Surface Chemistry of Ga(CH3)3on Pd(111)and Effect of Pre-covered H and O
2016-11-24LiangbingDingYunshengMaJieHuBohaoChenDepartmentofChemicalPhysicsUniversityofScienceandTechnologyofChinaHefei230026China
Liang-bing Ding,Yun-sheng Ma,Jie Hu,Bo-hao ChenDepartment of Chemical Physics,University of Science and Technology of China,Hefei 230026,China.
Surface Chemistry of Ga(CH3)3on Pd(111)and Effect of Pre-covered H and O
Liang-bing Ding,Yun-sheng Ma∗,Jie Hu,Bo-hao Chen
Department of Chemical Physics,University of Science and Technology of China,Hefei 230026,China.
The adsorption and decomposition of trimethylgallium(Ga(CH3)3,TMG)on Pd(111)and the effect of pre-covered H and O were studied by temperature programmed desorption spectroscopy and X-ray photoelectron spectroscopy.TMG adsorbs dissociatively at 140 K and the surface is covered by a mixture of Ga(CH3)x(x=1,2 or 3)and CHx(a)(x=1,2 or 3) species.During the heating process,the decomposition of Ga(CH3)3on clean Pd(111)follows a progressive Ga−C bond cleavage process with CH4and H2as the desorption products.The desorption of Ga-containing molecules(probably GaCH3)is also identified in the temperature range of 275−325 K.At higher annealing temperature,carbon deposits and metallic Ga are left on the surface and start to diffuse into the bulk of the substrate.The presence of precovered H(a)and O(a)has a significant effect on the adsorption and decomposition behavior of TMG.When the surface is pre-covered by saturated H2,CH4,and H2desorptions are mainly observed at~315 K,which is ascribed to the dissociation of GaCH3intermediate. In the case of O-precovered surface,the dissociation mostly occurs at~258 K,of which a Pd-O-Ga(CH3)2structure is assumed to be the precusor.The presented results may provide some insights into the mechanism of surface reaction during the film deposition by using trimethylgallium as precursor.
Ga(CH3)3,Pd(111),Adsorption,Decomposition,X-ray photoelectron spectroscopy,Temperature programmed desorption spectroscopy
I.INTRODUCTION
Recent progresses have been made progressively in the synthesis of solid thin film with atomic control in thickness and conformal structure by using atomic layer deposition techniques[1,2].Generally,the overall reaction involved in atomic layer deposition process can be divided into two self-limiting surface reactions occurring in a sequential fasion[1,2].In a recent perspective,it has been pointed out that several problems often have been overcome to obtain a clean,high-quality film by atomic layer deposition method,including the suppression of impurity,the control of the stoichiometry,the role of specific surface sites etc.[2].Therefore,the surface chemistry involved in atomic layer deposition process needs to be better understood at a molecular level.
Ga-based materials such as Ga2O3,GaAs,and GaN have been utilized in a variety of applications such as light emitting diodes,gas sensors,electronics,and transparent conducting oxides[1,3,4]. Trimethylgallium(TMG)has been widely used as an important organometallic precursor in film deposition techniques such as molecular beam epitaxy,metal-organic chemical vapor deposition and atomic layer epitaxy. For example,a recent investigation reported that highquality Ga2O3thin films have been prepared successfully utilizing TMG and ozone[4]. It is necessary to get a better understanding in the surface chemistry of Ga-containing precursors such as TMG on metal/semiconductor substrate.
Generally,the reaction and dissociation of TMG shows a complicated pattern on various substrates [4−14].Previous studies indicate that the adsorption and reaction of TMG is dependent on the orientation of Si substrates[13,14].On Si(111),TMG adsorbs mainly molecularly at room temperature as well as a small part of TMG dissociation resulting in-CH3groups bonded the Si substrate[14].Several desorption products were observed in the TDS results:the desorption of TMG occured at 423−473 K,C2H4,CH4,and CH3desorbed at 423−623 K,GaCH3desorption at 473−723 K and H2desorption at 773 K[14].In contrast,only CH4and H2were detected as decomposition products on Si(100), without the formation of C2H4and Ga(CH3)although a similar adsorption state was observed[13].It was proposed that the decomposition of TMG mostly follows an intramolecular process on Si(100).Upon heating,one of the methyl groups in TMG reacts with another methyl groups and produces methane and Ga-CH2fragment.The remaining methyl groups react with CH2fragment and release methane upon further annealing at higher temperature.At even higher temperature,CH species decompose to produce carbon and H2[13].Only few investigations have dealt with the adsorption and decomposition of TMG on metal substrate[9,11].Zhou, Liu,and coworkers have studied the interaction of TMG with Ni(111)and Pt(111)previously by temperature programmed desorption(TPD),static secondary ion mass spectrometry,high-resolution electron energy loss spectroscopy[9,11].It has been found that the adsorption and reaction on more active metal surface is largely different from that on semiconductor substrates. On Ni(111),TMG adsorbs dissociatively at a low temperature of 100 K and low exposure,leaving Ga,CH3, and Ga(CH3)2(DMG)on the surface[9].At higher exposure,TMG molecular adsorption is observed.Upon heating,TMG and DMG undergo stepwise dissociation of Ga−C bonds and the formed CH3fragment may undergo dehydrogenation below 150 K or hydrogenation to CH4at higher temperature(250 K).The final products after the complete decomposition at higher temperature are Ga and C[9].On Pt(111),TMG also adsorbs dissociatively even at a low temperature of 120 K via the cleavage of Ga−C bond,producing CH3(a)and Ga(CH3)2adsorbed on Pt substrate[11].After heating to higher temperature,the decomposition of TMG also proceeds through stepwise dissociation of Ga−C bonds while the formed methyl species undergo dehydrogenation or hydrogenation with CH4and H2as the only desorption products.
∗Author to whom correspondence should be addressed.E-mail: ysma@ustc.edu.cn
In the present study,the interaction of TMG with Pd(111)has been studied using TPD and X-ray photoelectron spectroscopy(XPS).It is found that TMG adsorb dissociatively on Pd(111)even at 140 K.Upon heating,TMG undergoes a stepwise dissociation with CH4and H2as the desorption products.The molecular desorption of Ga-containing species is also identified. Furthermore,the effect of precovered H and oxygen on the adsorption and decomposition behavior has also been investigated.The results are discussed in comparison with those obtained on clean Pd(111).
II.EXPERIMENTS
All experiments were performed in a home made stainless-steel ultrahigh vacuum(UHV)chamber with a base pressure of 2×10−10mbar.The UHV chamber was equipped with facilities for XPS,low energy electron diffraction(LEED),and differential-pumped TPD measurements. A Pd(111)single crystal(purchased from MaTeck)was mounted on the sample holder by two Ta wires spot-welded to the back side of the sample.The sample was cooled by liquid nitrogen and heated resistively.Temperatures could be controlled between 135 and 1200 K and were measured by a chromelalumel thermocouple spot-welded to the backside of the sample. Prior to the experiment,Pd(111)was cleaned by repeated cycles of Ar ion sputtering,oxidation(6.65×10−8mbar O2,T=900 K),and annealed until LEED gave a sharp diffraction pattern and no contaminants could be detected by XPS.
TMG(>99.999%,Jiangsu Nata Opto-Electronic Material Co.Ltd.)was introduced with N2as carrier gas by a line of sight stainless steel doser(diameter of 8 mm),which is positioned~1 cm in front of the sample. The hydrogen(>99.9%,Nanjing ShangYuan Industry Factory)and oxygen(>99.9%,Nanjing ShangYuan Industry Factory)were dosed by backfilling.The purity of all gases and reactants was checked by quadrupole mass spectrometer(QMS,Pfeiffer Vacuum QME 220) prior to experiments.All exposures were reported in Langmuir(1 L=1.33×10−8mbar·s)without correction of ion gauge sensitivity.Specially,the exposure of TMG was reported here without considering the enhancement factor of doser.
During the TPD experiments,the sample was positioned~1 mm away from the collecting tube of a differentially-pumped QMS and heated to 600 K with a heating rate of 3.0 K/s.XPS spectra were recorded by a hemispherical energy analyzer(PHBIOS 100 MCD, SPECS GmbH)with a pass energy of 20 eV using Al Kα radiation(hν=1486.6 eV).By using the XPS Peak 4.1 software,the C 1s XPS spectra was curve-fitted by a mixture of Gaussian and Lorentzian function after subtracting of a Shirley background.All binding energies are referenced to the Fermi level with the Pd 3d5/2peak of clean Pd substrate at 335.0 eV.For the annealing experiments,the substrate was heated to desired temperature and was held for 30 s.
III.RESULTS AND DISCUSSION
A.Adsorption and decomposition of Ga(CH3)3on clean Pd(111)
Figure1displaystheCH4(m/z=16)andH2(m/z=2)TPD spectraafterexposureofvarious amounts of Ga(CH3)3to clean Pd(111)at 140 K.In TPD spectra,CH4(m/z=16)and H2(m/z=2)are the only detected desorption products for all the studied exposures.The 15 amu signal is always detected with very similar peak shape,intensity and position with that of 16 amu,suggesting the desorption signal is due to CH4,not CH+3radical.The molecular desorption of Ga(CH3)x(x=1,2,or 3)as well as other hydrocarbon molecules(such as C2H4,C2H6etc.)was not observed under the present experimental condition.At exposure of 0.05 L,two CH4desorption peaks were observed:a broad one at 196 K with a low-temperature tail at 160 K and a narrow peak at 290 K,respectively.Since the desorption temperature for CH4adsorption on Pd(111)alone is below 100 K[15],the observed CH4desorption features should be reaction-rate limited.The following XPS results suggest that these three peaks should come from the decomposition/reaction of TMG and its dissociation intermediates such as dimethylgallium(Ga(CH3)2,DMG)and monomethylgallium(GaCH3(a),MMG)and will be discussed later.Simutaneously,four H2desorption peaks (2 amu)are detected at 160,200,294,and 366 K in the TPD spectra,respectively.The desorption temperatures of the former three features are similar with those of CH4desorption signals in the obtained TPD spectra,implying the same origin for both desorption products.The additional broad H2desorption signal around 366 K is possibly due to the associative desorption of H(a)on Pd(111)[16].With increasing TMG exposure, the desorption temperature of CH4peak at 160 K is kept almost constant while the CH4desorption peaks at 196 and 290 K shift to higher temperature gradually.Upon exposure of 0.2 L,the desorption of three CH4peaks appears at 160,256,and 320 K,respectively. In the case of 2 amu,except the signals accompanying with the 16 amu signal,an additional H2desorption was also observed at 407 K at TMG exposure of 0.2 L. This H2desorption feature exhibits a higher desorption temperature than the recombinative desorption,which is assigned to the dehydrogenation of CHxspecies[17, 18].
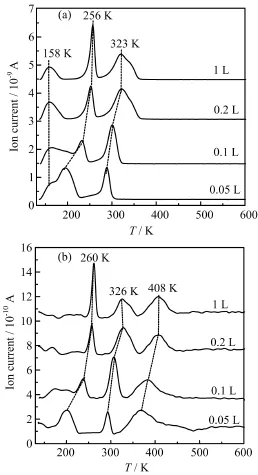
FIG.1(a)CH4(m/z=16)and(b)H2(m/z=2)TPD spectra following the indicated exposures of TMG on Pd(111) at 140 K.

FIG.2(a)C 1s and(b)Ga 2p3/2XPS spectra obtained after various TMG exposures on Pd(111)at 140 K.
The adsorption and decomposition of TMG on Pd(111)wasmonitored by XPS measurements. Figure 2 displays C 1s and Ga 2p3/2XPS spectra after Pd(111)was exposed to various amounts of TMG at 140 K.At low TMG exposure of 0.05 L,the Ga 2p3/2spectrum exhibits a small band at 1116.5 eV.Increasing the TMG exposure causes a small positive shift of the binding energy of Ga 2p3/2by 0.1 eV as well as an enhancement of the intensity.On Si(100),the binding energy of Ga 2p3/2is 1117.2 eV for molecular TMG and 1116.9 eV for chemisorbed Ga metal[13].Although the binding energy of Ga 2p3/2is not a sensitive parameter to differentiate TMG and its dissociation products (Ga(CH3)x,x=1,2,or 3),the observed positive shift of the binding energy of Ga 2p3/2with increasing exposure as well as the following annealing experiments may imply that TMG adsorbs dissociatively at low exposure leaving some Ga(CH3)x(x=1 or 2)species on the surface.At the same time,the C 1s spectrum is fitted by two peaks located at 283.3 and 284.4 eV at exposure of 0.05 L.The former feature is ascribed to the adsorbed TMG and its dissociation intermediate such as DMG/MMG,all of which have been reported to exhibit a similar binding energy(283.5 eV)after TMG adsorption on Si(100)[13,19].The latter peak at 284.4 eV is usually a characteristic of CHx(a)species on Pd sub-strate[17,20,21].So it may also suggest that TMG partly adsorbs dissociatively at 140 K,leaving CHx(a) and Ga(CH3)2(or Ga(CH3))species on the surface. Considering the atomic sensitivity factor[22],the ratio of C to Ga is~0.43 for 0.2 L exposure at 140 K compared to the value of 3 for molecular TMG.It also suggests that TMG adsorbs dissociatively on Pd(111) releasing CH4into gas phase.This assumption is further supported by the observation that CH4desorption starts immediately at the beginning of TDS measurement.The dissociative adsorption of TMG was also observed on Ni(111)and Pt(111)[9,11].On both metal surface,TMG partly decomposes into DMG and CH3after adsorption at 100−120 K,in contrast to molecular adsorption on Si substrate[12−14].So,it indicates that metal surfaces are more reactive towards TMG dissocation compared to semiconductor substrates.With increasing TMG exposure,the C 1s peak at 283.3 eV is largely enhanced,suggesting an increase of Ga(CH3)x(x=1,2,or 3)coverage.

FIG.3(a)C 1s Ga and(b)2p3/2XPS spectra obtained after Pd(111)was exposed to 0.2 L TMG at 140 K and followed by subsequent annealing to the indicated temperatures.(c) The corresponding integrated peak areas of Ga 2p3/2and C 1s components as a function of annealing temperature.
Figure 3 shows the C 1s and Ga 2p3/2XPS spectra and the corresponding integrated intensity acquired after Pd(111)was exposed to 0.2 L TMG at 140 K followed by annealing at various temperatures.As shown above,exposure of 0.2 L TMG gives rise to two features in the C 1s region:one main component at 283.4 eV and another one at 284.4 eV.These features are ascribed to the surface species such as Ga(CH3)x(x=1−3) and CHx(a)formed during the dissociative adsorption of TMG.Annealing to 200 K causes a slight decrease in the intensity of the C 1s feature at 283.4 eV,in consistent with CH4desorption at 160 K.After annealing at 275 K,the feature at 283.4 eV decreases gradually in intensity while the component at 284.4 eV is kept almost constant.It clearly indicates that the former feature is responsible for the CH4desorption peak at~256 K.Upon annealing at 375 K,the C 1s component at 283.4 eV disappears completely,corresponding to the CH4and H2desorption observed at~320 K(Fig.1). Further heating to 475 K induces a single C 1s peak at 284.6 eV,which is reasonably ascribed to carbonaceous C(a)left by the dehydrogenation of CHx(a)[23]. It agrees well with the H2desorption peak at 405 K observed in TPD spectra(Fig.1).Moreover,the intensity of C 1s decreases gradually above 375 K and almost no C 1s signal is detected at 600 K.It was explained by C diffusion into the bulk of Pd substrate since only H2desorption was observed above 350 K in the TPD spectra[17,24,25].In the case of Ga 2p3/2, the binding energy is kept constant at 1116.6 eV up to 275 K.Above 325 K,it shows a negative shift by 0.1 eV.In previous literatures,a simialr negative shift has been reported in Ga 2p3/2spectra when annealing TMG-adsorbed Si(100),which was explained by decomposition of Ga(CH3)xto metal Ga[13].So it may indicate that the Ga−C bond was broken completely above 375 K leaving adsorbed Ga(a)on the surface. In addition,a distinct attenuation of Ga 2p3/2peak isobserved in the temperature of 275−325 K.Since the desorption temperature of metallic Ga was reported to be above 800 K[11,13],it clearly suggests that some Ga-containing molecules(probably Ga(CH3)x)desorb from the surface along with reaction-limited CH4desorption although the TPD results did not detect any Ga(CH3)xdesorption.The absence of Ga(CH3)xsignal is probably due to its facile decomposition in the ionizer of mass spectrometer and/or the desorption signal is beyond the detection limit of QMS.The desorption of Ga-containing molecules on Pd(111)is a little surprising since there is no evidence for such desorption except for multilayer TMG on other metal surfaces such as Ni(111)and Pt(111)[9,11].However,the molecular desorption of MMG was observed above 473 K on Si(111)[14].Above 325 K,the intensity of Ga 2p3/2remains almost constant up to 500 K.At higher temperature,Ga starts to diffuse into Pd substrate and causes the formation of Pd−Ga alloy[26].
On the basis of the above TDS and XPS results, the adsorption and decomposition of TMG on Pd(111) was proposed as the following:TMG adsorbs dissociatively upon exposure at 140 K and the surface is covered by a mixed Ga(CH3)xand CHx(a)layer.It was reported that CH3(a)is very active and easily hydrogenates/dehydrogenates into CH3(g)below 200 K[15, 27].Therefore,the observed three reaction-limited CH4desorption peaks accompanied by H2desorption peaks in the TDS spectra are ascribed to the hydrogenation of CH3(a)formed by the stepwise dissociation of TMG. That is,the rate of CH4desorption is determined by the cleavage of Ga−C bond in adsorbed Ga(CH3)xspecies and the hydrogenation/dehydrogenation of CH3(a)occurs promptly after it is formed.At 160 K,TMG dissociates into DMG and CH3(a)and the latter species may hydrogenate/dehydrogenate after its formation releasing CH4and H2into gas phase[27].Around 250 K, DMG further decomposes into MMG and the resultant CH3(a)again formed CH4(g)and H2leaving CHx(a) species on the surface.In the temperature range of 250−320 K,the decomposition of MMG is accompanied by the molecular desorption of MMG,as supported by the decrease of the intensity of Ga 2p3/2signal in XPS spectra(Fig.3(c)).It should be noted that the formation of CH4(g)via the intermolecular/intramolecular reaction of Ga(CH3)xcannot be excluded on the basis of the above results.At a temperature of 375 K,only Ga(a)and CHx(a)are left on the surface.The CHx(a) species dehydrogenate and produce carbon deposits and H2(g)around 400 K.After complete decomposition of TMG,carbon deposits and metallic Ga are left on the surface,both of which start to diffuse into the bulk of Pd substrate at higher temperature.

FIG.4 CH4(m/z=16)and H2(m/z=2)TPD spectra for (a)clean Pd(111),(b)Pd(111)was exposed to saturated H2at 140 K,and(c)Pd(111)was exposed to saturated H2at 200 K followed by 0.2 L exposure of TMG at 140 K.
B.The effect of pre-covered H
The effect of pre-covered H for TMG adsorption and decomposition was studied by using TPD and XPS measurement. Previous reports show that the state of adsorbed H species on Pd samples depends on the substrate temperature during H2exposure[28,29].Increasing the substrate temperature usually induces H2diffusion into the bulk of the Pd sample[28,29].In the present study,two types of pre-covered H species were prepared by changing the substrate temperature before TMG adsorption on Pd(111)and then the results are compared with those on clean Pd(111).Figure 4 shows the CH4and H2TPD spectra after exposure of 0.2 L TMG on H-precovered Pd(111).When saturated H2was predosed at 140 K,CH4mostly desorbs at 315 K and a shoulder peak was observed at 330 K.The maximum of the main desorption peak is slightly lower than that of TMG adsorption on clean Pd(111)(322 K).Furthermore,the CH4desorption signal at 160 K is largely attenuated and the peak at 250 K completely disappears.Similarly,a single H2desorption peak was observed at 315 K accompanied by a shoulder peak at 330 K.Furthermore,the H2desorption peak at 407 K, which results from CHxdehydrogenation,is almost negligible.When H2was pre-exposed at 200 K,both CH4and H2desorption exhibit a single sharp peak at 315 K. Additionally,a small H2signal was also observed at340 K,assigned to recombinative desorption of H(a).
XPS measurements was also used to examine the surface intermediate and composition during TMG decomposition on H-precovered Pd(111).Figure 5(a)and(b) show C 1s and Ga 2p3/2spectra obtained during the annealing process of Pd(111)which was firstly pre-dosed to H2at 140 K and then exposed to 0.2 L TMG at the same temperature.Correspondingly,the changes of the intensity are displayed as a function of annealing temperature in Fig.5(c).When 0.2 L TMG was exposed to H-precovered Pd(111),the C 1s XPS spectra exhibit a broad band with the maximum at 283.4 eV together with a high-energy tail.Fitting the C 1s spectra gives rise to two components with the binding energy at 283.4 and 284.4 eV.Again,the former feature corresponds to Ga(CH3)x(a)while the latter results from CHx(a)produced by the decomposition of TMG.In the Ga 2p3/2region,a single peak was observed at 1116.6 eV,corresponding to adsorbed Ga(CH3)xspecies.Annealing at the temperature up to 250 K induces no significant changes of both C 1s and Ga 2p3/2features,in agreement with the observation that almost no desorption was detected in the TPD measurement(Fig.4).In the annealing temperature range of 275−325 K,the C 1s peak at 283.4 eV largely decreases in intensity.At the same time,the Ga 2p3/2peak experiences a negative shift to 1116.5 eV as well as a decrease in intensity. The above observations indicate that the Ga(CH3)x(a) species undergoes two competitive reaction channels between 275−325 K on H-precovered Pd(111):the desorption of Ga(CH3)xspecies versus the decomposition of Ga(CH3)x(a)with CH4and H2as the dissociation products.Above 375 K,only carbon deposits(C 1s peak at 284.6 eV)and metallic Ga(a)(Ga 2p3/2peak at 1116.5 eV)are left on the surface,both of which start to diffuse into the bulk of Pd substrate at higher temperature.
As discussed above,the rate-limiting step of CH4desorption is the formation of CH3(a)produced by the dissociation of Ga(CH3)xspecies.The presence of precovered-H clearly suppresses the CH4desorption peaks at~160 and 250 K,which are assigned to the hydrogenation of CH3(a)formed by the dissociation of TMG and DMG,respectively.And the situation becomes more significantly when the surface is precovered by subsurface H(a)(Fig.4).So it may suggest that the TMG mostly dissociates into MMG upon adsorption at 140 K in the presence of H(a)since H(a)can react easily with CH3(a)and promote the cleavage of Ga−C bond. As a result,the reaction channel of DMG is largely suppressed and the desorption and dissociation of MMG becomes dominant in subsequent annealing process.

FIG.5(a)C 1s and(b)Ga 2p3/2XPS spectra obtained after Pd(111)was first saturated by H2at 140 K,and then exposure of 0.2 L TMG at 140 K and subsequent annealing to the indicated temperatures.(c)The corresponding integrated peak areas of Ga 2p3/2and C 1s components as a function of annealing temperature.
C.TMG decomposition on O-precovered Pd(111)
TMG is not an appropriate Ga source for film deposition since unwanted residual carbon is left during TMGdeposition[13].On the other hand,one facile way to deposit Ga2O3film by atomic layer deposition is to provide various oxygen sources such as O2and O3during TMG interaction with the substrate,as demonstrated in Ref.[4].Therefore,in the present study,the effect of pre-covered oxygen on the adsorption and decomposition behavior of TMG was further examined.
Before TMG exposure,oxygen was exposed to Pd(111)at two different substrate temperatures of 140 and 300 K.It has been found that oxygen adsorbs dissociatively on Pd(111)above 200 K[30].Therefore, O2exposure at 140 K corresponds to molecular oxygen species while exposure at room temperature produces atomic oxygen.Figure 6 displays the CH4and H2TPD results obtained after Pd(111)were pre-covered by saturated O2(a)and O(a)followed by exposure of 0.2 L TMG at 140 K.In the presence of adsorbed O2(a),the CH4peak at 258 K gains in intensity and the others attenuate while the desorption temperatures of all the CH4desorption peaks remain almost unchanged with respect to the case of clean Pd(111).It suggests that the reaction channel at 250 K is preferred while the reaction channels for H2and CH4formation at 160 and 330 K are suppressed by preadsorbed O2.The situation becomes more significant for atomic O-precovered Pd(111).For both CH4and H2desorption,the desorption peak at 258 K is largely enhanced while that at 320 K disappears completely.Meanwhile,the H2desorption peak at 409 K due to CHxdehydrogenation remains almost constant.It should be mentioned that no O2desorption was observed in the studied temperature range(140−900 K).On clean Pd(111),it was reported that recombinative oxygen desorption occurs at 800 K[30].A small peak of H2O was detected at~500 K,which may be ascribed to OH disproportion. Such H2O desorption is consistent with an observation that Auger KLL peak of oxygen decreases in intensity in the temperature of 475−500 K(not shown).

FIG.6 CH4(m/z=16)andH2(m/z=2)TPD spectra for (a)clean Pd(111),(b)Pd(111)was exposed to saturated O2at 140 K and(c)Pd(111)was exposed to saturated O2at 300 K followed by 0.2 L exposure of TMG at 140 K.
The TMG decomposition behavior on O-precovered Pd(111)wasmonitored by XPS measurements. Figure 7 shows C 1s,Ga 2p3/2XPS spectra and the corresponding integrated intensity as a function of annealing temperature,which was recorded after Pd(111) was firstly exposed to saturated O2at 300 K and then to 0.2 L TMG at 140 K.The saturation coverage of O was reported to be 0.25 ML on Pd(111)at room temperature[30].The O 1s peak is not shown here since it is overlapped with Pd 3p3/2peaks and cannot provide the information of the chemical state of adsorbed O.Upon exposure of O-covered Pd(111)to TMG at 140 K,fitting the C 1s region gives rise to three C 1s features with binding energy at 282.6,283.3,and 284.4 eV,respectively.The C 1s peak at 282.6 eV is predominant after 0.2 L TMG adsorption on O-precovered Pd(111), very different from that observed on clean Pd(111)in which the main C 1s component is located at 283.4 eV(Fig.2(b)).As shown before,the feature at 283.3 and 284.4 eV can be attributed to Ga(CH3)x(a)and CHx(a)adsorbed on bare Pd substrate since O(a)coverage is only 0.25 ML and some Pd sites are still available for adsorption in the present study.The formation of oxygenated carbon species such as adsorbed methoxy(OCH3(a))and dioxymethylene(CH2O2(a)) can be ruled out as indicated by the absence of C 1s feature above 286 eV[20,31].At the same time,Ga 2p3/2shows a single peak at 1116.6 eV similar to the case on clean Pd(111),supporting Ga(CH3)xspecies, not metallic Ga,to be the predominant Ga species. When increasing the surface temperature up to 225 K, the C 1s feature at 282.6 eV attenuates largely while the intensities of those at 283.3 and 284.4 eV are kept almost constant.Simultaneously,the Ga 2p3/2peak shifts to a higher binding energy by 0.4 eV,suggesting Ga(a)is“oxidized”by preadsorbed oxygen.In comparison with the TDS results,the evolution of C 1s region clearly indicates that the surface species with a C 1s feature at 282.6 eV is responsible for the CH4and H2desorption at 258 K.After heating to 275 K,the C 1s feature at 282.6 eV disappears completely and two C 1s features are resolved to be located at 283.3 eV (Ga(CH3)x(a)species)and 284.4 eV(CHx(a)species),respectively.Upon heating to 425 K,the C 1s feature exhibits a broad peak at 284.6 eV,which was reasonably attributed to C(a)left by the dehydrogenation of CHxspecies.It agrees well with the H2desorption peak at 409 K.At higher temperature,C(a)starts to diffuse into the bulk and cause an attenuation of C 1s peak.In the case of Ga 2p3/2,the intensity remains almost constant up to 500 K,suggesting that the desorption of any Ga-containing species is negligible below that temperature.Moreover,the binding energy of Ga 2p3/2shows a negative shift in the temperature range of 425−500 K. Above that,Ga starts to diffuse into Pd substrate accompanied with the formation of Pd−Ga alloy,which cause a decrease in the intensity of Ga 2p3/2feature.
The origin of the C 1s feature at 282.6 eV is still not clear.Usually,carbide species shows a C 1s peak at 282.5 eV[22].In the case of TMG adsorption on Si(100),the C 1s feature at 282.5 eV was assigned to chemisorbed carbon[12,13].However,the assignment to carbide or chemisorbed carbon is not likely in the present study since TDS and XPS results indicate that this C 1s fature is related to simutaneous desorption of CH4and H2at 258 K.Furthermore,the dissociation temperature of this species is very close to that of Ga(CH3)2on bare Pd(111),as discussed above.Here, we tentatively assigned the C 1s peak at 282.6 eV to Pd-O-Ga(CH3)2species produced by dissociative adsorption of Ga(CH3)3on O-covered Pd(111).A similar structure was reported to be formed(Al-O-Al(CH3)2) in the reaction between trimethylaluminum and H2O during the controlled deposition of Al2O3film[32].
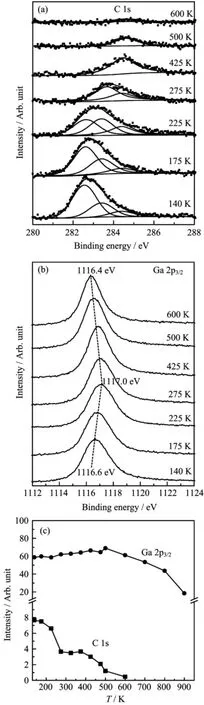
FIG.7(a)C 1s and(b)Ga 2p3/2XPS spectra obtained after Pd(111)was first saturated by O2at 300 K,and then exposure of 0.2 L TMG at 140 K and subsequent annealing to the indicated temperatures.(c)The corresponding integrated peak areas of Ga 2p3/2and C 1s components as a function of annealing temperature.
IV.CONCLUSION
TMG adsorbs dissociatively on clean Pd(111)at 140 K,leaving a mixture of Ga(CH3)xand CHx(a) species on the surface.Upon annealing,the dissociation of TMG may undergo a stepwise cleavage of Ga−C bond while CH3(a)hydrogenates/dehydrogenates immediately after its formation releasing CH4(g)and H2(g),which are observed at about 160,260,and 320 K, respectively. XPS results strongly suggest that the dissociation of MMG is accompanied by the molecular desorption of Ga(CH3)xspecies(probably GaCH3) at 320 K. Saturating the surface with H(a)significantly suppresses the dissociation channels at 160 and 260 K while simultaneous desorption of CH4and H2are mostly observed at 320 K.It is assigned to the dissociation products of MMG,which may be the predominate speices after TMG adsorption on H(a)-precovered Pd(111).Upon exposure of TMG on O(a)-precovered Pd(111),CH4and H2are the only desorption products in the subsequent heating. A Pd-O-Ga(CH3)2structure is proposed to be the decomposition intermediate. Furthermore,there is no evidence for the molecular desorption of Ga-containing species on O(a)-precovered Pd(111)in contrast to the case of clean andH(a)-precovered Pd(111).
V.ACKNOWLEDGMENTS
This work was supported by the National Basic Research Program of China(No.2013CB933102), the National Natural Science Foundation of China (No.21073172),and Fundamental Research Funds for the Central Universities.
[1]S.M.George,Chem.Rev.110,111(2009).
[2]F.Zaera,J.Phys.Chem.Lett.3,1301(2012).
[3]J.Lu,J.W.Elam,and P.C.Stair,Acc.Chem.Res. 46,1806(2013).
[4]D.J.Comstock and J.W.Elam,Chem.Mater.24, 4011(2012).
[5]K.Manandhar,M.Trenary,S.Otani,and P.Zapol,J. Vac.Sci.Technol.A 31,061405(2013).
[6]J.Nishizawa,J.Vac.Sci.Technol.B 14,136(1996).
[7]M.J.Bronikowski and R.J.Hamers,Surf.Sci.348, 311(1996).
[8]P.E.Gee,H.Qi,and R.F.Hicks,Surf.Sci.330,135 (1995).
[9]X.L.Zhou and J.M.White,Surf.Sci.273,322(1992).
[10]S.Shogen,Y.Matsumi,M.Kawasaki,I.Toyoshima, and H.Okabe,J.Appl.Phys.70,462(1991).
[11]Z.M.Liu,X.L.Zhou,and J.M.White,Appl.Surf. Sci.52,249(1991).
[12]F.Lee,T.R.Gow,and R.I.Masel,J.Electrochem. Soc.136,2640(1989).
[13]F.Lee,A.L.Backman,R.Lin,T.R.Gow,and R.I. Masel,Surf.Sci.216,173(1989).
[14]A.F¨orster and H.Luth,J.Vac.Sci.Technol.B 7,720 (1989).
[15]K.Watanabe and Y.Matsumoto,Surf.Sci.390,250 (1997).
[16]G.E.Gdowski and T.E.Felter,J.Vac.Sci.Technol. A 4,1409(1986).
[17]R.J.Levis,Z.Jiang,and N.Winograd,J.Am.Chem. Soc.111,4605(1989).
[18]D.Stacchiola,Y.Wang,and W.T.Tysoe,Surf.Sci. 524,173(2003).
[19]R.Lin and R.I.Masel,Surf.Sci.258,225(1991).
[20]R.J.Levis,Z.Jiang,and N.Winograd,J.Am.Chem. Soc.110,4431(1988).
[21]M.Rebholz and N.Kruse,J.Chem.Phys.95,7745 (1991).
[22]C.D.Wagner,W.M.Riggs,L.E.Davis,J.F. Moulder,and G.E.Muilenberg,Handbook of X-Ray Photoelectron Spectroscopy,Eden Proirie: Perkin-Elmer Coorpration,253(1979).
[23]H.Gabasch,E.Kleimenov,D.Teschner,S.Zafeiratos, M.Havecker, A.Knopgericke, R.Schlogl, D. Zemlyanov,B.Aszaloskiss,and K.Hayek,J.Catal. 242,340(2006).
[24]R.Levis,Z.Jiang,N.Winograd,S.Akhter,and J.M. White,Catal.Lett.1,385(1988).
[25]C.Morgan,N.Perkins,R.Holroyd,E.Fourre,F.Grillo, and A.MacDowall,J.Phys.Chem.B 109,2377(2004).
[26]C.Rameshan,W.Stadlmayr,S.Penner,H.Lorenz,L. Mayr,M.H¨avecker,R.Blume,T.Rocha,D.Teschner, A.Knop-Gericke,R.Schl¨ogl,D.Zemlyanov,N.Memmel,and B.Kl¨otzer,J.Catal.290,126(2012).
[27]J.J.Chen and N.Winograd,Surf.Sci.314,188(1994).
[28]G.E.Gdowski,T.E.Felter,and R.H.Stulen,Surf. Sci.181,L147(1987).
[29]H.Okuyama,W.Siga,N.Takagi,M.Nishijima,and T. Aruga,Surf.Sci.401,344(1998).
[30]X.Guo,A.Hoffman,and J.T.Yates,J.Chem.Phys. 90,5787(1989).
[31]A.C.Dillon,A.W.Ott,J.D.Way,and S.M.George, Surf.Sci.322,230(1995).
(Dated:Received on April 13,2016;Accepted on April 28,2016)
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Preparation of Bio-hydrogen and Bio-fuels from Lignocellulosic Biomass Pyrolysis-Oil
- Combination Computing of Support Vector Machine,Support Vector Regression and Molecular Docking for Potential Cytochrome P450 1A2 Inhibitors
- Working Condition Real-Time Monitoring Model of Lithium Ion Batteries Based on Distributed Parameter System and Single Particle Model
- Hydrodeoxygenation of Anisole over Ni/α-Al2O3Catalyst
- Highly Efficient and Selective Removal of Pb(II)ions by Sulfur-Containing Calcium Phosphate Nanoparticles
- Efficient Removal Phenol Red over Ternary Heterostructured Ag-Bi2MoO6/BiPO4Composite Photocatalyst
