三种配合物微晶的尺寸可控合成及其光催化性质
2016-11-28王晓祥
刘 祥 韩 晶*, 余 中 王晓祥
(1西安理工大学材料物理与化学系,西安710048) (2西安理工大学化学系,西安710048)
三种配合物微晶的尺寸可控合成及其光催化性质
刘祥1韩晶*,1余中2王晓祥1
(1西安理工大学材料物理与化学系,西安710048) (2西安理工大学化学系,西安710048)
以4,4′-二苯醚二甲酸和4,4′-联吡啶为配体,通过调节水热反应的条件,如三乙胺的加入量,反应时间和反应温度等因素,可控合成了尺寸从10到250 μm的Ni(Ⅱ)、Co(Ⅱ)和Zn(Ⅱ)3种配合物微晶,其尺寸、形貌和结构用扫描电镜和X射线粉末衍射进行了表征和分析。光催化性能测试表明3种配合物对有机染料罗丹明B、甲基橙和亚甲基蓝均具有杰出的降解能力,且随着配合物晶粒尺寸减小其催化能力明显提高。最引人注目的是尺寸为30 μm的Ni(Ⅱ)配合物,其光催化性降解RhB染料的能力已超越纳米TiO2。此外,X射线粉末衍射表明,在一次光降解过程后配合物结构保持稳定,可作为重复利用的光催化剂。
水热合成;配合物;尺寸可控;光催化
0 Introduction
The ability of using solar energy to eliminate the organic pollutants makes photocatalyst a practical technology for dealing with environmental pollution. Since the discovery of the first artificial photocatalyticsystem for pollutants degradation over TiO2,many metal oxides and sulfides including ZnO,WO3,CdS, ZnS have been identified as active photocatalysts for photodegradation of organic pollutants in gas or aqueous phase[1-4].However,the degradation efficiency of these developed photocatalysts are still low at present[5],perhaps limiting their practical applications in environmental purification.
On the other hand,metal-organic coordination polymers(MOFs)have generated considerable interests in both material science and environmental protection due to their potential applications in gas storage,heterogeneous catalysis,selective guest adsorption,and sensor technology[6-10].Furthermore, some MOFs may be used as potential photocatalysts due to their nature of semiconductors[11].Therefore, considerable efforts have been devoted to exploit the applications of MOFs as photocatalysts in the green degradation of organic dye contaminant to solve environmental issues[12-15].
Nanostructured materials,often characterized by a physical dimension of 1~100 nm(such as grain size) and a significant amount of surfaces and interfaces, have been attracting much interest because of surface effect and macro quantum effect.They demonstrate or anticipate unique physical and chemical properties compared to conventional materials.Also,it′s found that coordination polymers indicate the character of size effect[16-18].To date,several synthetic approaches have been developed for the syntheses of crystalline nano-sized MOFs such as microemulsions[19], surfactants[20],capping reagents[21],and hydrothermal methods[22].Kitagawa et al.[23]showed that highly crystalline nanorods with higher porosity comparable to that of bulk crystals could be prepared using coordination modulation method.Using reverse micelles as soft colloidal templates in the syntheses, Gao et al.[24]synthesized low-dimensional nanoscale Prussian blue analogue materials with more interesting magnetic behavior than that of the conventional large crystals.Wu et al.[25]reported two nano-sized rare earth complexes by using poly(vinyl pyrrolidone)as surfactant,which showed better fluorescence properties than those of the corresponding common complexes.These results indicate that the reduction of the particle size has a significant impact on performance.
Previously,we have synthesized and characterized three complexes[μ-O-Ni2(4,4′-bpy)2(oba)2]n(1), [Co2(4,4′-bpy)(oba)]n(2)and[Zn(4,4′-bpy)(oba)]n(3)[26]. It has been demonstrated that the powdered crystals of Ni(Ⅱ)complex exhibit outstanding photocatalytic performance for degradation of RhB,which is slightly inferior to that of nano-sized TiO2.Inspired by the improved performance originating from the size effect of nanomaterials and aimed at providing an insight on the correlation between crystallite size of MOFs and their photocatalytic activities,in this work,the controllable hydrothermal synthesis of various sizes of complexes were investigated in detail by regulating various reaction factors,such as the amount of NEt3, reaction time,temperature and cooling method.Their photocatalytic degradation activities and stabilities were further evaluated by UV-Vis spectra and X-ray powder diffraction analysis with RhB,MO and MB as model pollutants.Our research results show that the degradation efficiency of the Ni(Ⅱ)complex for RhB can be improved dramatically from 76.06%to 96.54% with the original average particle size of 200 μm down to 30 μm,while keeping the coordination structure unchanged.
1 Experimental
1.1Materials and instruments
All reagents and solvents were purchased from commercial sources and used as received without further purification.Powder X-ray diffraction(XRD) patterns were collected on a SHIMADZU LIMITED apparatus at 40 kV and 30 mA with a sealed tube X-ray generator producing Cu Kα radiation(λ=0.154 18 nm)over the 2θ range of 5°~35°at room temperature. The simulated pattern was obtained from single-crystal X-ray analysis.The solid-state diffuse-reflectance UVVis spectra for powder samples were recorded on a WFZ800-D2C UV-Vis spectrometer equipped with an integrating sphere by using BaSO4as reference.Thesize and morphology of samples were characterized by field-emission scanning electron microscopy(JSM-6700F).The photochemical reactor used in this study consist of a jacketed quartz tube with a high-pressure mercury lamp of 125 W placed inside,and equipped with the stirrer and ventilator
Nitrogen physisorption isotherms were measured at-77.30℃on a QUANTACHROME Autosorb1-MP volumetric instrument.Samples were outgassed in vacuum at room temperature for at least 24 h before the sorption measurements.Surface areas were estimated by applying the Brunauer-Emmett-Teller (BET)equation.The Barrett-Joyner-Halenda(BJH) method was applied to determine micropore size distributions.
For the degradation studies,three MOFs(25 mg) of varied size in a fresh aqueous solution of RhB,MO and MB(25 mL,2 mg·L-1)were stirred for 3 h to attain adsorption-desorption equilibrium of dyes on the sample surface.Then samples were collected and centrifuged to remove the catalyst particles prior to analysis on Zolix Scan Basic ultraviolet spectrophotometer.The degradation rate of dyes(D) could be obtained by D=(A0-At)/A0×100%,where A0and Atwere the absorbance intensity of original dyes aqueous solution before irradiation(t=0 min)and after irradiation for t min,respectively.
1.2Sample preparation
1.2.1Synthesis of[μ-O-Ni2(4,4′-bpy)2(oba)2]n(1)
To a mixture of of NiSO4·6H2O(0.131 4 g,0.5 mmol)in 10 mL deionized water,oba(0.129 1 g,0.5 mmol),4,4′-bpy(0.078 1 g,0.5 mmol)and different amount of NEt3were slowly added upon stirring at room temperature.The mixture was stirred vigorously until it became homogeneous.Then the mixture was allowed to be sealed in a 25 mL Teflon-lined stainless vessel and heated at certain temperature for different hours.Crystallization was completed by cooling,and the green cubic crystals was collected,washed three times with N,N-Dimethylformamide(DMF)and dried at 40℃in vacuum.Complex 1 was isolated in 75% yield on the basis of Ni.
1.2.2Synthesis of[Co2(4,4′-bpy)(oba)]n(2)
CoSO4·7H2O(0.084 3 g,0.3 mmol),oba(0.077 5 g, 0.3 mmol)and 4,4′-bpy(0.046 9 g,0.3 mmol)were heated at certain temperature for different hours in an analogous procedure for 1.Purple crystals were obtained with the yield of 78%based on Co.
1.2.3Synthesis of[Zn(4,4′-bpy)(oba)]n(3)
The synthesis of 3 was similar to that described for 1 except using ZnSO4·7H2O(0.144 0 g,0.5 mmol) instead of NiSO4·6H2O.Colorless crystals of 3 were obtained with the yield of 65%based on Zn.
2 Results and discussion
Hydrothermal method is recently demonstrated to be an appealing route for the synthesis of MOFs due to its simple operation,cheap fabrication and high yield.Thus,hydrothermal synthesis was employed in this work for the controllable syntheses of complexes with varied size by regulating the amount of NEt3, reaction time,and temperature.In order to maintain the original crystal structures,the molar ratio of metal to ligands was kept constant as 1∶1∶1,which was used in the syntheses of single crystals of complexes 1~3[26].
2.1Influence factors of crystal size
2.1.1Effect of the amount of NEt3on crystal size
Oba is a carboxylic acid ligand and its complexation with metal ions requires a base condition.In this study,the pH value was adjusted by the addition of NEt3because it could facilitate the deprotonation of oba and favor the formation of complexes with varied sizes.
To gain information on the precise influence of NEt3upon the crystal size of complex 1,we carried out sets of experiments where the additive amount of NEt3was selected as 50,100,150 and 200 μL in the syntheses.SEM images(Fig.1)show that addition of 50 μL NEt3affords square-like crystals with an average size of 120 μm(Fig.1a),while increasing the amount of NEt3to 100 μL leads to smaller cubic crystals close to 60 μm in size(Fig.1b).The crystals with average size of 30 μm(Fig.1c)are obtained in the presence of 150 μL NEt3.However,the crystal size increased suddenly to 200 μm when the volume of NEt3is added to 200 μL(Fig.1d).

(a)50 μL,(b)100 μL,(c)150 μL,(d)200 μLFig.1 SEM images of 1 synthesized with different amount of NEt3
The above observations clearly indicate an important effect of NEt3on the size of complex.At lower pH value,the concentration of deprotonated oba is very low,making the formation rate of MOFs rather low,so bulk crystals are formed easily.At higher pH value,more deprotonated oba ions are available for coordination with metal ions,resulting in increasingly accelerating nucleation rates and therefore decreasing crystal size.However,the extremely fast formation of nucleation will lead to the aggregation of small nuclei and thus results in the crystals with bigger sizes, which is consistent with the results of Fig.1d. Therefore,the optimal amount of NEt3was determined as 150 μL in the present system.
2.1.2Effect of reaction time on crystal size
It′s known that the originally-formed small particles may aggregate to form large uniform crystals with increasing reaction time.Thus,the effect of reaction time on crystal size and crystallization process of 1 was further investigated.The reaction time was set to 6,24,48 and 72 h,respectively,by keeping other factors constant.
The SEM image taken at an early reaction time (6 h)reveal that initial products are small particles with size less than 1 μm(Fig.2a.)With the reaction time increasing,the particles begin to aggregate into rod-like crystals with the width of ca.2 μm(Fig.2b). Over time,the number of aggregates with a rod shape increases(Fig.2c)and ultimately forms uniform cubic particles with smooth surface and the mean size is about 30 μm(Fig.2d).There are no further changes in morphology when the reaction time is more than 72h. These observations reveal a unique particle-to-cube transformation and indicate that the reaction time plays a significant role in the synthesis of Ni(Ⅱ)complexes with different morphologies and sizes.

Fig.2 SEM images of 1 synthesized with different reaction times
2.1.3Effect of temperature on crystal size
The temperature in this study was set to 120, 160 and 180℃where other parameters were invariant.The SEM observation suggests that the products are small spindle-shaped particles with the length of 20 μm at 120℃,but not crystallized completely(Fig.3a).As increasing the temperature to 160℃,the small spindle-shaped particles almostconvert into square-like crystals with the average size of 60 μm(Fig.3b).Further increasing the reaction temperature to 180℃,polyhedral crystalline grains appear with bigger sizes(Fig.3c).

Fig.3 SEM images of 1 synthesized with different temperatures
The above results suggest that temperature,as expected,is an important factor in regulating the size and morphology of 1.Although smaller particles yielded at lower temperature(120℃),the optimal temperature was determined as 160℃mainly due to the mono-dispersed uniform size of crystals synthesized at 160℃.
2.2Synthesis of the complexes 1~3 in varying size
On the basis of the above results,the optimal reaction time and temperature for 1 were determined as 72 h and 160℃,respectively.Uniform square-like crystals of 1 with different sizes of 30,60,120 and 200 μm were prepared by regulating the amount of base as listed in Table 1.
XRPD was performed to investigate whether the complexes structure changed with their sizes decreased.The XRPD patterns of the samples with different sizes are in good agreement with those simulated from single crystal of 1(Fig.4a),indicating that the crystal structures of complexes with varied sizes remain unchanged.
The size-controllable synthesis of complexes 2 and 3 were studied using the analogous processes to that of 1.The optimal reaction time and temperature were determined as 60 h and 180℃as well as 72 h and 160℃for complex 2 and 3,respectively(Table 1).
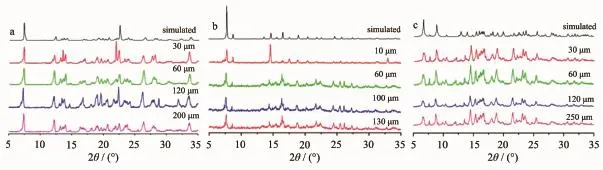
Fig.4 XRPD patterns of samples 1(a),2(b)and 3(c)with different sizes

Table1 Synthetic factors of 1~3
The XRPD patterns of 2(Fig.4b)and 3(Fig.4c.) demonstrate that all major peaks match quite well with those of the simulated for single crystals.The different diffraction intensities of complexes with varied sizes are due to the poly-crystal nature of the complexes samples. Moreover,the strong and sharp diffraction peaks for 2 and 3 indicate a good crystallinity.The average size of 2 calculated from the SEM images is ca.10,60,100 and 130 μm(Fig.5).Similarly,the average size of 3 was ca. 30,60,120 and 250 μm(Fig.6).

Fig.5 SEM images of complex 2 with different sizes

Fig.6 SEM images of complex 3 with different sizes
Although three kinds of complexes with sizes range from 10 to 250μm were successfully synthesized by hydrothermal method,nanoscale complexes were not fabricated successfully by using other methods,such as microemulsions and surfactants.
Adding capping reagents has been proved as an effective method to fabricate nanoparticles by Kitagawa[21].The mechanism of this method is to modulate the coordination equilibrium simply by adding capping reagents with the same chemical functionality as the linkers to impede the coordination interaction between metal ions and the organic linkers,which can generate a competitive situation that regulates the rate of framework extension and crystal growth.In this study,this strategy was also adopted and benzoic acid and sodium acetate were selected as capping reagents for controlling the crystal size,while the formation of nano-sized crystals was also unsuccessful.
2.3Photocatalytic properties of complexes 1~3
The diffuse reflective spectra of free ligands(oba and 4,4′-bpy)and complexes 1~3 were explored in solid state at room temperature and were shown in Fig.7.The peaks of 241,261 and 285 nm in the spectra of complexes 1~3 are attributed to the typical π-π*transitions of free ligands(238 nm for 4,4′-bpy and 294 nm for oba)and the peaks of 645 and 574 nm are assigned to the typical d-d transitions of Ni2+and Co2+,respectively.The newly appeared absorptions at 394,369 and 348 nm should be assigned to the ligand-to-metal charge transfer (LMCT).The values of the band gap were calculated as 2.63,2.68 and 2.94 eV based on the corresponding LMCT transitions for 1~3,respectively,indicating that three complexes might be potential photocatalysts[27].

Fig.7 UV-Vis spectra of complexes 1~3(a)and free ligands(b)in solid state
The photocatalytic activities of complexes 1~3 with different sizes were evaluated by degradation of organic dyes under UV irradiation.RhB,MO and MB were selected as the model dye contaminants due to their difficulties to be decomposed in waste water.The photocatalytic performances were monitored by the absorbance characteristic of the targets(RhB:555 nm, MO:463 nm and MB:668 nm),which were closely related to the structural changes of their chromophores.Furthermore,the photocatalytic performance of commercial TiO2(100 nm)was also assessed under the identical experimental conditions. Controlling experiments without catalyst in the presence of UV irradiation and with catalyst in the absence of UV irradiation were also carried out.The degradation rates of three dyes over complexes 1~3 in the absence of UV irradiation were 1.80%,2.45%and 1.59%,respectively,demonstrating a negligible adsorption effect of dyes on the complexes surfaces. The degradation rates of three dyes themselves in the presence of UV irradiation were 25.17%,16.59%and 14.13%,respectively.

Fig.8(a~c)Profiles of degradation of three dyes by TiO2as well as complex 1 with different sizes(②~⑤); (d)Color changes in degradations of three dyes by complex 1 with size of 30 μm
The complexes 1 with different sizes(②30 μm,③60 μm,④120 μm and⑤200 μm)were used ascatalysts to degrade three dyes.As illustrated in Fig. 8,the degradation rates of RhB,MO and MB were gradually improved with increasing the irradiation time in the presence of 1.
For the degradation of RhB for 60 min,the order of degradation rate was②(96.54%)>③(85.05%)>④(81.81%)>⑤(76.06%).The size of complex followed the order:②<③<④<⑤.The degradation rate of RhB followed the reverse order to that of the size.In another word,improved photocatalytic performance is realized by decrease of size of complex.
In detail,for complex 1 with size of 30 μm,the degradation rate of RhB attained 64.47%after irradiation for 10 min(TiO253.2%),and the degradation rate increased dramatically to 96.15% after 30 min irradiation(TiO290.62%)(Fig.8a).This result demonstrates apparently that the photocatalytic performance of complex 1 with size of 30 μm is superior to that by the nano-sized TiO2.
For MO and MB,the relationships between degradation rates and sizes of complex 1 were similar to that of RhB.Upon irradiation with UV light for 60 min, the degradation rates of MO and MB by complex 1 with size of 30 μm were 90.01%and 95.12%,respectively, which were comparable to those by the nano-sized TiO2(89.86%and 96.09%)(Fig.8b and 8c).
Fig.9shows the degradation rates of aqueous RhB,MO and MB in the presence of 2 with different sizes under UV light irradiation(②10 μm,③60 μm,④100 μm and⑤130 μm).After irradiation for 60 min,the degradation rates of RhB exhibited the following order:①TiO2(94.73%)>②(94.28%)>③(90.05%)>④(84.10%)>⑤(79.87%).The order of degradation rates of MO was②(94.68%)>①(89.86%)>③(86.04%)>④(64.37%)>⑤(59.34%)and the order of degradation rates of MB was①(96.09%)>②(87.18%)>③(80.12%)>④(76.03%)>⑤(73.68%).The above results indicate that the photocatalytic performance of 2 closely relates to the sizes of complexes.

Fig.9 Profiles of degradation of three dyes Rh B(a),MO(b)and MB(c)by nano-sized TiO2as well as complex 2 with different sizes(②~⑤)

Fig.10 Degradation profiles of three dyes RhB(a),MO(b)and MB(c)with nano-sized TiO2as well as complex 3 with different sizes(②~⑤)
Fig.10presents the degradation rate of three dyes over complex 3 as a function of irradiation time.As anticipated,the degradation rate increased with decrease of the size of complex.Upon irradiation for60 min,the degradation rate of MB by complex 3 with size of 30 μm was more than 95%.However,the degradation rates of RhB(87.16%)and MO(74.09%) by complex 3 with size of 30 μm were comparable lower than those by nano-sized TiO2.
As pointed out previously in the introduction part,the size effect of material is resulted from the increased surface areas.In degradation process, greater surface areas favor not only the adsorption of dyes on the catalyst surface but also the further interactions between catalyst and available dyes.To clarify whether the improved catalytic activities of the complexes in this study are originated from the increased surface area,nitrogen adsorption properties of complex 1 with different sizes were performed.The BET surface areas are ca.8,7,6 and 4 m2·g-1for the samples with sizes of 30,60,120 and 200 μm, respectively.Clearly,the smaller sized samples possess bigger surface area,which is agreement with theory.Thus,it′s concluded that the enhanced degradation abilities of complexes are ascribed to the increased surface areas by decreasing sizes of the complexes.
2.4Repeatable photocatalytic performances and stabilities of complexes 1~3
Whether the photocatalysts could retain their original structures and possess good photocatalytic properties after a cycle of photodegradation of dyes was crucial to their practical applications as reusable photocatalysts.Thus,XRPD was used to characterize the stabilities of three complexes after repeating the photocatalytic degradation once.
Fig.11shows that the XRPD patterns of the samples at the end of repeated bleaching experiments are almost the same as those of the as-prepared complexes except the difference in the diffraction width and some new weak peaks.The SEM images in Fig.12shows the grain sizes of complexes 1~3 after bleaching experiments decrease slightly and the surface morphologies become imperfect due to the vigorous stirring in degradation.Thus,the increment of crystal defects and the decrease of grain size appear for the reason of new weak peaks and diffraction-line broadening of XRPD.

Fig.11 XRPD patterns of samples before and after photodegradation of three dyes

Fig.12 SEM images of the samples after photodegradation of RhB
Table2presented the degradation rates of three dyes by three complexes in second cycle ofphotodegradation.The photocatalytic efficiencies of three complexes only decreased slightly in varying deg rees and thus could be repeatedly utilized.
因此,企业社会责任无论从理论研究层面还是从实践层面,都已然成为规范和指导企业伦理行为的“新范式”,在未来的企业发展方向上,不可能忽视和超越这个“新范式”。
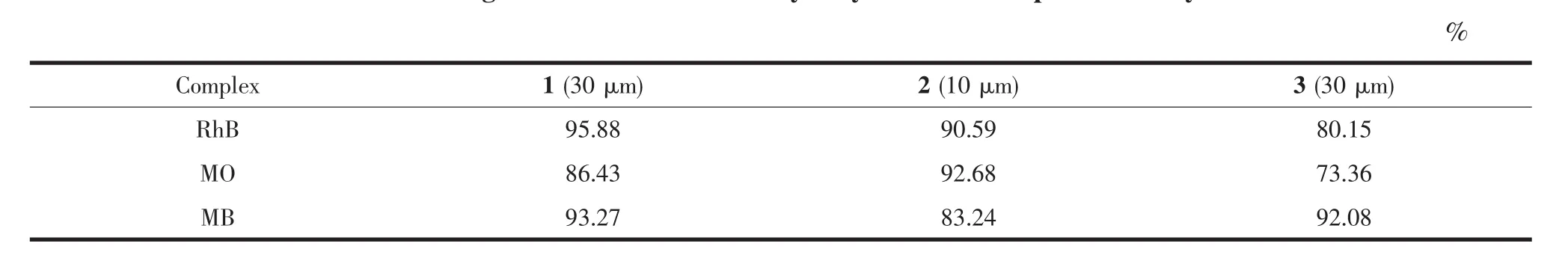
Table2 Degradation rates of three dyes by different samples in 2nd cycle
3 Conclusions
Three coordination polymers with sizes ranging from 10 to 250 μm were prepared via a hydrothermal method by regulating the amount of NEt3,reaction time and temperature.The relationship between synthetic factors and size of complex was investigated in detail by delicate experiments and SEM images. The results indicate that the photocatalytic activities of three complexes can be improved by decreasing sizes of complexes.Remarkably,Ni(Ⅱ)complex with size of 30μm performs excellent photocatalytic performance better than that of commercial nano-sized TiO2.Moreover,three complexes are stable after repeating the photocatalytic degradation of dyes and can be used repeatedly as promising photocatalysts in practice.
Acknowledgments:We would like to acknowledge financial support from the Project Sponsored by Natural Science Foundation of China(Grant No.21101125),The Project Sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars(Han Jing and Yu Zhong), Shaanxi Natural Science Foundation(Grant No.2011JM6007), and Scientific Research Program of Xi′an University of Technology(Grant No.2015CX001).
References:
[1]Thompson T L,Yates J T.Chem.Rev.,2006,106(10):4428-4453
[2]Mills A,Hunte S L.Chem.Rev.,1997,108:1-35
[3]Ayoub K,van Hullebusch E D,Cassir M,et al.J.Hazard. Mater.,2010,178(1/2/3):10-28
[4]Akpan U G,Hameed B H.J.Hazard.Mater.,2009,170(2/3): 520-529
[5]Du J J,Yuan Y P,Sun J X,et al.J.Hazard.Mater.,2011, 190(1/2/3):945-951
[7]Ferey G.Nat.Mater.,2003,2(3):136-137
[8]Zeng M H,Wang Q X,Tan Y X,et al.J.Am.Chem.Soc., 2010,132(8):2561-2563
[9]Liu W T,Ou Y C,Xie Y L,et al.Eur.J.Inorg.Chem., 2009,28:4213-4218
[10]ZHAO Nan(赵楠),DENG Hong-Ping(邓洪萍),SHU Mou-Hai(舒谋海),et al.Chinese J.Inorg.Chem.(无机化学学报),2010,26(7):1213-1217
[11]Silva C G,Corma A,Garcia H.J.Mater.Chem.,2010,20 (16):3141-3156
[12]Lin H,Maggard P A.Inorg.Chem.,2008,47(18):8044-8052
[13]Liao Z L,Li G D,Bi M H,et al.Inorg.Chem.,2008,47(11): 4844-4853
[14]Yu Z T,Liao Z L,Jiang Y S,et al.Chem.Commun.,2004, (16):1814-1815
[15]Yu Z T,Liao Z L,Jiang Y S,et al.Chem.Eur.J.,2005,11 (9):2642-2650
[16]Peng X,Manna L,Yang W,et al.Nature,2000,404(6773): 59-61
[17]Horn D,Rieger J.Angew.Chem,Int.Ed.,2001,40(23): 4330-4361
[18]Chen J,Herricks T,Xia Y.Angew.Chem,Int.Ed.,2005,44 (17):2589-2592
[19]Taylor K M L,Jin A,Lin W.Angew.Chem.Int.Ed.,2008, 120(40):7836-7839
[20]Guo L,Liu C,Wang R,et al.J.Am.Chem.Soc.,2004,126 (14):4530-4531
[21]Tsuruoka T,Furukawa S,Takashima Y,et al.Angew.Chem. Int.Ed.,2009,48(26):4739-4743
[22]FAN Ying-Hua(范英华),LUO Qin(雒琴),LIU Gui-Xia (刘桂霞),et al.Chinese J.Inorg.Chem.(无机化学学报), 2014,30(3):627-632
[23]Guo H,Zhu Y,Wang S,et al.Chem.Mater.,2012,24(3): 444-450
[24]Sun H L,Shi H,Zhao F,et al.Chem.Commun.,2005,(34):4339-4341
[25]WANG Jun(王俊),CAO Wei-Man(曹伟曼),YANG Hong (杨红),et al.J.Shanghai Normal Univ.(上海师范大学学报),2010,39(2):166-174
[26]Han J,Yu Z,He X,Li P,et al.Inorg.Chim.Acta,2012, 388(1):98-101
[27]Ji W J,Hu M C,Li S N,et al.CrystEngComm,2014,16(17): 3474-3477
Three Coordination Polymer Microcrystals:Size-Controlled Syntheses and Photocatalytic Properties
LIU Xiang1HAN Jing*,1YU Zhong2WANG Xiao-Xiang1
(1Department of Materials Physics and Chemistry,Xi′an University of Technology,Xi′an 710048,China)
(2Department of Chemistry,Xi′an University of Technology,Xi′an 710048,China)
Microcrystals of Ni(Ⅱ),Co(Ⅱ)and Zn(Ⅱ)coordination polymers based on 4,4′-oxybisbenzoic acid(oba)and 4,4′-bipyridine(4,4′-bpy)with sizes ranging from 10 to 250 μm were prepared hydrothermally by regulating the amount of NEt3,reaction time and reaction temperature.The crystal sizes,morphologies and structures were characterized and analyzed by scanning electron microscopy(SEM)and X-ray powder diffraction(XRPD). Photocatalytic measurements demonstrate that three polymers have excellent degradation capacities of organic dyes such as rhodamine B(RhB),methyl orange(MO)and methylene blue(MB)and the degradation rate increases with the decrease of catalyst sizes.Remarkably,the photocatalytic performance of Ni(Ⅱ)complex(30 μm)for RhB exceeds that of the nano-sized TiO2under same experimental conditions.In addition,XRPD studies show three coordination polymers are structurally stable after one cycle of photodegradation and thus can be utilized repeatedly as photocatalysts.
hydrothemal synthesis;coordination polymers;size-controlled;photocatalysis
O614.81+3;O614.81+2;O614.24+1
A
1001-4861(2016)11-1931-11
10.11862/CJIC.2016.253
2016-05-04。收修改稿日期:2016-09-27。
国家自然科学基金(No.21101125)、教育部留学回国人员启动基金、陕西省自然科学基金(No.2011JM6007)和西安理工大学科技创新计划(No.2015CX001)资助项目。
*通信联系人。E-mail:hanj@xaut.edu.cn
