Ramos, 2009). Synaptic plasticity is crucial in the formation of the learning and memory process (Zovkic et al., 2013; Poo et al., 2016). Our results in this study show that in the AD group compared with the normal control group, presynaptic mitochondria cristae were unclear; the postsynaptic densities were significantly thinner, the widths of synaptic clefts were significantly larger, the synaptic active zones were significantly shorter and the curvature of synaptic interface in CA1 area of the hippocampus was reduced. The percentage of flat synapses increased, but the concave and perforated synapses in CA1 area of the hippocampus were significantly reduced in the AD group. The above effects may greatly affect the synaptic transmission efficiency, causing significant learning and memory impairment to mice in the AD group. Compared with the AD group, the TSG group had clearer presynaptic mitochondria crista, significantly thicker postsynaptic density, significantly smaller width of synaptic cleft, significantly increased synaptic active zone and increased curvature of synaptic interface. The percentage of flat synapses was less, but the distribution of concave synapses and perforated synapses in CA1 area of the hippocampus was greater than in the AD group.
The NMDA receptor 2B (NR2B) is strongly associated with synaptic plasticity (Köhr, 2006). Müller et al. (2009) considered that both NR2A and NR2B subunits, NMDA receptor, are critical for long-term potentiation. Bliss called NR2B “a smart gene” and regards it a memory gene (Bliss and Collingridge, 1993; Corlew et al., 2008).
Tyrosine phosphorylation of NR2B requires the mediation of Src protein tyrosine kinases (PTKs), which is a family of membrane-bound non-receptor protein tyrosine kinases composed of 10 types with individual gene codes (Berg et al., 2013). From current studies on non-receptor PTKs, only Fyn is closely related to learning and memory functions. Mice with Fyn inhibition show a large number of neurological defects, including lack of long-term potentiation, impaired spatial memory, synaptic loss and uncoordinated hippocampal structures (Grant et al., 1992; Kojima et al., 1997; Minami et al., 2012). Mizuno et al. (2003) confirmed that mice with behavioral training had an increased activity of Fyn in the hippocampus. Fyn can also induce long-term potentiation in CA1 region through NMDA receptors (Lu et al., 1999; Trepanier et al., 2012). Therefore, Fyn and NR2B pathways are a key link in the learning and memory impairment process. Results of this study showed that compared with the AD group, NR2B and Fyn expressions were significantly higher in the TSG group, though not at normal levels. As a result, postsynaptic density became obviously thicker, which would promote synaptic transmission and therefore the signaling complex of NMDA receptors and change the permeability of ionic NMDA receptors to monovalent cation and calcium. These would enable the formation of long-term potentiation. Thus, TSG would improve the acquisition of learning and memory in the AD transgenic mice.
Change in the phosphorylation of some enzymes and substrate proteins (such as NMDA receptor and Src) will induce change in the molecular configuration, resulting in thicker or thinner postsynaptic densities (Li and Ju, 2012). Thicker postsynaptic density and smaller synaptic cleft will allow easier transmission of nerve impulse (Petzoldt and Sigrist, 2014). Conversely, if the synaptic cleft becomes wider, and the postsynaptic density becomes thinner, transmission of nerve impulses becomes more difficult. This is consistent with the significantly thinner postsynaptic density and wider synaptic cleft in the CA1 region of the hippocampus in mice in the AD group. Jones and Calverley (1991) have observed and demonstrated under different conditions that the curvature of synaptic interface is associated with different functionality of the synapses. Increased curvature of the synaptic interface indicates better synaptic transmission, while the reduced curvature of synaptic interface indicates poorer synaptic transmission and long-term potentiation is less probable. The change in percentage of flat synapses and concave synapses among the three types of synapses is also a key factor affecting the synaptic transmission efficiency. Concavesynapses, with their more concentrated interface, can allow the released neurotransmitters to arrive at the target point and improve the effectiveness of information transmission with neurotransmitters. Synaptic perforation also plays a significant role in synaptic plasticity and hippocampal synapse formation (Sorra et al., 1998). The loss of synapses in AD may include a loss of perforated synapses (Jones, 1993; Jones and Harris, 1995). It is held that synaptic perforation can increase the contact area between neurotransmitters and postsynaptic density to improve the efficiency of synaptic transmission.
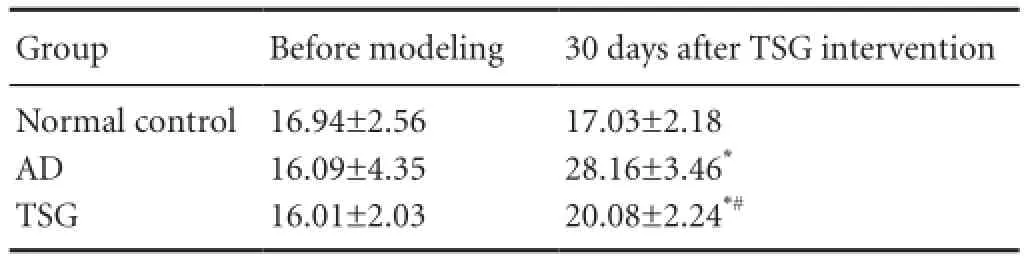
Table 1 Effects of TSG on learning and memory abilities of AD model mice in Y-maze test
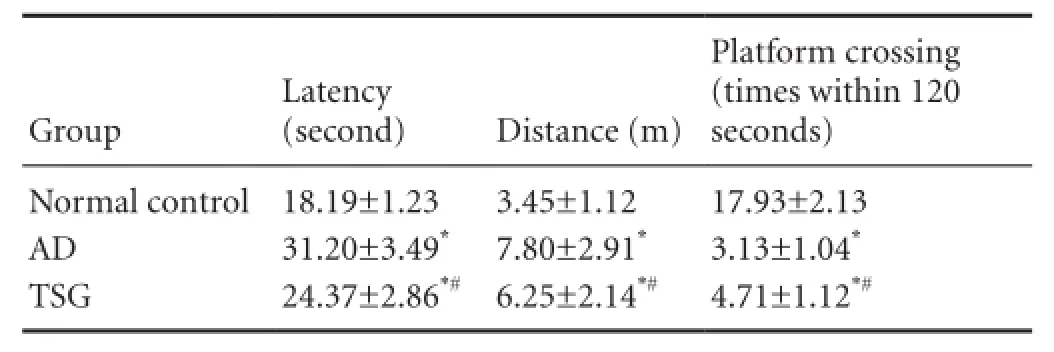
Table 2 Effects of TSG on learning and memory abilities of AD model mice in the Morris water maze test

Figure 1 Effect of TSG on synaptic ultrastructure in CA1 region of the hippocampus of AD model mice (× 40,000).

Table 3 Parameters for synaptic interface structure and average percentage of three types of synapse in the CA1 region of the mouse hippocampus

Figure 2 Effect of TSG on protein expression of Fyn and phosphorylation of NR2B in CA1 region of the hippocampus of AD model mice.
Our study demonstrated that the mechanism of TSG action may be the increased expressions of NR2B and Fyn proteins, which are of great significance to long-term potentiation. Fyn can cause the phosphorylation of NR2B, which is a major functional unit of the NMDA receptors and one of the main components of postsynaptic membranes. NR2B can bind other protein molecules and enzymes. The increased expressions of Fyn and NR2B can not only be good for the formation and functions of the NMDA receptor complex, but also it can cause thicker postsynaptic density, narrower synaptic cleft and so improve the effectiveness of synaptic transmission and long-term potentiation induction. With increased NR2B expression, more enzymes will be bound and anchored on the membrane, which could improve the activation of such enzymes and synaptic transmission.
TSG can reverse the distribution of structural parameters for concave synapses and perforated synapses in AD transgenic mice. That improves the information transmission via neurotransmitters resulting in significant relief from the learning and memory impairment caused by Aβ. These anatomical and behavioral benefits of TSG suggest it is a promising medicine for the future treatment of AD.
Author contributions: HBL and XQS conceived and designed the experiments. HBL, YL, ZQZ, LC, YW, XYZ and ZL performed the experiments. HBL, ZJL and XQS analyzed the data. HBL wrote the paper. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Allen N, Robinson AC, Snowden J, Davidson YS, Mann DM (2014) Patterns of cerebral amyloid angiopathy define histopathological phenotypes in Alzheimer’s disease. Neuropathol Appl Neurobiol 40:136-148. Alzheimer’s Association (2015) 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 11:332-384.
Bass B, Upson S, Roy K, Montgomery EL, Jalonen TO, Murray IV (2015) Glycogen and amyloid-beta: key players in the shift from neuronal hyperactivity to hypoactivity observed in Alzheimer’s disease? Neural Regen Res 10:1023-1025.
Berg LK, Larsson M, Morland C, Gundersen V (2013) Pre- and postsynaptic localization of NMDA receptor subunits at hippocampal mossy fibre synapses. Neuroscience 230:139-150.
Bliss TV, Collingridge GL (1993) A synaptic model of memory: longterm potentiation in the hippocampus. Nature 361:31-39.
Calabrese B, Shaked GM, Tabarean IV, Braga J, Koo EH, Halpain S (2007) Rapid, concurrent alterations in pre- and postsynaptic structure induced by naturally-secreted amyloid-beta protein. Mol Cell Neurosci 35:183-193.
Castello MA, Soriano S (2014) On the origin of Alzheimer’s disease. Trials and tribulations of the amyloid hypothesis. Ageing Res Rev 13:10-12.
Chiang K, Koo EH (2014) Emerging therapeutics for Alzheimer’s diseas. Annu Rev Pharmacol Toxicol 54:381-405.
Chin J, Palop JJ, Puoliväli J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E, Mucke L (2005) Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer’s disease. J Neurosci 25:9694-9703.
Colón-Ramos DA (2009) Synapse formation in developing neural circuits. Curr Top Dev Biol 87:53-79.
Corlew R, Brasier DJ, Feldman DE, Philpot BD (2008) Presynaptic NMDA receptors: newly appreciated roles in cortical synaptic function and plasticity. Neuroscientist 14:609-625.
D’Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Rev 36:60-90.
Evans NA, Facci L, Owen DE, Soden PE, Burbidge SA, Prinjha RK, Richardson JC, Skaper SD (2008) Abeta(1-42) reduces synapse number and inhibits neurite outgrowth in primary cortical and hippocampal neurons: a quantitative analysis. J Neurosci Methods 175:96-103.
Folch J, Petrov D, Ettcheto M, Abad S, Sánchez-López E, García ML, Olloquequi J, Beas-Zarate C, Auladell C, Camins A (2016) Current research therapeutic strategies for Alzheimer’s disease treatment. Neural Plast 2016:8501693.
Güldner FH, Ingham CA (1980) Increase in postsynaptic density material in optic target neurons of the rat suprachiasmatic nucleus after bilateral enucleation. Neurosci Lett 17:27-31.
Grant SG, O’Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER (1992) Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science 258:1903-1910.
Hermann D, Mezler M, Müller MK, Wicke K, Gross G, Draguhn A, Bruehl C, Nimmrich V (2013) Synthetic Aβ oligomers (Aβ(1-42) globulomer) modulate presynaptic calcium currents: prevention of Aβ-induced synaptic deficits by calcium channel blockers. Eur J Pharmacol 702:44-55.
Holtzman DM, John CM, Goate A (2011) Alzheimer’s disease: the challenge of the second century. Sci Transl Med 3:77sr71.
Jones DG (1993) Synaptic plasticity and perforated synapses: their relevance for an understanding of abnormal synaptic organization. APMIS Suppl 40:25-34.
Jones DG, Devon RM (1978) An ultrastructural study into the effects of pentobarbitone on synaptic organization. Brain Res 147:47-63.
Jones DG, Calverley RKS (1991) Perforated and non-perforated synapses in rat neocortex: three-dimensional reconstructions. Brain Res 556:247-258.
Jones DG, Harris RJ (1995) An analysis of contemporary morphological concepts of synaptic remodelling in the CNS: perforated synapses revisited. Rev Neurosci 6:177-219.
Köhr G (2006) NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res 326:439-446.
Kojima N, Wang J, Mansuy IM, Grant SGN, Mayford M, Kandel ER (1997) Rescuing impairment of long-term potentiation in fyn-deficient mice by introducing Fyn transgene. Proc Natl Acad Sci U S A 94:4761-4765.
Kumar A, Singh A, Ekavali (2015) A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep 67:195-203.
Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL (2007) Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci 27:796-807.
Langa KM (2015) Is the risk of Alzheimer’s disease and dementia declining? Alzheimers Res Ther 7:34.
Lau CG, Takeuchi K, Rodenas-Ruano A, Takayasu Y, Murphy J, Bennett MVL, Zukin RS (2009) Regulation of NMDA receptor Ca2+signalling and synaptic plasticity. Biochem Soc Trans 37:1369-1374.
Li H, Liu MF, Liu JG, Liu LT, Guan J, Cai LL, Hu J, Wei Y (2012) Effect of Huannao Yicong prescription extract on β-amyloid precursor protein metabolic signal transduction-related protein in brain tissue of dementia model transgenic mouse. Zhong Xi Yi Jie He Xue Bao 18:683-689.
Li ST, Ju JG (2012) Functional roles of synaptic and extrasynaptic NMDA receptors in physiological and pathological neuronal activities. Curr Drug Targets 13:207-221.
Liang ZH, Cheng XH, Ruan ZG, Wang H, Li SS, Liu J, Li GY, Tian SM (2015) Protective effects of components of the Chinese herb grassleaf sweetflag rhizome on PC12 cells incubated with amyloid-beta42. Neural Regen Res 10:1292-1297.
Lu YF, Kojima N, Tomizawa K, Moriwaki A, Matsushita M, Obata K, Matsui H (1999) Enhanced synaptic transmission and reduced threshold for LTP induction in fyn-transgenic mice. Eur J Neurosci 11:75-82.
Luo H, Li Y, Guo J, Liu Z, Zhang Z, Wang Y, Liu Z, Shi X (2015) Tetrahydroxy stilbene glucoside improved the behavioral disorders of APP695V717I transgenic mice by inhibiting the expression of Beclin-1 and LC3-II. J Tradit Chin Med 35:295-300.
Luo HB, Yang JS, Shi XQ, Fu XF, Yang QD (2009) Tetrahydroxy stilbene glucoside reduces the cognitive impairment and overexpression of amyloid precursor protein induced by aluminum exposure. Neurosci Bull 25:391-396.
Müller T, Albrecht D, Gebhardt C (2009) Both NR2A and NR2B subunits of the NMDA receptor are critical for long-term potentiation and long-term depression in the lateral amygdala of horizontal slices of adult mice. Learn Mem 16:395-405.
Manczak M, Reddy PH (2013) Abnormal interaction of oligomeric amyloid-β with phosphorylated tau: implications to synaptic dysfunction and neuronal damage. J Alzheimers Dis 36:285-295.
Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, Brayne C, Medical Research Council Cognitive Function and Ageing Collaboration (2013) A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet 382:1405-1412.
Minami SS, Clifford TG, Hoe HS, Matsuoka Y, Rebeck GW (2012) Fyn knock-down increases Aβ, decreases phospho-tau, and worsens spatial learning in 3xTg-AD mice. Neurobiol Aging 33:825.e815-824.
Mizuno M, Yamada K, He J, Nakajima A, Nabeshima T (2003) Involvement of BDNF receptor TrkB in spatial memory formation. Learn Mem 10:108-115.
Morris GP, Clark IA, Vissel B (2014) Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer’s disease. Acta Neuropathol Commun 2:135.
Mota SI, Ferreira IL, Rego AC (2014) Dysfunctional synapse in Alzheimer’s disease - A focus on NMDA receptors. Neuropharmacology 76 Part A:16-26.
Mucke L, Selkoe DJ (2012) Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med 2:a006338.
Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R (2014) Engineering a memory with LTD and LTP. Nature 511:348-352.
O’Keefe J, Dostrovsky J (1971) The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34:171-175.
Parodi J, Sepúlveda FJ, Roa J, Opazo C, Inestrosa NC, Aguayo LG (2010) Beta-amyloid causes depletion of synaptic vesicles leading to neurotransmission failure. J Biol Chem 285:2506-2514.
Paxinos G, Franklin KB (2001) The Mouse Brain in Stereotaxic Coordinates. New York: Academic Press.
Petzoldt AG, Sigrist SJ (2014) Synaptogenesis. Curr Biol 24:R1076-1080.
Poo MM, Pignatelli M, Ryan TJ, Tonegawa S, Bonhoeffer T, Martin KC, Rudenko A, Tsai LH, Tsien RW, Fishell G, Mullins C, Gonçalves JT, Shtrahman M, Johnston ST, Gage FH, Dan Y, Long J, Buzsáki G, Stevens C (2016) What is memory? The present state of the engram. BMC Biol 14:40.
Qiu C, von Strauss E, Bäckman L, Winblad B, Fratiglioni L (2013) Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology 80:1888-1894.
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. New Engl J Med 362:329-344.
Robakis NK (2014) Cell signaling abnormalities may drive neurodegeneration in Familial Alzheimer disease. Neurochem Res 39:570-575.
Santana I, Farinha F, Freitas S, Rodrigues V, Carvalho Å (2015) The epidemiology of dementia and Alzheimer disease in portugal: estimations of prevalence and treatment-costs. Acta Med Port 28:182-188.
Satizabal CL, Beiser AS, Chouraki V, Chêne G, dufouil C, Seshadri S (2016) Incidence of dementia over three decades in the framingham heart study. New Engl J Med 374:523-532.
Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL (2007) Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci 27:2866-2875.
Sivanesan S, Tan A, Rajadas J (2013) Pathogenesis of Abeta oligomers in synaptic failure. Curr Alzheimer Res 10:316-323.
Sorra KE, Fiala JC, Harris KM (1998) Critical assessment of the involvement of perforations, spinules, and spine branching in hippocampal synapse formation. J Comp Neurol 398:225-240.
Spires-Jones TL, Hyman BT (2014) The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 82:756-771.
Trepanier CH, Jackson MF, MacDonald JF (2012) Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J 279:12-19.
Zeng C, Xiao JH, Chang MJ, Wang JL (2011) Beneficial effects of THSG on acetic acid-induced experimental colitis: involvement of upregulation of PPAR-γ and inhibition of the Nf-Κb inflammatory pathway. Molecules 16:8552-8568.
Zhang JK, Yang L, Meng GL, Fan J, Chen JZ, He QZ, Chen S, Fan JZ, Luo ZJ, Liu J (2012) Protective effect of tetrahydroxystilbene glucoside against hydrogen peroxide-induced dysfunction and oxidative stress in osteoblastic MC3T3-E1 cells. Eur J Pharmacol 689:31-37.
Zhang L, Yu S, Zhang R, Xing Y, Li Y, Li L (2013) Tetrahydroxystilbene glucoside antagonizes age-related α-synuclein overexpression in the hippocampus of APP transgenic mouse model of Alzheimer’s disease. Restor Neurol Neurosci 31:41-52.
Zovkic IB, Guzman-Karlsson MC, Sweatt JD (2013) Epigenetic regulation of memory formation and maintenance. Learn Mem 20:61-74.
Copyedited by Ann Dawes E, Hindle A, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.191223
*Correspondence to:
杂志排行
中国神经再生研究(英文版)的其它文章
- Blood microRNAs as potential diagnostic and prognostic markers in cerebral ischemic injury
- Recovery of corticospinal tract injured by traumatic axonal injury at the subcortical white matter: a case report
- Sigma-1 receptor and neuroprotection: current outlook and potential therapeutic effects
- Intra-axonal protein synthesis - a new target for neural repair?
- Nanobiomaterials for neural regeneration
- Cell transplantation for the treatment of spinal cord injury — bone marrow stromal cells and choroid plexus epithelial cells
