The pleiotropic effects of tissue plasminogen activator in the brain: implications for stroke recovery
2016-12-01JuliaA.Grummisch,NafisaM.Jadavji,PatriceD.Smith
The pleiotropic effects of tissue plasminogen activator in the brain: implications for stroke recovery
Tissue plasminogen activator (tPA) use in the treatment of ischemic stroke: tPA is a serine protease that catalyzes the breakdown of blood clots. Because of its thrombolytic properties, tPA is used to treat specific types of stroke, including ischemia, but is contraindicated for treatment of hemorrhagic stroke or head trauma. Although a life saving and powerful ‘clot buster’, tPA has a short therapeutic window. When administered outside of this prescribed timeframe, research suggests that tPA can produce neurotoxic effects in the brain, due in part to activation of several signalling processes associated with cell apoptosis, degradation of the extracellular matrix, and increase in the permeability of the neurovascular unit (Yepes et al., 2009). Concerted research has been dedicated toward understanding the mechanisms mediating the impact of tPA on the brain, using both in vivo and in vitro animal models. Despite the success of repeated clinical trials and a growing research base pointing to the involvement of several underlying cellular mechanisms mediating the effect of tPA, including an emerging role for mammalian target of rapamycin (mTOR)-dependent signaling, the precise impact of tPA on neurons at the cellular level remains unclear. Furthermore, the widespread clinical use of tPA, for the treatment of acute ischemic stroke, supports the critical nature of establishing a better understanding of the underlying molecular processes mediating the effects of tPA in the brain. This perspective briefly examines current research focusing on the underlying mechanisms mediating the proposed effects of tPA on the brain.
The pleiotropic role of tPA in neuronal viability: tPA is an enzyme that catalyzes the conversion of plasminogen to plasmin and plays a key role in regulating the extracellular matrix (Yu et al., 2016). tPA crosses the blood-brain barrier and is made up of several unique functional domains that contribute to its pleiotropic functions, including interacting with target sequences within substrates of other molecules, binding to membranes, proteins and phospholipids, regulating catalytic activity, interacting with fibrin, plasminogen, and platelet-derived growth factors, and facilitating the cleavage of the NR1 subunit of the NMDA receptor (Yepes et al., 2009). tPA can also act as a cytokine, thereby contributing to multiple molecular cascades (Lin et al., 2010), as well as inducing a variety of responses in the context of neuropathology, including ischemia. These functions of tPA can serve to either protect or harm neurons, depending on the temporal and spatial parameters. In this regard, current research supports a potential role for a plasminogen-dependent effect in the intravascular space and a plasminogen-dependent and -independent effect within the area of cerebral insult (Yepes et al., 2009). At the molecular level, tPA promotes neuroprotection; in a study involving murine postnatal primary cortical neurons in culture, it was found that tPA administration produces a time-dependent neuroprotective effect via activation of mTOR signaling (Grummisch et al., 2016). Postnatal cortical neurons treated with tPA were more likely to survive compared to neurons treated with a control. These results illustrate that the natural sequence of events that promotes cell death in early postnatal neurons could be postponed, at least to some degree, with tPA treatment.
Within the context of well-established models of stroke, current research also points to a neuroprotective role for tPA, particularly in regard to cortical neuron survival. Supporting the potential neuroprotective role of tPA, a recent study using hypoxia, has shown that tPA promotes neuroprotection, and was devoid of neurotoxic effects (Haile et al., 2012). Wu and colleagues also exposed neurons to hypoxic conditions and discovered, through liquid chromatography and tandem mass spectrometry, that the endogenous release of tPA or treatment with recombinant tPA promoted neuron survival via activation of the mTOR pathway (Wu et al., 2012). This study also showed that tPA induces the uptake of glucose under ischemic conditions and that this response improves neurological outcome following the induction of ischemic stroke (Wu et al., 2012). In a separate study, Wu et al. (2013) investigated the effect of tPA on excitotoxin-induced neuronal death, and found that either the genetic overexpression of endogenous tPA or treatment with exogenous recombinant tPA rendered neurons resistant to the harmful effects induced by the excitotoxicity. An extracellular signal-regulated protein kinases 1 and 2 (ERK1/2)-mediated activation of cAMP response element binding protein (CREB) was found to underlie the neuroprotective effects observed (Wu et al., 2013). In a separate study, using an oxygen and glucose deprivation (OGD) model, a rapid release of tPA from cerebral cortical neurons but not from astrocytes was observed, and the tPA-induced glucose uptake was via AMPK activation in astrocytes and endothelial cells; this was followed by the synthesis and release of lactic acid by the astrocytes that contributed to a neuronal survival effect (An et al., 2014).
Potential functional benefits of tPA treatment: As further evidence of its beneficial effects, tPA has been shown to play a critical role in promoting neurite outgrowth, synaptic plasticity, learning and memory (Jeanneret and Yepes, 2016). Recent research has branched out to focus on the deleterious effects of cerebral ischemia on the morphology and function of dendritic spines, specialized structures associated with synaptic transmission, and their capacity for recovery following an ischemic event in the presence or absence of tPA (Jeanneret and Yepes, 2016). Results from recent work in this area revealed that when neurons are treated with tPA or a similar serine protease, urokinease-type plasminogen activator (uPA), a robust induction of synaptic plasticity through promotion of dendritic spine morphology and functional recovery is achieved following metabolic stress due to acute ischemic injury (Jeanneret and Yepes, 2016). This work provides a clearer picture of the molecular properties of tPA and suggests a more substantiated basis for establishing its capability for promoting functional recovery following ischemia, in addition to its role as a thrombolytic agent.
Future directions: Several underlying cellular mechanisms have been identified that play a role in the maintenance of neuronal survival, using both in vivo and in vitro models. A growing body of research evidence has pointed to the precise importance of the mTOR pathway in promoting neuroprotection as a result of tPA treatment. Specifically, in a recent comprehensive review focused on the proposed neuroprotective role of tPA in the CNS, tPA-induced neuroprotective effect appears to be mediated by mTOR-dependent signalling. Howere, the role of mTOR signaling in tPA-induced neuroprotection requires further investigation, especially given the widespread clinical use of tPA as a therapeutic for stroke. An illustration of three principal inter-linked signalling pathways typically involved in neuroprotection is provided in Figure 1. Possible combinatorial interactions between different pathways could result in the regulation and maintenance of cell survival, growth and proliferation; however, these mechanisms remain to be fully elucidated and could be critical in unlocking the precise function of tPA in facilitating pro-survival effects in neurons. It has been recently shown that tPA enhances postnatal cortical neuron survival via an mTOR-dependent mechanism, although the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway also appeared to play a role in promoting cell survival (Grummisch et al., 2016), see Figure 2.
A burgeoning body of literature has pointed to the applicabilityand efficacy of adjunctive therapies, using tPA within an integrative context with other agents, to promote synergistic and more robust tPA-induced neuroprotection within the clinical setting. For example, in a recent animal-based study, Yu and colleagues found that following embolic middle cerebral artery occlusion (MCAO), an animal model of cerebral ischemia, combined treatment with tPA and 2-(4-Methoxyphenyl)ethyl-2-acetamido-2-deoxy-β-d-pyranoside (SalA-4g), a salidroside analog, resulted in neuroprotective effects, compared to treatment with tPA alone (Yu et al., 2016). Combined treatment resulted in reduced neurological deficits, infarct volume, intracerebral bleeding, edema formation, neuronal loss and cellular apoptosis four hours following MCAO (Yu et al., 2016). Within the human population, a series of endovascular clinical trials have also highlighted the potential of using supplemental therapies in concert with tPA to improve functional independence (Lees et al., 2010). These developments shed light on the early yet promising potential of adjunctive therapies for future advances in ischemic therapy and recovery with or in combination with tPA. Future research focusing on identifying the molecular and cellular events underlying the beneficial effects of tPA may provide insight into methods for further optimizing combinatorial treatment strategies for stroke.

Figure 1 The JAK/STAT, Raf/MEK/ERK, and PI3K/PTEN/Akt/mTOR pathway cascades.
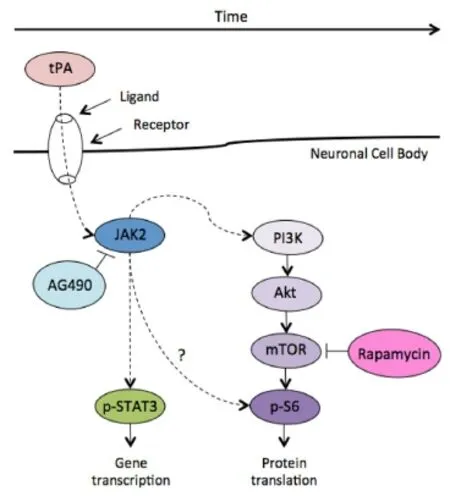
Figure 2 Proposed signaling mechanism underlying the neuroprotective role of tPA.
Conclusion: Further understanding of the impact of tPA on neurons could open new doors towards enhancing the efficacy of tPA clinically; particularly the promise of combinatorial therapies. The results from the current research literature foster a framework to better understand the protective effects of tPA within the brain and the underlying mechanisms mediating its effect. Further research is warranted to improve the current understanding of the non-thrombolytic functions of tPA in the brain and to ultimately identify novel therapies that will improve functional outcomes for individuals suffering from ischemic stroke.
Julia A. Grummisch, Nafisa M. Jadavji, Patrice D. Smith*
Department of Neuroscience, Carleton University, Ottawa, Ontario, Canada
*Correspondence to: Patrice D. Smith, Ph.D., Patrice.Smith@carleton.ca.
Accepted: 2016-09-01
orcid: 0000-0002-0731-2803 (Patrice D. Smith)
How to cite this article: Grummisch JA, Jadavji NM, Smith PD (2016) The pleiotropic effects of tissue plasminogen activator in the brain: implications for stroke recovery. Neural Regen Res 11(9):1401-1402.
References
An J, Haile WB, Wu F, Torre E, Yepes M (2014) Tissue-type plasminogen activator mediates neuroglial coupling in the central nervous system. Neuroscience 257:41-48.
Grummisch JA, Jadavji NM, Smith PD (2016) tPA promotes cortical neuron survival via mTOR-dependent mechanisms. Mol Cell Neurosci 74:25-33.
Haile WB, Wu J, Echeverry R, Wu F, An J, Yepes M (2012) Tissue-type plasminogen activator has a neuroprotective effect in the ischemic brain mediated by neuronal TNF-alpha. J Cereb Blood Flow Metab 32:57-69.
Jeanneret V, Yepes M (2016) The plasminogen activation system promotes dendritic spine recovery and improvement in neurological function after an ischemic stroke. Transl Stroke Res doi:10.1007/s12975-016-0454-x.
Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, Tilley BC, Davis SM, Donnan GA, Hacke W; ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group, Allen K, Mau J, Meier D, del Zoppo G, De Silva DA, et al. (2010) Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 375:1695-1703.
Lin L, Bu G, Mars WM, Reeves WB, Tanaka S, Hu K (2010) tPA activates LDL receptor-related protein 1-mediated mitogenic signaling involving the p90RSK and GSK3beta pathway. Am J Pathol 177:1687-1696.
Wu F, Echeverry R, Wu J, An J, Haile WB, Cooper DS, Catano M, Yepes M (2013) Tissue-type plasminogen activator protects neurons from excitotoxin-induced cell death via activation of the ERK1/2-CREB-ATF3 signaling pathway. Mol Cell Neurosci 52:9-19.
Wu F, Wu J, Nicholson AD, Echeverry R, Haile WB, Catano M, An J, Lee AK, Duong D, Dammer EB, Seyfried NT, Tong FC, Votaw JR, Medcalf RL, Yepes M (2012) Tissue-type plasminogen activator regulates the neuronal uptake of glucose in the ischemic brain. J Neurosci 32:9848-9858.
Yepes M, Roussel BD, Ali C, Vivien D (2009) Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends Neurosci 32:48-55.
Yu S, Liu X, Shen Y, Xu H, Yang Y, Ding F (2016) Therapeutic benefits of combined treatment with tissue plasminogen activator and 2-(4-methoxyphenyl) ethyl-2-acetamido-2-deoxy-beta-d-pyranoside in an animal model of ischemic stroke. Neuroscience 327:44-52.
10.4103/1673-5374.191204
杂志排行
中国神经再生研究(英文版)的其它文章
- Blood microRNAs as potential diagnostic and prognostic markers in cerebral ischemic injury
- Recovery of corticospinal tract injured by traumatic axonal injury at the subcortical white matter: a case report
- Sigma-1 receptor and neuroprotection: current outlook and potential therapeutic effects
- Intra-axonal protein synthesis - a new target for neural repair?
- Nanobiomaterials for neural regeneration
- Cell transplantation for the treatment of spinal cord injury — bone marrow stromal cells and choroid plexus epithelial cells
