Copper partitioning between granitic silicate melt and coexisting aqueous fluid at 850°C and 100 MPa
2016-11-21ShuilongWangHuiLiLinboShangXianwuBiXinsongWangWenlinFan
Shuilong Wang·Hui Li·Linbo Shang·Xianwu Bi· Xinsong Wang·Wenlin Fan
Copper partitioning between granitic silicate melt and coexisting aqueous fluid at 850°C and 100 MPa
Shuilong Wang1,3·Hui Li2·Linbo Shang1·Xianwu Bi1· Xinsong Wang1·Wenlin Fan1
Experiments on the partitioning of Cu between different granitic silicate melts and the respective coexisting aqueous fluids have been performed under conditions of 850°C,100 MPa and oxygen fugacity(f O2)buffered at approaching Ni-NiO(NNO).Partition coefficients of Cu(DCu=cfluid/cmelt)were varied with different alumina/alkali mole ratios[Al2O3/(Na2O+K2O),abbreviated as Al/ Alk],Na/K mole ratios,and SiO2mole contents.The DCuincreased from 1.28±0.01 to 22.18±0.22 with the increase of Al/Alk mole ratios(ranging from 0.64 to 1.20)and Na/K mole ratios(ranging from 0.58 to 2.56).The experimental results also showed that DCuwas positively correlated with the HCl concentration of the starting fluid. The DCuwas independent of the SiO2mole content in the range of SiO2content considered.No DCuvalue was less than 1 in our experiments at 850°C and 100 MPa,indicating that Cu preferred to enter the fluid phase rather than the coexisting melt phase under most conditions in the melt-fluid system,and thus a significant amount of Cu could be transported in the fluid phase in the magmatichydrothermal environment.The results indicated that Cu favored partitioning into the aqueous fluid rather than themelt phase if there was a high Na/K ratio,Na-rich,peraluminous granitic melt coexisting with the high Cl-fluid.
✉ Shuilong Wang
shuilongwang0@126.com
✉ Linbo Shang
shanglinbo@vip.gyig.ac.cn
1State Key Laboratory of Ore Deposit Geochemistry,Institute of Geochemistry,Chinese Academy of Sciences,Guiyang 550081,China
2Geophysical Exploration Institute of Heilongjiang Province,Harbin 150036,China
3University of Chinese Academy of Sciences,Beijing 100039,China
Cu·Experimental study·Partition coefficient· Granitic silicate melt·Aqueous fluid
1 Introduction
The porphyry copper deposit is the most important source of Cu in the world,as it accounts for about 50%-60%of the world’s Cu production(Sinclair 2007).Porphyry copper deposits are spatially and temporally related to felsic and to intermediate porphyritic intrusions.This has led many investigators to examine the relationship between magmatic activity and hydrothermal ore genesis(Frank et al.2011).Studies are in general agreement that magma provides the heat and metallogenic materials for the porphyry deposits(Sillitoe 1979,1989,2010;Sinclair 2007).It has been hypothesized that the majority of the Cu found in the deposits was derived from the melt(Sinclair 2007). Most researchers believed that the porphyry deposits were formed when the metals in the ore-forming fluid were precipitated due to the boiling and/or un-mixing of fluid(Sillitoe 2010).The close spatial relationship between the felsic porphyry rocks and the ore bodies strengthens the melt-ore link,but the details of the Cu transport in the porphyry environment are still not clear over a range of pressure,temperature,compositions of fluids and silicate melts.
The transformation of silicate melt and fluid from the original melt resulted in the element partitioning behavior between the melt and fluid phases(Audétat et al.2000;Kamenetsky et al.2004).This is an important step for the formation of the porphyry ore deposits(Hedenquist andLowenstern 1994;Shinohara 1994;Candela and Piccoli 1995;Barnes 1997;Ulrich et al.1999).During phase transformation,the fluid and silicate melt compositions immediately influence the element partitioning behavior between the silicate melt and fluid(Bodnar et al.1985;Heinrich et al.1999;Kamenetsky et al.2004;Rusk et al. 2004),causing the variation of element species and concentrations in fluid,followed by the formation of various kinds of hydrothermal deposits(Halter and Webster 2004). Thus,it is important to know the partitioning behavior of Cu between granitic silicate melts and coexisting fluids.
The partitioning behavior of metals between silicate melts and fluids can be affected by a number of parameters,including temperature,pressure,oxygen fugacity,and compositions of fluid and melt.Previous studies on Cu partitioning between aqueous fluids and silicate melts have focused on various aqueous fluid systems at~700-1000°C and 50-400 MPa(Khitarov et al.1982;Candela and Holland 1984;Keppler and Wyllie 1991;Williams et al.1995;Bai and Koster van Groos 1999;Simon et al.2006;Frank et al.2011;Zajacz et al.2012). Their data show that the DCu(fluid/melt)values can vary from 0.1 to 433,with most of them being greater than 1. The available experimental data of Cu partitioning between fluids and melts show that the Cu partitioning into the fluid phase is significantly enhanced with the presence of chloride(NaCl/KCl or HCl)in the system,with positive correlations between the DCu(fluid/melt)values and the Clconcentrations in the fluids(Khitarov et al.1982;Candela and Holland 1984;Keppler and Wyllie 1991;Williams et al.1995;Bai and Koster van Groos 1999;Simon et al. 2006;Frank et al.2011).Data of Simon et al.(2006)demonstrate that the presence of sulfur enhances the partitioning of Cu from melts into magmatic volatile and brine phases,as thevalue of the S-bearing system is five times of that of the S-free system.Recently,Tattitch et al.(2015)reported that the DCu(brine/vapor)values increase from 25(±6)at XCO2=0.10,to 100(±30)at XCO2=0.38 in the CO2-bearing vapor-brine-melt system.
In natural systems,analyses of Cu-rich,low-density fluid bubbles,trapped in melt inclusions in quartz of silicic volcanic rocks from the 1912 Valley of Ten Thousand Smokes,Alaska(Lowenstern 1993)and in the CO2-dominated vapor bubbles from quartz phenocrysts of rhyolites from Pantelleria,Sicily(Lowenstern et al.1991),show the D(vapor/melt)values for Cu to be from 100 to 1000.Rare natural data on Cu partitioning between fluids and silicic melts in granitic-pegmatite(Zajacz et al.2008)and magmatic-hydrothermal(Audétat and Pettke 2003;Vikent’ev et al.2012)systems agree with the range of experimentally measured values in the laboratory.
However,there is a lack of experimental data about the influence of silicate melt compositions[Al/(Na+K),Na/ K,SiO2]on the partitioning behavior of Cu between the melt and fluid phases.Only some data reported by Bai and Koster van Groos(1999)show that the decrease of ASI of the silicate melt results in a decrease of the DCuvalues for the Cl-bearing system.In the experiments in this paper,the fluid-melt partition coefficients of Cu were determined as a function of the melt compositions(e.g.,Al/Alk mole ratios,Na/K mole ratios,and SiO2mole contents,where the Al/ Alk mole ratio is the molar ratio of Al2O3divided by the sum of Na2O+K2O)and the concentrations of chlorine in the co-existing fluids at 850°C,100 MPa,in order to constrain the Cu partitioning behavior between the melt and fluid phases in a fluid-melt system evolved from the representative porphyry magma.
2 Experimental methods
2.1Starting materials
The starting chemical compositions of the individual haplogranite gels were determined by X-ray fluorescence(XRF)and are provided in Table 1.The method used to compound the haplogranitic gels is presented in detail by Hamilton and Henderson(1968).The gels were prepared from reagent grade TEOS,Na2CO3,KHCO3,and Al2O3. The Al/Alk mole ratios and Na/K mole ratios of these haplogranitic gels are varied around a basic point with composition of(Qtz0.38Ab0.33Or0.29),which is the ternary minimum composition of the water-saturated melt,with the liquids at a temperature of about 710°C and a vapor pressure of 100 MPa(Tuttle and Bowen 1958;Zhang 1992).In order to conform to Henry’s Law,a small amount of Cu2O powder was added for the synthesis of the haplogranite gels.
The haplogranitic gel was fused to glass in the Pt crucibles in a silicon-molybdenum electric oven at 1300°C for 2 h.The glasses were then mounted onto a polymer casting resin,polished,and examined for the compositional homogeneity by using a scanning electron microscope(Fig.1).The data show that major elements(Si,Al,Na,K)were distributed homogeneously in the glass,indicating that the glasses were compositionally homogeneous.
2.2Experimental apparatus
Experiments were conducted using an externally-heated cold-seal rapid quench pressure vessel(RQV)(Fig.2),which mainly includes three parts(reaction system,pressure system,and temperature control system).The vessel of the reaction system has 30 mm in its outer diameter,8 mm in its inner diameter,450 mm in length,with the distilled water as the pressure medium of the reactionsystem.The exterior cooling kettle(~200 mm long)of the vessel is cooled by a water-circulating jacket during the entire experimental run,in order to protect the vessel during the rapid quench.The vessel was removed from the furnace and tilted vertically to allow the capsule to slide to the cool-end of the vessel and quenched to room temperature in 1 min at the end of the experiment.A manual spiral pump was used to enhance the pressure of the pressure system.Valves and buffers were used to keep a constant pressure condition.Pressures were measured with a Bourdon-type gauge,which was manufactured by the Jinan Great Wall Instrumentation Factory and has an uncertainty of±5 MPa.The temperature control system,produced by the Shanghai Automation Instrumentation Co.,Ltd.,has applied an XTMD-1000P controller with a temperature error of±1°C.A calibrated sheathed Pt-Pt90Rh10 thermocouple was inserted into a shallow hole at the end of the vessel to connect the reaction system and the temperature control system.There is a constant temperature zone in the reaction system to keep the same conditions during various experiments.The Ni-rich alloy of the vessel(GH49,containing ca.60 wt%Ni)and a Ni-based filler rod generated an oxygen fugacity that was intrinsically buffered near the NNO at temperatures of~800-850°C and a water pressure of 100 MPa(Chou 1987;Taylor et al.1992).

Table 1 Major element(wt%)and Cu(μg/g)concentrations of haplogranitic gels

Fig.1 A SEM image of haplogranitic glass.Pores(such as A and B)are produced by rapid quench.They are distributed randomly in the haplogranitic glass
2.3Experimental procedures
Experiments were conducted in gold capsules with a length of~60 mm,outer diameter of 5 mm,and inner diameter of 4.5 mm.Approximately 200 mg of haplogranite powder and 200 μl solution were loaded into each capsule.The capsule was crimped and bathed in ice water while being sealed with an oxyacetylene torch to minimize volatile losses,with less than±0.5 mg being lost from the capsules after sealing.They were then baked in an oven overnight at 110°C to check for leakage.If the weight loss was less than±0.5 mg,the capsules were placed into the externallyheated cold-seal rapid quench pressure vessel,sealed,pressurized to about one-third of the desired final pressure,and then heated to the desired temperature and pressure. Experiments were done for several days at constanttemperature and pressure and finally quenched isobarically within 1 min.

Fig.2 Sketch of the RQV experimental apparatus
The capsules were then removed from the vessels,cleaned,and weighed to check for any leakage that may have happened during the experiments.Capsules that had a weight loss of over±0.5 mg were discarded.Then,the eligible capsules were pierced with a stainless steel needle and the solution was removed with a microsyringe into a 10 ml test tube.The solution was then weighed and diluted with 5 wt%HNO3to a volume of 10 ml,with an uncertainty of±1%.After the solution was removed,the glass was obtained by splitting the capsule.Then,the inside of the capsule and the outside of the glass bead were cleaned with 5 wt%HNO3.The cleaning solution was moved to a 50 ml test tube with an error of±0.1 ml,similar to the concentration of Cu in the aqueous fluid.Therefore,achieving equilibrium between the haplogranite melt and fluid in the capsule was an important precondition.A series of experiments have been done to check how long the equilibrium could be reached for the partition of elements(Candela and Holland 1984;Williams et al.1995;Frank et al.2003,2011;Chen 1989;Tang 2003).Based on the data from the studies above,each of our experiments had to be run for 5 days,in order to make sure the equilibrium between the haplogranite melt and the fluid in the capsule was reached.
3 Analytical techniques
The major elements of the glasses were analyzed by using a PANalytical Axios-advance XRF spectrometer on fused lithium-tetraborate glass pellets.Analytical precision,as determined by the Chinese National standard GSR-1,was generally around 1%-5%.The Cu concentrations of the haplogranitic gels and recovered quenched aqueous fluids were analyzed by using a Finnigan MAT ELEMENT inductively coupled plasma source mass spectrometer(ICP-MS)following the procedures described by Qi et al.(2000).Part of the product glasses were milled to 200 mesh in an agate mortar,then 50 mg of the glass powder was dissolved by 1 ml of purified HF and HNO3in a Teflon crucible,and the solutions also were analyzed by using ICP-MS.Rhodium was used as an internal standard to monitor signal drift during counting.The international standards GBPG-1 and OU-6 were used for analytical quality control.The relative analytical precision was generally less than 5%.
Simon et al.(2007)verified that compositions of aqueous fluids trapped as quartz-hosted inclusion and glasshosted inclusion,and those recovered from the capsule after quench are generally consistent at the 2δ uncertainty level,though those compositions were analyzed by using three entirely different instrumental techniques(i.e.,LAICP-MS,INAA and AAS).Therefore,in our experiments,the Cu contents of the recovered quenched aqueous fluids analyzed with the ICP-MS represent the concentration of Cu in the fluids at high temperature and pressure,while the Cu contents of the produced glasses indicate those of the melts.
During the quenching process,a small amount of the solutions included in some pores or inclusions of the glasses could influence the actual Cu concentrations of the melts.In order to estimate the influence,a small portion of product glass was mounted onto a polymer casting resin,polished,and observed under optical microscope.Microscopic observations verified that the glass was homogeneous and crystal-free,with many pores or inclusions(Fig.3).Among these pores or inclusions,only 1%-5% of them in volume are actual fluid inclusions,which were checked using Laser Raman Spectrometry.Raman spectra indicate that these inclusions contain some water(Fig.4),in which Cu and other components,such as NaCl,KCl and HCl,could be presented.Therefore,it is estimated thatthere is an uncertainty of about±5%for the Cu concentrations of the melts.

Fig.3 Photomicrograph of pores or inclusions in product glass.The dark areas like A or B are mostly pores produced by quenching of the melts(95%).Only 1%-5%of them(C and D)contain water verified by Raman spectroscopy
Fig.4 Raman spectrum of water contained in a pore in product glass
4 Results and discussion
4.1Influence of Al/Alk mole ratio of the melt
In Group A of Table 2,the L1 series gels with various Al/ Alk mole ratios and similar Na/K mole ratios were used as starting melts and the 0.1 mol/l HCl solution was used as the starting fluid.Cu partition coefficients vary from 1.28±0.01 to 10.09±0.10 when Al/Alk mole ratios range from 0.64 to 1.20.Evidently,the DCuis positively correlated with the Al/Alk mole ratios(Fig.5).This infers that Cu partitioning is distinctly affected by the melt composition changing from peralkaline to peraluminous.It can be seen that the higher Al/Alk mole ratios for the melts,the more Cu partitions into the coexisting aqueous fluids. However,the higher the alkali contents of the melts,the more Cu partitions into the melts.
The network of silicate melts constitutes of the basic structure of magma.In silicate melts,Si4+and Al3+are called network-former cations,while Na+and K+are called network-modifier cations.The ratio between the network-modifier cation and network-former cation defines the degree of melt polymerization.The increase of Al/Alk mole ratios means an increase of the degree of polymerization but a relative decrease of non-bridging oxygen(NBO)available.Some elements,such as Cu,Pb,Zn,Mo,and so on,form six-coordinate(or>6)complexes in the silicate melts(Bai and Koster van Groos 1999),with a positive correlation to the NBO(Farges et al.1991,1992). Therefore,with the increase of the Al/Alk mole ratios,the solubility of the element Cu in the silicate melts is reduced,resulting in an increase of the partition coefficient of Cu between fluids and melts.
4.2Influence of Na/K mole ratio of the melt
The influences of the Na/K mole ratio on the Cu partition coefficients are listed in Group B of Table 2.In Group B,the L2 series gels with various Na/K mole ratios and similar Al/Alk mole ratios were used as the starting melts and the 0.1 mol/l HCl solution was used as the starting fluid.The Cu partition coefficients vary from 1.35±0.01 to 22.18±0.22,when the Na/K ratios range from 0.58 to 2.56.Evidently,the DCuis positively correlated with the Na/K mole ratio(Fig.6).This implies that Cu is more favorable to be partitioned into the aqueous fluid for a Narich melt-fluid system than a K-rich melt-fluid system.
The standard enthalpy of formation of Na2O and K2O are-416 and-363.17 kJ/mol,respectively.In addition,the Pauling radius of Na+(95 pm)is smaller than that of K+(133 pm),while the electronegativity of Na+is larger than that of K+(Huheey et al.2006).Based on these parameters,the structure of a melt becomes more and more stable with the increase Na/K mole ratio,resulting in the decrease of capability for accommodating Cu in the melt phase.Hence,Cu tends to be enriched in the fluids rather than the melt.In addition,the Cu partition coefficients are increased with an increase of the Na/K ratios of the melt. This agrees with the fact that the Na ion is in favor of the Cu enrichment in fluids by Holland(1972).
4.3Influence of the SiO2mole content
The influences of the SiO2mole content on the Cu partition coefficients are listed in Group C of Table 2.In Group C,the L3 series gels with similar Al/Alk mole ratios and Na/K mole ratios were used as the starting melts and the 0.1 mol/ l HCl solution was used as the starting fluid.The Cu partition coefficients vary from4.48±0.04to 13.30±0.13,with the SiO2mole content ranging from 75.44±3.84 to 85.61±3.95.It can be seen in Fig.7 thatthere is no linear correlation between the Cu partition coefficient and the SiO2mole content.

Table 2 Data for the partitioning of Cu between various silicate melts and coexisting aqueous fluids

Fig.5 Relationship between DCuand Al/Alk of melt
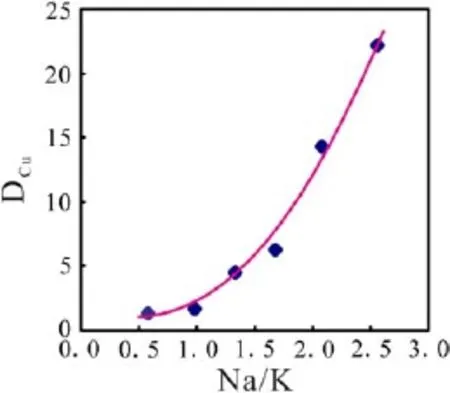
Fig.6 Relationship between DCuand Na/K mole ratio

Fig.7 Relationship between DCuand SiO2mole content
The SiO2mole content is the most important parameter controlling the various kinds of structures of silicate melts. The capability for accommodating elements varies widely with different kinds of melt structures(from island silicates to framework silicates).In this study,all silicate melts are supersaturated with SiO2.Consequently,the SiO2mole content of the melt is not obviously influenced by the Cu partition coefficients between the liquid and melt.
4.4Influence of HCl concentration in starting fluids
In Group D,L1-3 gels with Al/Alk mole ratio of 0.97 and Na/K mole ratio of 1.34 were used as the starting melt and different HCl solutions were used as the starting fluids(Table 2).The Cu partition coefficients vary from 2.98±0.03 to 17.61±0.17,with the HCl concentrations of solutions ranging from 0.01 to 2.00 mol/l.The results of this study are consistent with those of previous ones(Khitarov et al.1982;Candela and Holland 1984;Keppler and Wyllie 1991;Williams et al.1995;Bai and Koster van Groos 1999;Simon et al.2005;Frank et al.2011).It can be seen in Fig.8 that there is a positive linear correlation between the Cu partition coefficient and the Cl concentration of the fluid.

Fig.8 Relationship between DCuand HCl concentrations in starting fluids
Volatile elements,particularly F and Cl,play an important role in magmatic hydrothermal ore-forming systems(Webster 1990).For example,halogens affect various processes,such as the timing of vapor saturation,the compositional variations by complexing with metals,and types of mineralization in hydrothermal metallogenic system(Hu et al.2008).Chlorine prefers to be distributed in aqueous fluids with a maximum Cl distribution coefficient of 117(Webster 1992a,b;Bureau et al.2000).Cu prefers to complex with Cl in aqueous fluids(Khitarov et al.1982;Candela and Holland 1984;Keppler and Wyllie 1991;Williams et al.1995;Bai and Koster van Groos 1999;Simon et al.2005;Frank et al.2011),so the Cu partitioning coefficients are increased with an increase of Cl concentrations in the fluids.
5 Implications
The metallogenic system of the porphyry ore deposits is complicated,as the compositions of melts,vapor,and brine fluids are extremely varied throughout the ore-forming process.Our data have demonstrated that the melt composition is also an important factor governing the partitioning behavior of Cu between fluids and melts,besides the chlorine and sulfur in fluids.Whole rock analyses of ore-bearing porphyry rocks obviously show their Al/Alk and Na/K mole ratios are both larger than 1 in Fig.9(Zhou 2011;Wang 2013;Hou et al.2003).These geological and experimental results imply that Cu prefers to partition into the aqueous fluids to form Cu-rich ore-forming fluids in the system of peraluminous granitic melt or Na-rich melt coexisting with HCl-rich aqueous.Fluids(vapor and/or brine)can be exsolved form magmatic melts when they are saturated in the melts under conditions of certain temperatures and pressure(Robb 2013).The total quantity of Cu scavenged from the melts by the exsolved fluids can easily supply enough Cu to form a world-class Cu-porphyry deposit(Sinclair 2007).
In natural systems,the exsolution of the magmatic volatile phase(MVP),which always occur during the later stage of the fractional crystallization of the magma,resulted in the partition of Cu between the MVP and melt(Candela 1997).Therefore,the contents Al2O3,Na2O,and K2O(or Na2O+K2O)of the primary melt cannot be used to evaluate its metallogenic potentiality,as those contents in the melt are variable during the fractional crystallization process.Our study presents that the parameters(Al/Alk,Na/K mole ratios)of evolved magmas are more suitable for indicating the metallogenic potentiality of the melt.TheDCu(fluid/melt)values(1.28-22.18)presented by our work are consistent with the values obtained by previous researchers from the natural samples(Lowenstern et al. 1991;Lowenstern 1993;Audétat and Pettke 2003;Vikent’ev et al.2012;Zajacz et al.2008).Therefore,it is believed that our Cu partition coefficients between fluids and silicate melts could be used as a parameter to estimate the possible the amount of Cu derived from source magma of porphyries in a geological model,such as that shown by Pokrovski et al.(2013).

Fig.9 Plots of Al/Alk and Na/K mole ratios versus SiO2contents of Copper deposits(Data cited:Zhou 2011;Wang 2013;Hou et al.2003)Al/Alk and Na/K are calculated in mole content.Al/Alk is the mole ratio of Al2O3divided by the sum of Na2O+K2O
Herein,our Cu partitioning data obtained from the above experiments have been applied to discuss how copper could be efficiently removed from a silicate melt into an evolving fluid in the fluid-melt system.We selected the following parameters for the simulation:mean Cu partitioning value(10),volume of granite melts(100 km3),density of melts(2.5 g/cm3),content of water(5 wt%),Cu concentration in granite melts(50 ppm).In addition,the system is supposed to be closed so that the Cu amounts are limited to the given volume of the melts.After the equilibration of Cu in the fluid and melt two-phases,the calculated Cu mass in fluid is 4.17×106tons.The model indicates that 33.3%of the Cu would be removed from the melt into the fluid.This calculation reveals that a large amount of Cu could be extracted from the silicate melt into the coexisting fluid,even if the Cu partitioning values are relatively small(such as 1-10).This implies that the variation of compositions of silicate melts could result in the large variation of Cu mass transferred from the melt into the fluid.
Simultaneously,considering the influence of fluid compositions(such as S,CO2and HCl)on the Cu partition coefficients(Simon et al.2006;Zajacz et al.2008;Frank et al.2011;Tattitch et al.2015;this study),a Cu partitioning value of 50 without exaggeration has been assumed to calculate the efficiencies of Cu removal from the melt into the fluid.On the basis of the calculation,8.93×106tons out of the total 1.25×107tons of Cu in the melt could be extracted from the melt into the fluid,showing the efficiency of removal to be about 71.5%.This shows the extent of Cu partition between the fluid and silicate melt phases,which should play a dominant controlling role for the formation of porphyry Cu deposits.Such calculations,while representing a simplification of nature’s processes,suggest that a partition coefficient may have a significant influence on the behavior of copper in magmatic systems.
6 Conclusions
The results of our experiments have shown that the Cu partition coefficients show a positive correlation with the concentration of HCl in the starting fluid.Furthermore,the composition of a melt evidently constrains the Cu partitioning between the granitic silicate melt and coexisting aqueous fluid.Cu partition coefficients are increased with the increases of Na/K and Al/Alk mole ratios in the melt,indicating that Cu is more favorable to be partitioned into the fluid phase in the peraluminous granitic melt(especially Na-rich)-fluid system compared to the peralkaline and K-rich granitic melt-fluid system.
References
Audétat A,Pettke T(2003)The magmatic-hydrothermal evolution of two barren granites:a melt and fluid inclusion study of the Rito del Medio and Canada Pinabete plutons in northern New Mexico(USA).Geochim Cosmochim Acta 67(1):97-121
Audétat A,Günther D,Heinrich CA(2000)Causes for large-scale metal zonation around mineralized plutons:fluid inclusion LAICP-MS evidence from the Mole Granite,Australia.Econ Geol 95(8):1563-1581
Bai TB,Koster van Groos AF(1999)The distribution of Na,K,Rb,Sr,Al,Ge,Cu,W,Mo,La,and Ce between granitic melts and coexisting aqueous fluids.Geochim Cosmochim Acta 63(7):1117-1131
Barnes HL(1997)Geochemistry of hydrothermal ore deposits,vol 1. Wiley,New York
Bodnar RJ,Burnham CW,Sterner SM (1985)Synthetic fluid inclusions in natural quartz.III.Determination of phase equilibrium properties in the system H2O-NaCl to 1000°C and 1500 bars.Geochim Cosmochim Acta 49(9):1861-1873
Bureau H,Keppler H,Métrich N (2000)Volcanic degassing of bromine and iodine:experimental fluid/melt partitioning data and applications to stratospheric chemistry.Earth Planet Sci Lett 183(1):51-60
Candela PA(1997)A review of shallow,ore-related granites: textures,volatiles,and ore metals.J Petrol 38(12):1619-1633
Candela PA,Holland HD(1984)The partitioning of copper and molybdenum between silicate melts and aqueous fluids. Geochim Cosmochim Acta 48(2):373-380
Candela PA,Piccoli PM(1995)Model ore-metal partitioning from melts into vapor and vapor/brine mixtures.Magmas Fluids Ore Depos 23:101-127
Chen SQ(1989)Experimental study on geochemistry of copper and molybdenum.Dissertation,Institute of Geochemistry,Chinese Academy of Sciences(in Chinese)
Chou IM(1987)Oxygen buffer and hydrogen sensor techniques at elevated pressures and temperatures.Hydrotherm Exp Tech,61-99
Farges F,Ponader CW,Brown GE(1991)Structural environments of incompatible elements in silicate glass/melt systems:I.Zirconium at trace levels.Geochim Cosmochim Acta 55(6):1563-1574
Farges F,Ponader CW,Calas G,Brown GE(1992)Structural environments of incompatible elements in silicate glass/melt systems:II.U IV,U V,and U VI.Geochim Cosmochim Acta 56(12):4205-4220
Frank MR,Candela PA,Piccoli PM(2003)Alkali exchange equilibria between a silicate melt and coexisting magmatic volatile phase: an experimental study at 800°C and 100 MPa.Geochim Cosmochim Acta 67(7):1415-1427
Frank MR,Simon AC,Pettke T,Candela PA,Piccoli PM(2011)Gold and copper partitioning in magmatic-hydrothermal systems at 800°C and100 MPa.Geochim Cosmochim Acta 75(9):2470-2482
Halter WE,Webster JD (2004)The magmatic to hydrothermal transition and its bearing on ore-forming systems.Chem Geol 210(1):1-6
Hamilton DL,Henderson CMB(1968)The preparation of silicate compositions by a gelling method.Miner Mag 36(282):832-838
Hedenquist JW,Lowenstern JB(1994)The role of magmas in the formation of hydrothermal ore deposits.Nature 370(6490):519-527
Heinrich CA,Günther D,Audétat A,Ulrich T,Frischknecht R(1999)Metal fractionation between magmatic brine and vapor,determined by microanalysis of fluid inclusions.Geology 27(8):755-758
Holland HD(1972)Granites,solutions,and base metal deposits.Econ Geol 67(3):281-301
Hou ZQ,Mo XX,Gao YF,Qu XM,Meng XJ(2003)Adakite,a possible host rock for porphyry copper deposits:case studies of porphyry copper belts in Tibetan plateau and in Northern Chile.Miner Depos 22(1):1-12(in Chinese with English abstract)
Hu X,Bi X,Hu R,Shang L,Fan W(2008)Experimental study on tin partition between granitic silicate melt and coexisting aqueous fluid.Geochem J 42(2):141-150
Huheey JE,Keiter EA,Keiter RL,Medhi OK(2006)Inorganic chemistry:principles of structure and reactivity.Pearson Education India,New Delhi
Kamenetsky VS,Naumov VB,Davidson P,Van Achterbergh E,Ryan CG(2004)Immiscibility between silicate magmas and aqueous fluids:a melt inclusion pursuit into the magmatic-hydrothermal transition in the Omsukchan Granite(NE Russia).Chem Geol 210(1):73-90
Keppler H,Wyllie PJ(1991)Partitioning of Cu,Sn,Mo,W,U,and Th between melt and aqueous fluid in the systems haplogranite-H2O-HCl and haplogranite-H2O-HF.Contrib Miner Petrol 109(2):139-150
Khitarov NI,Malinin SD,Lebedev EB,Shibieva NP(1982)Partition of Zn,Cu,Pb and Mo between the fluid phase and silicate melt of granitic composition under high-temperature and pressure. Geokhimiya 8:1094-1107
Qi Liang,Hu Jing,Gregoire DC(2000)Determination of trace elements in granites by inductively coupled plasma mass spectrometry.Talanta 51(3):507-513
Lowenstern JB(1993)Evidence for a copper-bearing fluid in magma erupted at the Valley of Ten Thousand Smokes,Alaska.Contrib Miner Petrol 114(3):409-421
Lowenstern JB,Mahood GA,Rivers ML,Sutton SR(1991)Evidence for extreme partitioning of copper into a magmatic vapor phase. Science 252(5011):1405-1409
Pokrovski GS,Borisova AY,Bychkov AY(2013)Speciation and transport of metals and metalloids in geological vapors.Rev Miner Geochem 76(1):165-218
Robb L(2013)Introduction to ore-forming processes.Wiley,London
Rusk BG,Reed MH,Dilles JH,Klemm LM,Heinrich CA(2004)Compositions of magmatic hydrothermal fluids determined by LA-ICP-MS of fluid inclusions from the porphyry coppermolybdenum deposit at Butte,MT.Chem Geol 210(1):173-199
Shinohara H(1994)Exsolution of immiscible vapor and liquid phases from a crystallizing silicate melt:implications for chlorine and metal transport.Geochim Cosmochim Acta 58(23):5215-5221
Sillitoe RH(1979)Some thoughts on gold-rich porphyry copper deposits.Miner Depos 14(2):161-174
Sillitoe RH(1989)Gold deposits in western Pacific island arcs:the magmatic connection.Econ Geol Monogr 6:274-291
Sillitoe RH(2010)Porphyry copper systems.Econ Geol 105(1):3-41
Simon AC,Frank MR,Pettke T,Candela PA,Piccoli PM,Heinrich CA(2005)Gold partitioning in melt-vapor-brine systems. Geochim Cosmochim Acta 69(13):3321-3335
Simon AC,Pettke T,Candela PA,Piccoli PM,Heinrich CA(2006)Copper partitioning in a melt-vapor-brine-magnetite-pyrrhotite assemblage.Geochim Cosmochim Acta 70(22):5583-5600
Simon AC,Frank MR,Pettke T,Candela PA,Piccoli PM,Heinrich CA,Glascock M (2007)An evaluation of synthetic fluid inclusions for the purpose of trapping equilibrated,coexisting,immiscible fluid phases at magmatic conditions.Am Miner 92(1):124-138
Sinclair WD(2007)Porphyry deposits.Miner Depos Can 5:223-243
Tang QL(2003)Experimental research on the partitioning coefficients of copper between silicate melts and liquid coexisting. Dissertation,Institute of Geochemistry,Chinese Academy of Sciences(in Chinese with English abstract)
Tattitch BC,Candela PA,Piccoli PM,Bodnar RJ(2015)Copper partitioning between felsic melt and H2O-CO2bearing saline fluids.Geochim Cosmochim Acta 148:81-99
Taylor JR,Wall VJ,Pownceby MI(1992)The calibration and application of accurate redox sensors.Am Miner 77(3-4):284-295
Tuttle OF,Bowen NL(1958)Origin of granite in the light of experimental studies in the system NaAlSi3O8-KAlSi3O8-SiO2-H2O.Geol Soc Am Mem 74:1-146
Ulrich T,Guenther D,Heinrich CA(1999)Gold concentrations of magmatic brines and the metal budget of porphyry copper deposits.Nature 399(6737):676-679
Vikent’ev IV,Borisova AY,Karpukhina VS,Naumov VB,Ryabchikov ID(2012)Direct data on the ore potential of acid magmas of the Uzel’ginskoe ore field(Southern Urals,Russia).In:Doklady earth sciences,vol.443,No.1.MAIK Nauka/Interperiodica,pp.401-405
Wang D(2013)The character of volatile abundances and its constraint to the metallogenesis of Cenozoic alkaline-rich magma in the Jinshajiang-Red-River belt.Dissertation,Institute of Geochemistry,Chinese Academy of Sciences(in Chinese with English abstract)
Webster JD(1990)Partitioning of F between H2O and CO2fluids and topaz rhyolite melt.Contrib Miner Petrol 104(4):424-438
Webster JD(1992a)Fluid-melt interactions involving Cl-rich granites:experimental study from 2 to 8 kbar.Geochim Cosmochim Acta 56(2):659-678
Webster JD(1992b)Water solubility and chlorine partitioning in Clrich granitic systems:effects of melt composition at 2 kbar and 800°C.Geochim Cosmochim Acta 56(2):679-687
Williams TJ,Candela PA,Piccoli PM (1995)The partitioning of copper between silicate melts and two-phase aqueous fluids:an experimental investigation at 1 kbar,800°C and 0.5 kbar,850°C.Contrib Miner Petrol 121(4):388-399
Zajacz Z,Halter WE,Pettke T,Guillong M(2008)Determination of fluid/melt partition coefficients by LA-ICPMS analysis of coexisting fluid and silicate melt inclusions:controls on element partitioning.Geochim Cosmochim Acta 72(8):2169-2197
Zajacz Z,Candela PA,Piccoli PM,Wälle M,Sanchez-Valle C(2012)Gold and copper in volatile saturated mafic to intermediate magmas:Solubilities,partitioning,and implications for ore deposit formation.Geochim Cosmochim Acta 91:140-159
Zhang BD(1992)Physical chemic of granite related to uranium ore forming.Atomic Energy Press,Beijing,pp 9-18(in Chinese)
Zhou Q(2011)Petrogenesis and metallogeny for the dexing porphyry copper deposits.Dissertation,Nanjing University(in Chinese with English abstract)
1 February 2015/Revised:9 April 2016/Accepted:6 June 2016/Published online:9 August 2016
©Science Press,Institute of Geochemistry,CAS and Springer-Verlag Berlin Heidelberg 2016
杂志排行
Acta Geochimica的其它文章
- Element geochemical characteristics of a soil profile developed on dolostone in central Guizhou,southern China:implications for parent materials
- Dissipation of polycyclic aromatic hydrocarbons in crop soils amended with oily sludge
- Geochemistry of sedimentary rocks from Permian-Triassic boundary sections of Tethys Himalaya:implications for paleo-weathering,provenance,and tectonic setting
- Standard-sample bracketing calibration method combined with Mg as an internal standard for silicon isotopic compositions using multi-collector inductively coupled plasma mass spectrometry
- Study on oil-source correlation by analyzing organic geochemistry characteristics:a case study of the Upper Triassic Yanchang Formation in the south of Ordos Basin,China
- The genetic relationship between Habo alkaline intrusion and its surrounding deposits,Yunnan Province,China:geological and S-Pb isotopic evidences
