CdS/FeP复合光催化材料界面结构与性质的理论研究
2016-11-22赵宗彦
赵宗彦 田 凡
(1昆明理工大学材料科学与工程学院,昆明650093;2云南大学材料科学与工程学院,云南省微纳材料与技术重点实验室,昆明650504)
CdS/FeP复合光催化材料界面结构与性质的理论研究
赵宗彦1,2,*田凡1
(1昆明理工大学材料科学与工程学院,昆明650093;2云南大学材料科学与工程学院,云南省微纳材料与技术重点实验室,昆明650504)
构建同质异相或异质结构是提高光催化材料性能的有效途径之一,尤其是对于CdS这类具有光腐蚀的材料,这种方法还能起到提高光催化材料稳定性的作用。因此目前制备CdS基复合光催化材料得到了广泛的研究,但是目前对其中的一些基本问题和关键因素仍需要进一步探讨和解释。本文采用第一性原理方法对CdS/FeP复合光催化材料中异质结构的界面微观结构和性质进行深入研究。计算结果表明,由于在界面上部分悬挂键被饱和,界面模型呈现出与体相或表面模型不同的电子结构特征,并且有界面态的存在。在CdS/ FeP异质结构的界面处,CdS和FeP的能带都相对向下移动,而且FeP的能带(费米能级)插入到CdS的导带下方;同时在界面达到平衡态之后,异质结构的内建电场由FeP层指向CdS层,因而能够实现光生电子-空穴对在CdS/FeP界面处的空间有效分离,这对于光催化性能的增强极其有利。此外,构建CdS/FeP异质结构也能够进一步增强CdS在可见光区的光吸收。本文研究结果为构建具有异质结构的高效复合光催化材料提供了机理解释和理论支持。
光催化;硫化镉;异质结构;界面微观结构;界面性质;密度泛函理论计算
1 Introduction
As strategic resource for social and economic development, energy always shows its importance and irreplaceable1.After longterm over-exploitation and excessive consumption,the fossil energy,including:coal,petroleum,and natural gas,will be exhausted in a few years2,3.What′s more,a series of environmental problems caused by fossil energy utilization could not be ignored, for example,greenhouse gas emissions,water resources pollution, and so on4.Severe energy crisis and environmental pollution makes the development of renewable energy(i.e.sustainable utilization,environment friendly,high efficiency,and cheap)is particularly necessary.In the renewable energy resources,solar energy is inexhaustible and clean.And thus,the technology of its efficient utilization is getting more and more attention from all over the world,becoming the important focus,and the corresponding progress increasingly blooming5.Photocatalysis is just one of the promising technologies of solar energy utilization:to produce hydrogen from water splitting,to produce hydrocarbon fuel from CO2reducing,to degrade the organic pollutants.So, scientists expect that it could help people to do away with dependence on fossil fuels in the future6.
In 1972,Japanese scientists,Fujishima and Honda7observed water splitting phenomenon on the TiO2photoelectrode under UV-light irradiation.Their work provided preliminary evidence for the hydrogen production from photocatalytic water splitting,and opened the prelude of research on artificial photosynthesis to convert solar energy into chemical energy.Unfortunately,the technological development of photocatalysis is still facing two major obstacles to date:narrow spectral response and low quantum efficiency,leading to a long way to achieve large-scale and low-cost solar energy industrial utilization.Cadmium sulfide (CdS)is a typical visible-light driven semiconductor photocatalyst with a band gap of~2.4 eV,which is well overlapping with the spectrum of sunlight,and could theoretically utilize more 40%of solar energy.Furthermore,its conduction band edge is more negative than the H+/H2redox potential,implying that it is thus able to evolve hydrogen from water splitting under sunlight irradiation8,9.However,CdS has a fatal drawback in practical applications,i.e.its stability is much worse10.This phenomenon is ascribed from its anodic decomposition(the so-called photocorrosion)when CdS is in aqueous solution by long-time light irradiation.In order to improve its stability and activity,to construct hetero-structure(combined with cocatalyst or other semiconductors)has been proposed,which has been proved as an effective modification method.For example:Pt/CdS composite photocatalyst has higher photocatalytic activity than Ru/CdS for hydrogen evolution reaction11;ETS-4 loading not only promotes the efficiency of hydrogen production from water splitting under visible-light irradiation,but also enhances the stability of CdS12. Yan et al.13reported that an artificial photocatalyst(Pt-PdS/CdS) can achieve a quantum efficiency up to 93%in photocatalytic H2production under visible-light irradiation,and is very stable under the photocatalytic reaction conditions.
Metal cocatalyst(especially noble metal)loading is often used to improve the photocatalytic activity of CdS.However,noble metal is scarce and has high production cost,which is against by the original intention to develop low-cost renewable energy resources.Recently,to replace noble metal cocatalyst with cheap materials for CdS photocatalyst has been attracted more and more attention9,14.On the other hand,the stability and catalytic properties of metal phosphide,such as,MoP,InP,Ni2P,CoP,and FeP, also attract extensive concern,and have been widely investigated, owing to their inexpensive and earth-abundant compositions and highly active hydrodesulfurization catalysis reactions15.Cao et al.16reported at the first time that Ni2P nanoparticles present high photocatalytic hydrogen-generating activity and excellent stability in lactic acid aqueous solution under visible light LED irradiation using CdS nanorods as a photosensitizer.Callejas et al.17synthesized uniform,hollow morphology FeP/TiO2composite,which exhibits the highest hydrogen-evolution reaction activities reported to date in both acidic and neutral-pH aqueous solutions,indicating that FeP is a highly earth-abundant material for efficiently facilitating the hydrogen-evolution reaction both electrocatalytically and photocatalytically.Zhang et al.18also considered FeP as a promising alternative to Pt-based catalysts for the hydrogenevolution reaction,in order to develop inexpensive and highly efficient non-precious-metal electrocatalysts.Motivated by above encouraging experimental observations,we adopt density functional theory(DFT)to further investigate the interfacial structure and properties,in order to deepen the understanding of CdS/FeP composite photocatalyst with hetero-structure.Using theoretical simulations,the atomic-scale interfacial microstructure and the electronic-scale interface properties will be provided.And then, based on the calculated results,the detailed mechanism of CdS/ FeP hetero-structure to improve the photocatalytic performance will be discussed.We hope these findings could provide someuseful reference for the development of efficient photocatalyst with hetero-structure in the future.
2 Computational methods and model
All of the DFT calculations in the present work are carried out by Cambridge Serial Total Energy Package(CASTEP)codes that are included into the software of Materials Studio19.CASTEP is a quantum mechanics-based program designed specifically for solid-state materials science.For solid-state materials,the interactions between nucleus and electrons are approximately treaded by the Born-Oppenheimer approximation,Hartee-Fock selfconsistent field theory,and periodic potential method.Thus,the interaction between ion cores(i.e.Cd:[Kr],S:[Ne],Fe:[Ar],P: [Ne])are treated by the ultrasoft pesudopotential(USP)20.For expanding the Kohn-Sham wave functions,the energy cutoff is chosen as 330 eV.The exchange-correlation effects of valence electrons(i.e.Cd:4d105s2,S:3s23p4,Fe:3d64s2,P:3s23p3)were described by the revised Perdew-Burke-Ernzerhof for solid (PBEsol)within generalized gradient approximation(GGA)21.In order to overcome the well-known shortcoming of conventional GGA method that underestimates the band gap value of semiconductor by~50%,the GGA+U method is chosen to obtain accurate electronic structure22.The value of effective U is set as 3.6 eV for the Cd-d and Fe-d states,which is obtained by comparison between the calculated results and experimental measurement of band gap value of CdS.A 1×2×1 mesh in the irreducible Brillouin zone was set for Monkhorst-Pack scheme kpoints grid sampling,and a 90×30×360 mesh was set for the fast Fourier transformation.In the geometry optimization process,the minimization algorithm was chosen the Broyden-Fletcher-Goldfarb-Shanno(BFGS)scheme23.The convergence standard was set as follows:the force on the atoms was less than 0.3 eV·nm-1,the stress on the atoms was less than 0.05 GPa,the atomic displacement was less than 1×10-4nm,and the energy change per atom was less than 1×10-5eV.
To simulate the interfacial structure and properties,the combination model of slab plus vacuum layer was adopted in the present work.The slab contains eight CdS layers and eight FeP layers,in which the two components were boned together. Moreover,the slabs ware separated by a 2 nm-thickness vacuum layer to avoid the mirror self-interaction along the interfacial normal direction.Firstly,the bulk CdS and FeP crystals were fully optimized both cell′s parameters and atomic coordination,and then the corresponding electronic structure and optical properties were calculated.Secondly,the surface with specific direction was cleaved from the optimized bulk phase with eight stoichiometric CdS or FeP layers,and then the atomic coordination was optimized,while the cell′s parameters were restricted.At the same time,the below four CdS layers are constrained to mimic the bulk effects for surface.Then the corresponding electronic structure and optical properties are calculated.Thirdly,two separated slabs of CdS and FeP are combined together,in which the supercell sizes are set as the averages two components.The final supercell sizes parallel to the interface are chose as the average of two components:1.6598 nm×0.5868 nm.Furthermore,the interfacial model is also separated by more than a 2 nm-thickness vacuum layer,and the size of this model along the normal direction interface is up to 7 nm.The total atoms reached to 256.After the interfacial model constructing,the atomic coordination of all atoms in this model are optimized by the BFGS scheme as mentioned above,except those atoms in the below four CdS layers.By this way,the interfacial stress could be reduced to a minimum.Finally,the electronic structure and optical properties are calculated for the interface,based on the optimized model.
3 Results and discussion
3.1Interfacial micro-structure
By the geometry optimizing,we could obtain the accurate lattice constants of bulk CdS and FeP.For diamond-structure(i.e. zincblende structure)CdS(space group:F4ˉ3m),the calculated lattice constants are listed as following:a=b=c=0.5868 nm,α= β=γ=90°,which are very agreement with experimental measurement(a=b=c=0.5811 nm,α=β=γ=90°)24.For orthorhombic-structure FeP(space group:Pnma),the calculated lattice constants are listed as following:a=0.5027 nm,b=0.2962 nm, c=0.5663 nm,α=β=γ=90°,which are very agreement with experimental measurement(a=0.5191 nm,b=0.3009 nm,c= 0.5792 nm,α=β=γ=90°)25.The calculated results indicate that the computational methods are reasonable and credible in the present work.The knowledge of CdS semiconductor atomic surface structure is very important for the tailoring of heterostructure.Anumber of experimental and theoretical investigations have been carried out on CdS semiconductor and its surfaces26,27. In general,the non-polar(101)zincblende surface of CdS semiconductor shows an outward relaxation of the surface-layer anions and an inward relaxation of the surface-layer cations.Our present work well reproduced above conventional phenomenon.
To construct hetero-structure model,the important factor is the crystal lattice matching.If the crystal lattice mismatching is too large,the interface is unstable due to the larger interfacial stress. In spite of the large crystal lattice mismatching could be decreased by constructing larger supercells,but the interface stress could not be obviously reduced.In the present work,we found the interface combined by the(101)plane CdS and the(103)plane of FePcould meet the above requirement.The two-dimensional lattice constants of the(101)plane of CdS is listed as following:u=0.4149 nm,v=0.5868 nm,γ=90°;and those of the(103)plane of FeP is listed as following:u=1.6108 nm,v=0.2962 nm,γ=90°.For the interface of 4×1(101)CdS/1×2(103)FeP,the degree of crystal lattice mismatching is:Δu=3.04%,Δv=0.93%,Δγ=0, which are less than 5%,suggesting that the(101)plane of CdS and the(103)plane of FeP could form a stable interface.
After all atomic coordination optimizing,the interfacial model is presented in Fig.1(a).One can see that the atomic relaxation at the interface is very significant:all the displacements of atoms at the interface are larger than 0.05 nm,and the variation of distancesbetween layers is larger than 0.02 nm.At the same time,the dangling bonds at the interface have been partially saturated. Using the formula defined by Xu et al.28,the adhesion energy(Eadh) of CdS/FeP is also calculated as-7.3 eV·nm2.This litter negative value indicates that the formation process of interface is exothermic and the formation of interface bonds stabilizes the interface.Therefore,the CdS/FeP hetero-structure is composed by the(101)plane of CdS and the(103)plane of FeP has small interface stress,and thus is stable.

Fig.1 (a)Side view of CdS(101)/FeP(103)interface model, (b)average electrostatic potential,and(c)average electron density difference along the interfacial normal direction
3.2Electronic structure
In order to explore the evolution of electronic structure of our interfacial model from bulk,to surface,and to interface,the total and partial density of states(DOS)are illustrated in Fig.2.As shown in Fig.2(a),in the case of CdS@Bulk,the calculated band gap is 2.406 eV,which is very consistent with experimental measurement(~2.4 eV).The upper valence band(VB)is dominantly consisted by the S-3p states.The lower conduction band (CB)is dominantly consisted by the Cd-5s states.Below VB and above CB,another band is consisted by the hybridized state between S-3p and Cd-5s states.Compared with bulk electronic structure,in the cases of surface and interface,above-mentioned main features are also exhibited.In the case of CdS@Surface,the lower band of VB is relatively upward shifting and overlapping with the middle band of VB,while in the case of CdS@Bulk the lower band of VB is relatively separated with the middle band of VB.An opposite situation could be found for CB of CdS@Surface case:the lower CB is relatively separated from the middle band of CB,which is overlapping in the bulk case.These variations are arising from the existence of dangling bonds on the surface,and the obvious surface relaxation.Although the dangling bonds are partially saturated,these variations maybe partially disappeared in the case of CdS@Interface.However,owing to the different bonding ways and chemical environments at the interface,there areobviousdifferentelectronicstructuresinthecaseofCdS@Interface,especially the interfacial states in the band gap,and upper band of VB or the lower band of CB.For the case of FeP,as shown in Fig.2(b),bands near the Fermi energy level(EF)are overlapping with each other,suggesting that FeP is a metallic compound. Furthermore,the bonding information of the three models is similar,which are consisted by the hybridized states between Fe-3d states and P-3p states.The presented obvious differences are as following:(i)in the case of FeP@Surface,the energy bands are concentrated to the EF;(ii)the band gap states(or interfacial states)are more obvious in the case of FeP@Interface,as similar with the case of CdS@Interface,which means that the interfacial states are obvious and important for CdS/FeP hetero-structure.

Fig.2 Calculated total and partial density of states of(a)CdS and(b)FePin different systems:bulk,surface,and interface
When CdS(101)surface and FeP(103)surface are contact together to form the interface,the atoms at the interface will be relaxed,in order to reduce the interfacial stress and saturate the dangling bonds,as shown in Fig.1(a).Thus,the interfacial states are presenting in Fig.2.In order to further understand the interfacial states,we plotted the layer-resolution total DOS for the CdSand FeP in the interface model in Fig.3,which are respectively compared with the bulk total DOS of CdS or FeP.In the first two layers,the feature of electronic structure is obviously different with that of bulk phase.While,in the case of 3rd-6th layers,the feature of electronic structure is similar with that of bulk phase. This phenomenon indicates that the interfacial states are localized at the limited layers at the interface that are obviously relaxed. Another important phenomenon can be observed:the relative energy band shifting in the case of interface model.It is obviously seen that the energy bands of CdS and FeP are relatively downshifting compared with those of bulk phase.
For the electronic structure of interface model,to align the energy bands between two components is the most important task. In the present work,we used the method that is defined by Zhang29and Chen30et al.to estimate the valence band offset ΔEVbetween CdS and FeP.The energy-level differences between valence band maximum(VBM)and core levels for CdS and FeP(i.e.are firstly calculated for the bulk models.For FeP, the VBM means the Fermi energy level in the present work.Then, for the interface model,the core-level difference(ΔEC′,C)between CdS layers and FeP layers is calculated.According to these data, the valence band offset ΔEV(CdS/FeP)can be derived by the following equations:ΔEV(CdS/FeP)=By this way,we estimated the shifting relative value of band:~0.3 eV for CdS and~1.1 eV for FeP in order to quantitatively evaluate the energy band shifting.
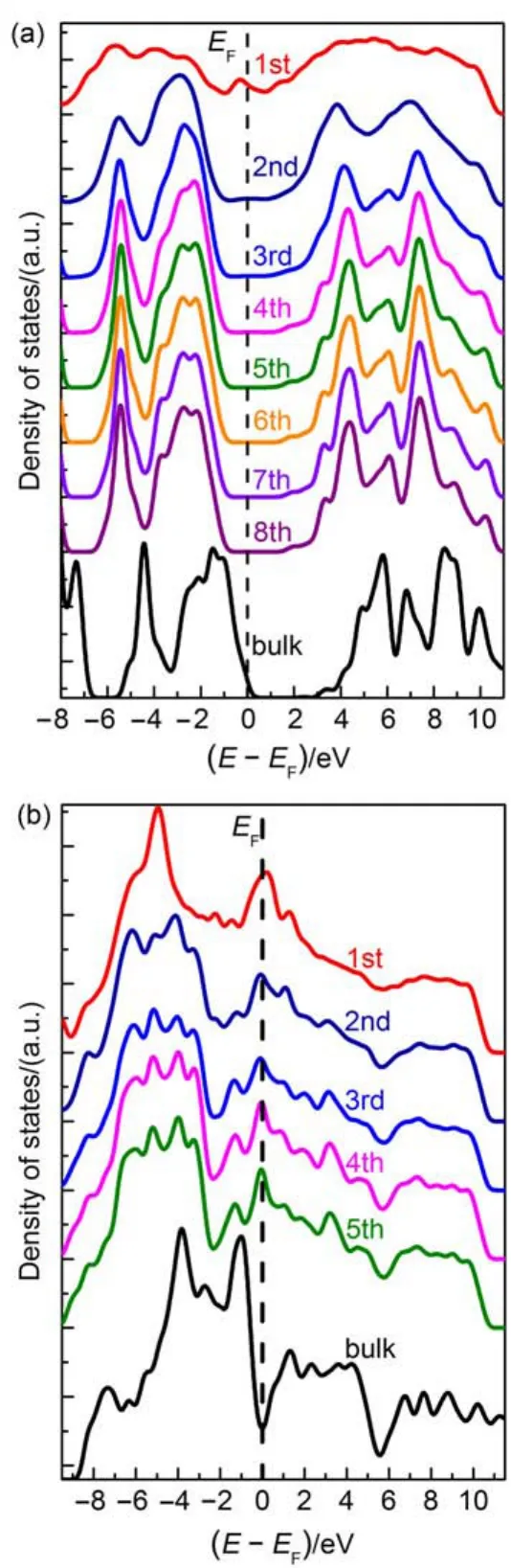
Fig.3 Calculated layer-resolution total density of states of CdS(a)and FeP(b)in the CdS/FePmodel compared with the total density of states of bulk phase
The electrostatic potential along the interfacial normal direction was illustrated in Fig.1(b),in compared with that of CdS surface along the[101]direction or FeP surface along the[103]direction. The obvious feature is the potential of CdS slab(with average potential of-9.74 eV)is higher than that of FeP slab(with average potential of-17.52 eV).Therefore,the built-in electric field points from FeP slab to CdS slab under equilibrium,after the interface is formed and stable.Compared with bulk counterpart, the average potential of CdS slab is increased by~0.76 eV,while that of FeP slab is decreased by~0.01 eV.For the clean unrelaxed CdS(101)surface,the work function is 5.458 eV,which is higher than that of the clean unrelaxed FeP(103)surface(ΦFeP=4.692 eV).So,when they contact together,the electrons will be transferred from CdS slab to FeP slab,resulting the existence of space charge region(or depletion layer).It is could be confirmed the average electron density difference along the interfacial normal direction as shown in Fig.1(c).The result of electron transfer is the Fermi energy(EF,CdS)and the vacuum energy level(EVac,CdS)of CdS slab is downwards shifting,as well as the EF,FePof FeP slab,until the EF,CdSand the EF,FePare aligned.Finally,the EF,CdS/FePis located at below the bottom of CB of CdS by~0.9 eV.From Fig.1(c),the thickness of space charge region could be estimated by about 0.8 nm.Based on above calculated data and discussion,we proposed the energy band diagram of CdS(101)/FeP(103)interface as illustrated in Fig.4.Because of the existence of built-in electric field,the energy band edges of CdS are shifted downwards,which is called as the band bending.The degree of energy band bending (VBB)is defined as the difference of work function between CdS (101)surface and FeP(103)surface,VBB=ΦCdS-ΦFeP=0.766 eV. The presence of the space charge region can prevent more electrons flow from CdS slab to FeP slab.Thus,under the equilibrium conditions,the photo-generated electron-hole pairs can be spatially separated by the CdS/FeP interface.

Fig.4 Proposed energy band diagram of CdS/FePhetero-structure
3.3Optical properties
Fig.5 illustrated the calculated absorption spectra of different CdS and FeP systems along the[101](for CdS)or[103](for FeP) directions.The optical properties of different systems are determined by their composition,crystal structure,and electronic structure.For example,the fundamental absorption band edge of bulk CdS is located about 550 nm,which is determined by the ~2.4 eV band gap.While the absorption spectra of bulk FeP has no obvious absorption band edge in the visible-light region. Furthermore,the absorption coefficient of FeP is obviously larger than that of CdS,owing to its intrinsic metallic or semi-metallic characters.Compared to bulk CdS,the fundamental absorption band edge of CdS(101)surface along the normal direction is obviously blue-shifting.In the case of FeP(103)surface,the absorption coefficient is significantly decreased.In the case of CdS/ FeP composite photocatalyst,the optical properties have significant difference compared with bulk CdSor bulk FePin the visiblelight region.Importantly,the absorption coefficient of CdS/FeP composite photocatalyst is obviously increasing in the visible-light region in comparison with CdS(101)surface or bulk CdS.This calculated result suggests that the FeP loading could enhance the visible-light absorption of CdS.

Fig.5 Calculated absorption spectra of CdS or FePin different systems:bulk,surface,and interface
4 Conclusions
To in-depth investigate the interfacial properties of CdS/FeP composite photocatalyst with hetero-structure,its atomic-scale structure,electronic structure,and optical properties are calculated by density functional theory in the present work.Firstly,the interface is consisted by the CdS(101)crystalline plane and FeP (103)crystalline plane,which have slight lattice mismatching(less than 5%)and could form a stable interface.Owing to partially saturated dangling bonds,the electronic structure of interface model exhibits both the features of bulk and surface references. At the interface of CdS/FeP hetero-structure,the energy bands of CdS and FeP are relatively down-shifting,and the energy band of FeP inserts at the below of conduction band of CdS,which is very favorable for the improvement of photocatalytic performance. Moreover,the built-in electric field of hetero-structure points from FeP layer to CdS layer under equilibrium,so the photo-generated electron-hole pairs can be spatially separated by the CdS/FeP interface,which is the improvement mechanism for photocatalytic performance.Based on the calculated results,the energy band diagram of CdS(101)/FeP(103)interface is proposed.In addition, to construct CdS/FeP hetero-structure also can further improve the absorption properties of CdS in visible-light region.
References
(1)Guo,Q.;Zhou,C.Y.;Ma,Z.B.;Ren,Z.F.;Fan,H.J.;Yang,X. M.Acta Phys.-Chim.Sin.2016,32,28.[郭庆,周传耀,马志博,任泽峰,樊红军,杨学明.物理化学学报,2016,32,28.] doi:10.3866/PKU.WHXB201512081
(2) Chang,X.X.;Gong,J.L.Acta Phys.-Chim.Sin.2016,32,2. [常晓侠,巩金龙.物理化学学报,2016,32,2.]doi:10.3866/ PKU.WHXB201510192
(5) Schultz,D.M.;Yoon,T.P.Science 2014,343,1239176. doi:10.1126/science.1239176
(8) Sun,W.T.;Yu,Y.;Pan,H.Y.;Gao,X.F.;Chen,Q.;Peng,L.M. J.Am.Chem.Soc.2008,130,1124.doi:10.1021/ja0777741
(9) Zong,X.;Yan,H.;Wu,G.;Ma,G.;Wen,F.;Wang,L.;Li,C. J.Am.Chem.Soc.2008,130,7176.doi:10.1021/ja8007825
(10) Yang,S.;Wen,X.;Zhang,W.;Yang,S.J.Electrochem.Soc. 2005,152,G220.doi:10.1149/1.1859991
(11) Sathish,M.;Viswanathan,B.;Viswanath,R.P.Int.J.Hydrog. Energy 2006,31,891.doi:10.1016/j.ijhydene.2005.08.002
(12) Guan,G.;Kida,T.;Kusakabe,K.;Kimura,K.;Fang,X.;Ma,T.; Abe,E.;Yoshida,A.Chem.Phys.Lett.2004,385,319. doi:10.1016/j.cplett.2004.01.002
(13) Yan,H.;Yang,J.;Ma,G.;Wu,G.;Zong,X.;Lei,Z.;Shi,J.;Li, C.J.Catal.2009,266,165.doi:10.1016/j.jcat.2009.06.024
(14) Walter,M.G.;Warren,E.L.;McKone,J.R.;Boettcher,S.W.; Mi,Q.;Santori,E.A.;Lewis,N.S.Chem.Rev.2010,110,6446. doi:10.1021/cr1002326
(15) Song,H.;Wang,J.;Wang,Z.;Song,H.;Li,F.;Jin,Z.J.Catal. 2014,311,257.doi:10.1016/j.jcat.2013.11.021
(16) Cao,S.;Chen,Y.;Wang,C.J.;He,P.;Fu,W.F.Chem. Commun.2014,50,10427.doi:10.1039/C4CC05026F
(17) Callejas,J.F.;McEnaney,J.M.;Read,C.G.;Crompton,J.C.; Biacchi,A.J.;Popczun,E.J.;Gordon,T.R.;Lewis,N.S.; Schaak,R.E.ACS Nano 2014,8,11101.doi:10.1021/ nn5048553
(18) Zhang,Z.;Hao,J.;Yang,W.;Lu,B.;Tang,J.Nanoscale 2015, 7,4400.doi:10.1039/C4NR07436J
(19) Clark,S.J.;Segall,M.D.;Pickard,C.J.;Hasnip,P.J.;Probert,M.J.;Refson,K.;Payne,M.C.Z.Kristallogr.2005,220,567. doi:10.1524/zkri.220.5.567.65075
(21) Perdew,J.P.;Ruzsinszky,A.;Csonka,G.I.;Vydrov,O.A.; Scuseria,G.E.;Constantin,L.A.;Zhou,X.;Burke,K.Phys. Rev.Lett.2008,100,136406.doi:10.1103/ PhysRevLett.100.136406
(22) Anisimov,V.I.;Zaanen,J.;Andersen,O.K.Phys.Rev.B 1991, 44,943.doi:10.1103/PhysRevB.44.943
(23) Pfrommer,B.G.;Câté,M.;Louie,S.G.;Cohen,M.L. J.Comput.Phys.1997,131,233.doi:10.1006/jcph.1996.5612
(24)Yeh,C.Y.;Lu,Z.W.;Froyen,S.;Zunger,A.Phys.Rev.B 1992, 46,10086.doi:10.1103/PhysRevB.46.10086
(25) Rundqvist,S.;Nawapong,P.C.Acta Chem.Scand.1965,19, 1006.doi:10.3891/acta.chem.scand.19-1006
(26) Lin,C.M.;Tsai,M.H.;Yang,T.J.;Chuu,D.S.Phys.Rev.B 1997,56,9209.doi:10.1103/PhysRevB.56.9209
(27) Schröer,P.;Krüger,P.;Pollmann,J.Phys.Rev.B 1993,48, 18264.doi:10.1103/PhysRevB.48.18264
(28) Xu,X.;Sun,X.;Sun,B.;Peng,H.;Liu,W.;Wang,X.J.Colloid Interface Sci.2016,473,100.doi:10.1016/j.jcis.2016.03.059
(29) Zhang,S.B.;Wei,S.H.;Zunger,A.J.Appl.Phys.1998,83, 3192.doi:10.1063/1.367120
(30) Chen,S.;Yang,J.H.;Gong,X.G.;Walsh,A.;Wei,S.H.Phys. Rev.B 2010,81,245204.doi:10.1103/PhysRevB.81.245204
Theoretical Study of the Interfacial Structure and Properties of a CdS/FeP Composite Photocatalyst
ZHAO Zong-Yan1,2,*TIAN Fan1
(1Faculty of Materials Science and Engineering,Kunming University of Science and Technology,Kunming 650093,P.R.China;2Yunnan Key Laboratory of Micro/Nano Materials&Technology,School of Materials Science and Engineering, Yunnan University,Kunming 650504,P.R.China)
An effective method for improving the performance of a photocatalyst is to construct a suitable hetero-/homo-structure.This strategy can also lead to improvements in the stability of the photocatalysts that suffer with photo-corrosion(such as CdS).The preparation of CdS-based composite photocatalysts has therefore been widely studied.Unfortunately,however,some of the fundamental and more significant aspects of this strategy still need to be evaluated in greater detail.In this study,we have evaluated the interfacial microstructure and properties of a CdS/FeP composite photocatalyst with a hetero-structure using a series of the firstprinciples calculations.The results revealed that the electronic structure of the interface model exhibited different features compared with the bulk and surface models,because of the partially saturated dangling bonds. However,several obvious interfacial states were observed.At the interface of the CdS/FeP hetero-structure, the energy bands of CdS and FeP were relatively down-shifted,whereas the energy band of FeP was inserted below the conduction band of CdS.Furthermore,the direction of the built-in electric field of the hetero-structureprojected out from the FeP layer towards the CdS layer under the equilibrium conditions.The photo-generated electron-hole pairs were therefore spatially separated by the CdS/FeP interface,which was favorable for improving the photocatalytic performance.The construction of a CdS/FeP hetero-structure can also lead to further improvements in the absorption properties of CdS in the visible-light region.The results of this study have provided mechanical explanations and theoretical support for the construction of highly efficient composite photocatalyst with hetero-structures.
April 19,2016;Revised:July 13,2016;Published online:July 13,2016.
.Email:zzy@kmust.edu.cn;Tel:+86-871-65109952.
Photocatalysis;Cadmium sulfide;Hetero-structure;Interfacial micro-structure;Interfacial property;Density functional theory calculation
O647
10.3866/PKU.WHXB201607131
The project was supported by the National Natural Science Foundation of China(21473082),and 18th Yunnan Province YoungAcademic and Technical Leaders Reserve Talent Project(2015HB015).
国家自然科学基金(21473082)和云南省第18批中青年学术和技术后备人才项目(2015HB015)资助©Editorial office ofActa Physico-Chimica Sinica
(3)Hamakawa,Y.Renew.Energy 1994,5,34.10.1016/0960-1481(94)90352-2
(4) Kamat,P.V.J.Phys.Chem.C 2007,111,2834.10.1021/ jp066952u
(6) Qu,Y.;Duan,X.Chem.Soc.Rev.2013,42,2568.10.1039/ C2CS35355E
(7) Fujishima,A.;Honda,K.1972,238,37.10.1038/238037a0
(20) Vanderbilt,D.Phys.Rev.B 1990,41,7892.10.1103/ PhysRevB.41.7892
