氧化鱼粉对肉仔鸡生长性能、消化道结构和功能的影响
2016-11-15廖瑞波闫海洁刘国华常文环吝常华黄向阳蔡辉益
廖瑞波 闫海洁 张 姝 刘国华 常文环 刘 伟 吝常华 黄向阳 蔡辉益
(中国农业科学院饲料研究所,农业部饲料生物技术重点开放实验室,北京100081)
氧化鱼粉对肉仔鸡生长性能、消化道结构和功能的影响
廖瑞波闫海洁*张姝刘国华常文环刘伟吝常华黄向阳蔡辉益**
(中国农业科学院饲料研究所,农业部饲料生物技术重点开放实验室,北京100081)
本试验旨在探索氧化的鱼粉蛋白质对肉仔鸡生长性能、消化道结构和功能的影响。选用健康、体重相近的1日龄肉公鸡180只,随机分为3个组,每组6个重复,每个重复10只鸡。3个组分别饲喂对照饲粮(CON组)、含2%正常鱼粉饲粮(FM组)和含2%氧化鱼粉饲粮(OFM组),试验期21 d。结果表明:1)与CON组与FM组相比,OFM组显著降低肉仔鸡体重和平均日增重(P<0.05),显著提高肉仔鸡料重比(P<0.05);2)与CON组与FM组相比,OFM组肉仔鸡出现持续的腹泻症状,且14和21日龄肉仔鸡粪便pH显著降低(P<0.05),粪便中水分含量显著提高(P<0.05);3)对于21日龄肉仔鸡回肠的绒毛高度、隐窝深度和绒毛高度/隐窝深度,3个组间无显著差异(P>0.05);4)肉仔鸡嗉囊中谷胱甘肽、氧化型谷胱甘肽、脂质过氧化物和丙二醛含量和谷胱甘肽/氧化型谷胱甘肽值在3个组间无显著差异(P>0.05),而OFM组与CON组和FM组相比显著提高了肉仔鸡回肠中脂质过氧化物和丙二醛含量(P<0.05)。结果提示,氧化鱼粉对肉仔鸡回肠形态结构没有显著影响,但显著破坏了回肠的氧化还原平衡状态,造成肉仔鸡腹泻,影响消化道的功能,显著降低早期肉仔鸡的生长性能。
肉仔鸡;氧化鱼粉;消化道;生长性能
鱼粉在加工和储藏期间发生的抗氧化和促氧化反应对鱼粉品质有重要影响[1]。鱼粉中的脂质氧化造成的高温对蛋白质品质有毒害作用[2],但脂质氧化本身对鱼粉蛋白质品质影响方面的研究较少[3]。脂质过氧化物(LPO),如羟基壬烯酸、丙二醛(MDA)和丙烯醛可以与赖氨酸、组氨酸和半胱氨酸残基反应[4-6],更重要的是氧化反应是一系列链式反应,反应产生的自由基可能直接攻击蛋白质。而蛋白质氧化定义为由自由基直接诱导的或由氧化反应次级产物诱导的蛋白质共价修饰[7]。
鱼粉富含不饱和脂肪酸,容易发生氧化性酸败,脂肪的酸败加速了蛋白质的变质[8]。低脂肪鱼和高脂肪鱼在温度为40~115 ℃下加热20 min,蛋白质中自由巯基(—SH)含量线性降低,同时二硫键(S—S)含量增加[9]。鱼粉即便是在低温储藏(低于0 ℃)条件下,也出现巯基含量降低的情况[10]。虽然如此,巯基氧化转化为二硫键需要温度高于50 ℃,95 ℃时反应迅速,在20 min后达到平衡状态[9]。蛋白质内部的巯基转换为二硫键形成交联会产生不溶性的蛋白质胶体结构[11],而蛋白质的生化结构[自由巯基含量、巯基/(巯基+二硫键)值]的改变会造成饲粮中蛋白质消化率的改变[12]。Tang等[13]报道,氧化的大豆蛋白产生自由基,会降低大鼠消化道的抗氧化状态,导致氧化还原状态失衡。Wu等[14]报道,氧化的大豆蛋白降低肉仔鸡前段消化道中多种酶的活性。故推测氧化的饲料蛋白质可能引发肉仔鸡消化道功能改变,影响生长性能。而鱼粉富含不饱和脂肪酸,在加工和储藏过程中易发生氧化性酸败[1],加速鱼粉蛋白质的变质[2]。Timm-Heinrich等[10]也认为鱼粉中的油脂可能会诱导蛋白质氧化,发生共价修饰[7]。因此,本试验旨在通过研究氧化鱼粉对肉仔鸡生长性能、消化道结构和功能的影响,探究氧化鱼粉对肉仔鸡不利影响的机理,为生产实践中缓解这种不利影响提供理论基础。
1 材料与方法
1.1试验材料
本研究以国产鱼粉为基础试验材料,参照Lagrain等[15]报道的方法,按每20 g鱼粉放入100 mL含有8 mL/L双氧水的蒸馏水的比例,95 ℃加热1 h,之后自然晾晒风干粉碎制成。试验中使用的正常鱼粉和氧化鱼粉为相同种类鱼粉。
1.2试验设计
选用健康体重相近[(37.47±0.77) g]1日龄爱拔益加肉公鸡(北京华都肉鸡公司提供)180只,采用单因素完全随机设计,将试验肉仔鸡分为3个组,每组6个重复,每个重复10只鸡。对照(CON)组、鱼粉(FM)组和氧化鱼粉(OFM)组分别饲喂对照饲粮、含2%正常鱼粉的饲粮和含2%氧化鱼粉的饲粮。饲粮营养水平参照Aviagen公司品种推荐标准,饲粮组成及营养水平见表1。

表1 饲粮组成及营养水平(风干基础)
1)预混料为千克饲粮提供 The premix provided the following per kg of diets:VA 12 500 IU,VD33 500 IU,VE 25 mg,VB13.5 mg,VB28.5 mg,VB65 mg,VB120.03 mg,VK32.5 mg,烟酸 nicotinic acid 30 mg,D-泛酸D-pantothenic acid 15 mg,叶酸 folic acid 1.0 mg,生物素 biotin 0.1 mg,Zn (as zinc sulfate) 110 mg,Mn (as manganese sulfate) 110 mg,Fe (as ferrous sulfate) 80 mg,Cu (as copper sulfate) 8 mg,I (as potassium iodide) 0.35 mg,Se (as sodium selenite) 0.15 mg。
2)粗蛋白质为测定值,其余为计算值。Crude protein was a measured value, while the others were calculated values.
1.3饲养管理
试验期为21 d,所有肉鸡自由采食和饮水,采用层笼饲养,光照制度采用1 h(黑暗)/23 h(灯光)。人工控温,育雏期前4 d舍内温度控制在33 ℃左右,以后每周降低2 ℃,直至22 ℃。7日龄免疫新支二联苗(滴鼻点眼),14日龄免疫法氏囊疫苗(饮水)。随时观察、记录鸡只的采食和健康状况。
1.4样品采集和指标测定
1.4.1鱼粉中自由巯基含量
巯基含量测定采用Ellman法,具体操作参照Beveridge等[16]、Buttkus[17]报道的方法。
1.4.2生长性能
试验期间每日记录鸡只健康状况。21日龄07:00以重复为单位称量试验鸡的空腹重。根据试验记录,以重复为单位计算体重、平均日增重(ADG)、平均日采食量(ADFI)和料重比(F/G)。
1.4.3粪便pH和水分含量
收集14和21日龄新鲜粪便,混匀,测定pH(Testo 206便携式pH计)和水分含量。
1.4.4回肠形态,嗉囊和回肠氧化状态指标
21日龄时每个重复按照平均体重选取1只鸡,电击处死,打开腹腔,取2段2 cm左右的回肠和3 cm2的嗉囊样品。
一段回肠组织用生理盐水冲洗肠道,浸入4%的甲醛溶液固定,固定后做石蜡切片。将已固定好的组织进行修整、脱水、包埋、切片和苏木精-伊红(HE)染色制成切片,100×光镜下用显微测微尺测定绒毛高度、隐窝深度,并计算绒毛高度/隐窝深度(V/C)值。
嗉囊和另外一段回肠组织称重、研磨制成组织匀浆液。利用紫外分光光度计(赛默飞尔科技公司,Nano Drop 2000)测定组织中总蛋白用于生化指标分析。组织中谷胱甘肽(GSH)和氧化型谷胱甘肽(GSSG)含量分别使用南京建成生物工程研究所A061和A061-1试剂盒测定,方法参考Tietze[18],两者的差值为还原型谷胱甘肽;MDA含量使用南京建成生物工程研究所A003-1试剂盒测定,方法为硫代巴比妥酸(TBA)法,参考Todorova等[19];LPO含量采用TBA法,使用南京建成生物工程研究所A106试剂盒测定。
1.5数据统计与分析
所有试验数据使用Excel 2007进行整理,使用SPSS 19.0统计软件进行单因素方差分析(one-way ANOVA),组间平均值采用LSD多重比较进行差异显著性检验。数据采用平均值±标准误来表示,以P<0.05为差异显著。
2 结 果
2.1鱼粉中自由巯基含量
经测定,正常鱼粉中自由巯基含量为31.11 μmol/g,使用8 mL/L双氧水加工制成的氧化鱼粉中自由巯基含量为13.32 μmol/g,自由巯基含量降低了57.18%。
2.2氧化鱼粉对肉仔鸡生长性能的影响
氧化鱼粉对肉仔鸡生长性能的影响见表2。对1~21日龄肉仔鸡而言,3个组间ADFI无显著差异(P>0.05)。CON和FM 2个组间肉仔鸡体重、ADG和F/G无显著差异(P>0.05)。与CON组和FM组相比,OFM组的肉仔鸡体重、ADG显著降低(P<0.05),而F/G显著提高(P<0.05)。
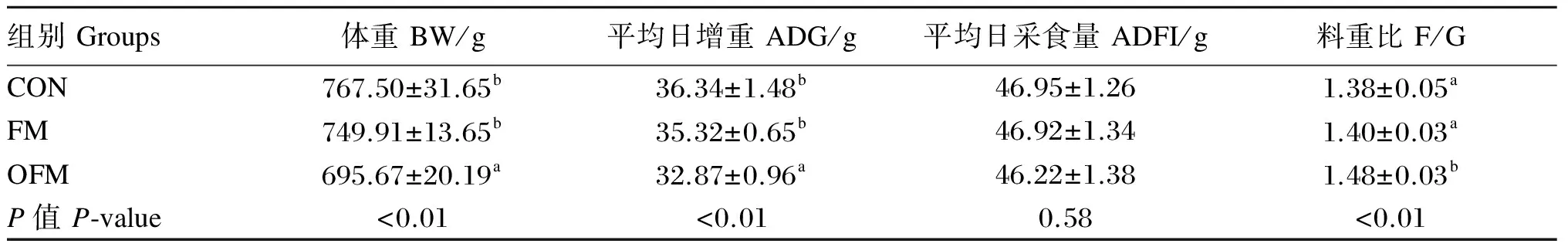
表2 氧化鱼粉对肉仔鸡生长性能的影响
同列数据肩标相同字母或无字母表示差异不显著(P>0.05),不同字母表示差异显著(P<0.05)。下表同。
In the same column, values with the same or no letter superscripts mean no significant difference (P>0.05), while with different letter superscripts mean significant difference (P<0.05). The same as below.
2.3氧化鱼粉对肉仔鸡粪便pH和水分含量的影响
氧化鱼粉对肉仔鸡粪便pH和水分含量的影响见表3。与CON组和FM组相比,OFM组的粪便pH显著降低(P<0.05),水分含量显著提高(P<0.05)。且饲喂含氧化鱼粉的饲粮后,14和21日龄肉仔鸡粪便的水分含量分别为77.88%和75.93%。

表3 氧化鱼粉对肉仔鸡粪便pH和水分含量的影响
2.4氧化鱼粉对肉仔鸡回肠形态的影响
如表4所示,对21日龄肉仔鸡回肠绒毛高度,隐窝深度和V/C值而言,3个组间无显著差异(P>0.05)。
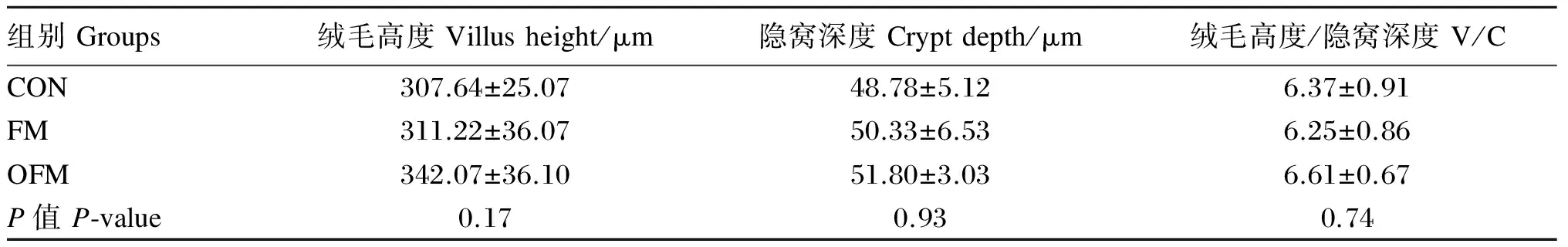
表4 氧化鱼粉对肉仔鸡回肠形态的影响
2.5氧化鱼粉对嗉囊、回肠氧化状态的影响
如表5所示,与CON组和FM组相比,OFM组未显著影响21日龄肉仔鸡嗉囊中的GSH、GSSG含量和GSH/GSSG值以及LPO和MDA含量(P>0.05)。与其他2种饲粮相比,饲喂正常鱼粉饲粮肉仔鸡回肠中GSH含量显著提高(P<0.05)。与CON组和FM组相比,OFM组肉仔鸡回肠中LPO和MDA含量显著提高(P<0.05)。

表5 氧化鱼粉对肉仔鸡嗉囊和回肠氧化状态的影响
3 讨 论
3.1鱼粉中自由巯基含量
自由巯基转换成二硫键是蛋白质氧化过程中的显著变化,可以作为检测蛋白质氧化的指标。同时Ellman法是测定蛋白质中自由巯基含量广泛采用的方法,因此本研究采用Ellman法测定氧化前后鱼粉中自由巯基含量。研究表明,即便是低温储藏(低于0 ℃),鱼粉也会出现巯基含量降低的情况[10]。低脂肪鱼和高脂肪鱼在温度为40~115 ℃下加热20 min,蛋白质中自由巯基含量线性降低,二硫键含量增加[9]。自由巯基含量可以用于评判饲料或原料质量的高低。Sunde等[20]报道高品质的饲料蛋白质中自由巯基含量显著高于低品质饲料中相应的含量。Aslaksen等[21]报道,鱼粉饲粮中二硫键含量在23.3~32.7 nmol/mg prot。而Timm-Heinrich等[10]发现初始鱼粉中巯基含量在16.5~17.3 μmol/g,低温条件下存储12 d后巯基含量为12.9~14.9 μmol/g。而本试验中正常鱼粉中巯基含量为31.11 μmol/g,氧化后自由巯基含量为13.32 μmol/g,与前人研究结果差别可能是由于用于加工鱼粉的鱼种类的差异。此外,自由巯基的氧化仅是蛋白质中氨基酸侧链氧化中的一种,除侧链氧化外,蛋白质氧化也可能造成蛋白质骨架的氧化或蛋白质碎片化[22]。
3.2氧化鱼粉对肉仔鸡生长性能的影响
蛋白质的氧化修饰诱导蛋白质一系列结构性改变[23],会降低蛋白的品质和消化率[24-26],造成其营养价值的损失[27]以及生物利用率的降低[28],这些因素均是肉仔鸡生长性能降低的原因。Laohabanjong等[29]发现与高度酸败鱼粉[硫代巴比妥酸反应物(TBARS)=62.31 mg/kg]相比,中度酸败鱼粉(TBARS=22.52 mg/kg)显著降低黑虎虾最终体重,日增重和生长速率最低。该结果暗示影响生长性能重要的因素可能并非脂质过氧化,而可能是脂质过氧化造成的蛋白质氧化。本研究中饲喂氧化鱼粉的肉仔鸡体重和ADG显著降低,F/G显著提高。Wu等[14]发现经过高温烘烤的分离大豆蛋白,其巯基含量显著降低,而饲喂烘烤的大豆蛋白造成肉仔鸡平均体重显著降低,这与本试验结果相一致。
3.3氧化鱼粉对肉仔鸡粪便pH和水分含量的影响
医学研究表明,粪便的外观和一致性代表了一个重要的症状学线索[30],可以用于疾病的诊断。参照Ogunji等[31]报道的粪便视觉评分,氧化鱼粉组粪便疏松,有少量圆锥形粪便;该组粪便水分含量高于75%,尽管未观察到游离水的存在,但该组肉仔鸡表现出持续的腹泻症状。而Engberg等[32]用氧化的植物油(9%的菜籽油,2%的大豆油,均为156 mEq O2/kg)饲喂肉仔鸡,并未观察到腹泻症状。Yuan等[33]用氧化鱼油(786.5 mEq O2/kg)饲喂断奶仔猪,也未报道动物出现腹泻症状。因此,氧化鱼粉是诱导肉仔鸡腹泻的因素。而肠道完整性受损伤也会造成水分重吸收量的降低,进一步造成腹泻。
本研究中,OFM组肉仔鸡粪便pH显著降低。微生物的代谢产物(乳酸、乙酸、丙酸和丁酸)是降低肠道黏膜pH[34]和粪便pH[35]的主要原因。通常认为中度酸性的粪便是正常的,而粪便过度酸性可能暗示消化道的某些病变[36]。断奶仔猪的研究表明,粪便的pH与腹泻有关联[37],并通常将粪便pH的降低作为渗透性腹泻的诊断指标[38]。家禽方面,尚无类似报道,因此无法依据粪便pH判定腹泻类型。但粪便表观状态、水分含量、pH的结果表明氧化鱼粉造成肉仔鸡消化道的功能障碍。
3.4氧化鱼粉对肉仔鸡回肠形态的影响
诸多研究将饲粮成分对肠道形态(绒毛高度、隐窝深度和V/C值)的影响作为评价动物健康的一项指标[39-42],甚至有研究表明消化道局部或系统性炎症反应与小肠绒毛高度有关联[43-44]。尽管2%的氧化鱼粉显著影响前期肉仔鸡的生长性能,但回肠形态结构并未受到氧化鱼粉的影响。前人研究报道认为,蛋白质氧化对消化道结构有损伤作用。如Chen等[26]报道,100 ℃加热8 h的大豆蛋白显著降低前段消化道和回肠的相对重量,氧化的蛋白质可能会对肉仔鸡消化道和器官的发育有负面影响;Xie等[45]报道蛋白质氧化的终极产物诱导炎性肠病(IBD)病人的肠道组织损伤。
3.5氧化鱼粉对嗉囊、回肠氧化状态的影响
消化道指胃肠道,包括前段的消化器官和后段的肠道。嗉囊是肉鸡体内微生物活动重要的器官之一[46-47],同时在肉鸡采食3 h后,约1/2的食物会停留在嗉囊中[48]。因此除回肠外,本试验也观察氧化鱼粉对嗉囊的影响,研究结果显示氧化鱼粉对嗉囊中氧化状态无显著影响,而显著提高前期肉仔鸡回肠的中LPO和MDA的含量。
对肉仔鸡而言,消化道的抗氧化能力在维持消化道正常功能方面也发挥着重要作用[49]。本研究结果表明,氧化鱼粉诱导的消化道应激状态,破坏了肉仔鸡消化道中氧化还原平衡。这与前人使用氧化的饲料原料得到的研究结果一致,Tang等[13]报道氧化的大豆蛋白造成大鼠消化道中氧化平衡状态破坏,降低抗氧化能力;Yuan等[33]报道,氧化鱼油诱导断奶仔猪氧化应激,造成血清和肝脏中抗氧化酶活性降低,MDA含量提高。Ferretti等[50]报道,进入消化道上皮细胞的面筋蛋白抗原片段通过激活核转录因子-κB(NF-κB),诱导促炎症细胞因子和环氧化酶2(COX2)、诱导型一氧化氮合酶(iNOS)的转录,造成前列腺素和一氧化氮(NO)代谢产物大量产生,最终导致上皮细胞的氧化应激。Xie等[45]也报道蛋白质氧化的终极产物通过氧化介导的通路诱导人消化道上皮细胞死亡。结合回肠形态观察的结果,2%的氧化鱼粉仅改变了肉仔鸡回肠中的氧化平衡状态,并未造成回肠上皮细胞的损伤。
4 结 论
氧化鱼粉对肉仔鸡回肠形态结构没有显著影响,但显著破坏了回肠的氧化还原平衡状态,造成肉仔鸡腹泻,影响消化道的功能,显著降低早期肉仔鸡的生长性能。
[2]LAKSESVELA B.Protein value and amino-acid balance of condensed herring solubles and spontaneously heated herring meal.Chick experiments[J].The Journal of Agricultural Science,1958,51(2):164-176.
[3]OPSTVEDT J.Influence of residual lipids on the nutritive value of fish meal:Ⅶ.Effect of lipid oxidation on protein quality of fish meal[J].Acta Agriculturae Scandinavica,1975,25(1):53-71.
[4]REQUENA J R,FU M X,AHMED M U,et al.Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoprotein[J].Biochemical Journal,1997,322(1):317-325.
[5]KIM J G,SABBAGH F,SANTANAM N,et al.Generation of a polyclonal antibody against lipid peroxide-modified proteins[J].Free Radical Biology and Medicine,1997,23(2):251-259.
[6]UCHIDA K,STADTMAN E R.Quantitation of 4-hydroxynonenal protein adducts[J].Methods in Enzymology,1994,233:371-380.
[7]SHACTER E.Quantification and significance of protein oxidation in biological samples[J].Drug Metabolism Reviews,2000,32(3/4):307-326.
[8]张磊.鱼粉特性的研究[D].硕士学位论文.无锡:江南大学,2008:74.
[9]OPSTVEDT J,MILLER R,HARDY R W,et al.Heat-induced changes in sulfhydryl groups and disulfide bonds in fish protein and their effect on protein and amino acid digestibility in rainbow trout (Salmogairdneri)[J].Journal of Agricultural and Food Chemistry,1984,32(4):929-935.
[10]TIMM-HEINRICH M,EYMARD S,BARON C P,et al.Oxidative changes during ice storage of rainbow trout (Oncorhynchusmykiss) fed different ratios of marine and vegetable feed ingredients[J].Food Chemistry,2013,136(3/4):1220-1230.
[11]HWANG D C,DAMODARAN S.Synthesis and properties of fish protein-based hydrogel[J].Journal of the American Oil Chemists’ Society,1997,74(9):1165-1171.
[12]RUNGRUANGSAK-TORRISSEN K,RUSTAD A,SUNDE J,et al.Invitrodigestibility based on fish crude enzyme extract for prediction of feed quality in growth trials[J].Journal of the Science of Food and Agriculture,2002,82(6):644-654.
[13]TANG X,WU Q P,LE G W,et al.Effects of heat treatment on structural modification andinvivoantioxidant capacity of soy protein[J].Nutrition,2012,28(11/12):1180-1185.
[14]WU D W,CHEN X,YANG X,et al.Effects of heat treatment of soy protein isolate on the growth performance and immune function of broiler chickens[J].Poultry Science,2014,93(2):326-334.
[15]LAGRAIN B,THEWISSEN B G,BRIJS K,et al.Mechanism of gliadin-glutenin cross-linking during hydrothermal treatment[J].Food Chemistry,2008,107(2):753-760.
[16]BEVERIDGE T,TOMA S J,NAKAI S.Determination of SH- and SS-groups in some food proteins using Ellman’s reagent[J].Journal of Food Science,1974,39(1):49-51.
[17]BUTTKUS H.The sulfhydryl content of rabbit and trout myosins in relation to protein stability[J].Canadian Journal of Biochemistry,1971,49(1):97-107.
[18]TIETZE F.Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione:applications to mammalian blood and other tissues[J].Analytical Biochemistry,1969,27(3):502-522.
[19]TODOROVA I,SIMEONOVA G,KYUCHUKOVA D,et al.Reference values of oxidative stress parameters (MDA,SOD,CAT) in dogs and cats[J].Comparative Clinical Pathology,2005,13(4):190-194.
[20]SUNDE J,EIANE S A,RUSTAD A,et al.Effect of fish feed processing conditions on digestive protease activities,free amino acid pools,feed conversion efficiency and growth in Atlantic salmon (SalmosalarL.)[J].Aquaculture Nutrition,2004,10(4):261-277.
[21]ASLAKSEN M A,ROMARHEIM O H,STOREBAKKEN T,et al.Evaluation of content and digestibility of disulfide bonds and free thiols in unextruded and extruded diets containing fish meal and soybean protein sources[J].Animal Feed Science and Technology,2006,128(3/4):320-330.
[22]BERLETT B S,STADTMAN E R.Protein oxidation in aging,disease,and oxidative stress[J].Journal of Biological Chemistry,1997,272(33):20313-20316.
[23]HEADLAM H A,DAVIES M J.Markers of protein oxidation:different oxidants give rise to variable yields of bound and released carbonyl products[J].Free Radical Biology and Medicine,2004,36(9):1175-1184.
[24]GATELLIER P,SANTÉ-LHOUTELLIER V,PORTANGUEN S,et al.Use of meat fluorescence emission as a marker of oxidation promoted by cooking[J].Meat Science,2009,83(4):651-656.
[25]PROMEYRAT A,GATELLIER P,LEBRET B,et al.Evaluation of protein aggregation in cooked meat[J].Food Chemistry,2010,121(2):412-417.
[26]CHEN X,CHEN Y P,WU D W,et al.Effects of heat-oxidized soy protein isolate on growth performance and digestive function of broiler chickens at early age[J].Asian-Australasian Journal of Animal Sciences,2015,28(4):544.
[27]TIRONI V A,TOMS M C,AN M C.Lipid and protein deterioration during the chilled storage of minced sea salmon (Pseudopercissemifasciata)[J].Journal of the Science of Food and Agriculture,2007,87(12):2239-2246.
[28]SAEED S,GILLIES D,WAGNER G,et al.ESR and NMR spectroscopy studies on protein oxidation and formation of dityrosine in emulsions containing oxidised methyl linoleate[J].Food and Chemical Toxicology,2006,44(8):1385-1392.
[29]LAOHABANJONG R,TANTIKITTI C,BENJAKUL S,et al.Lipid oxidation in fish meal stored under different conditions on growth,feed efficiency and hepatopancreatic cells of black tiger shrimp (Penaeusmonodon)[J].Aquaculture,2009,286(3/4):283-289.
[30]RIEGLER G,ESPOSITO I.Bristol scale stool form.A still valid help in medical practice and clinical research[J].Techniques in Coloproctology,2001,5(3):163-164.
[31]OGUNJI P A,BREWER R N,ROLAND D A,et al.Effect of dietary sodium chloride,protein,and strain difference upon water consumption and fecal moisture content of broiler breeder males[J].Poultry Science,1983,62(12):2497-2500.
[32]ENGBERG R M,LAURIDSEN C,JENSEN S K,et al.Inclusion of oxidized vegetable oil in broiler diets.Its influence on nutrient balance and on the antioxidative status of broilers[J].Poultry Science,1996,75(8):1003-1011.
[33]YUAN S B,CHEN D W,ZHANG K Y,et al.Effects of oxidative stress on growth performance.Nutrient digestibilities and activities of antioxidative enzymes of weanling pigs[J].Asian-Australasian Journal of Animal Sciences,2007,20(10):1600-1605.
[34]TARAS D,VAHJEN W,MACHA M,et al.Performance,diarrhea incidence,and occurrence ofEscherichiacolivirulence genes during long-term administration of a probioticEnterococcusfaeciumstrain to sows and piglets[J].Journal of Animal Science,2006,84(3):608-617.
[35]HUANG Y,YOO J S,KIM H J,et al.Effects of dietary supplementation with blended essential oils on growth performance.Nutrient digestibility,blood profiles and fecal characteristics in weanling pigs[J].Asian-Australasian Journal of Animal Sciences,2010,23(5):607-613.
[36]OSUKA A,SHIMIZU K,OGURA H,et al.Prognostic impact of fecal pH in critically ill patients[J].Critical Care,2012,16(4):R119.
[37]YOON C P,LOH T C,CHEONG Y H.Effects of organic acids and natural herbs on performance and incidence of diarrhoea in post-weaning pigs[J].Malaysian Journal of Animal Science,2002,7(2):25-30.
[38]FINE K D,SCHILLER L R.AGA technical review on the evaluation and management of chronic diarrhea[J].Gastroenterology,1999,116(6):1464-1486.
[39]BRUNSGAARD G.Effects of cereal type and feed particle size on morphological characteristics,epithelial cell proliferation,and lectin binding patterns in the large intestine of pigs[J].Journal of Animal Science,1998,76(11):2787-2798.
[40]HOWARD M D,GORDON D T,PACE L W,et al.Effects of dietary supplementation with fructooligosaccharides on colonic microbiota populations and epithelial cell proliferation in neonatal pigs[J].Journal of Pediatric Gastroenterology and Nutrition,1995,21(3):297-303.
[41]CHANG W H,LI J J,ZHANG S,et al.Effects of glucocorticoid-induced stress on absorption of glycylsarcosine in jejunum of broilers[J].Poultry Science,2015,94(4):700-705.
[42]LI Y,CAI H Y,LIU G H,et al.Effects of stress simulated by dexamethasone on jejunal glucose transport in broilers[J].Poultry Science,2009,88(2):330-337.
[43]JIANG Z Y,SUN L H,LIN Y C,et al.Effects of dietary glycyl-glutamine on growth performance,small intestinal integrity,and immune responses of weaning piglets challenged with lipopolysaccharide[J].Journal of Animal Science,2009,87(12):4050-4056.
[44]LEE K W,LEE S H,LILLEHOJ H S,et al.Effects of direct-fed microbials on growth performance,gut morphometry,and immune characteristics in broiler chickens[J].Poultry Science,2010,89(2):203-216.
[45]XIE F,SUN S,XU A,et al.Advanced oxidation protein products induce intestine epithelial cell death through a redox-dependent,c-jun N-terminal kinase and poly (ADP-ribose) polymerase-1-mediated pathway[J].Cell Death and Disease,2014,5(1):e1006.
[46]GABRIEL I,LESSIRE M,MALLET S,et al.Microflora of the digestive tract:critical factors and consequences for poultry[J].World’s Poultry Science Journal,2006,62(3):499-511.
[47]WENK C.The role of dietary fibre in the digestive physiology of the pig[J].Animal Feed Science and Technology,2001,90(1/2):21-33.
[48]SVIHUS B,HETLAND H,CHOCT M,et al.Passage rate through the anterior digestive tract of broiler chickens fed on diets with ground and whole wheat[J].British Poultry Science,2002,43(5):662-668.
[49]DENG Y X,CUI H M,PENG X,et al.Dietary vanadium induces oxidative stress in the intestine of broilers[J].Biological Trace Element Research,2012,145(1):52-58.
[50]FERRETTI G,BACCHETTI T,MASCIANGELO S,et al.Celiac disease,inflammation and oxidative damage:a nutrigenetic approach[J].Nutrients,2012,4(4):243-257.
*Contributed equally
**Corresponding author, professor, E-mail: caihuiyi@caas.cn
(责任编辑田艳明)
Effects of Oxidized Fish Meal on Growth Performance, Gut Structure and Functions of Broilers
LIAO RuiboYAN Haijie*ZHANG ShuLIU GuohuaCHANG WenhuanLIU WeiLIN ChanghuaHUANG XiangyangCAI Huiyi**
(Key Laboratory of Feed Biotechnology of Ministry of Agriculture, Feed Research Institute, Chinese Academy of Agricultural Sciences, Beijing 100081, China)
This experiment was conducted to study the effects of oxidized fish meal on the growth performance, gut structure and functions of broilers. A total of 180 male broilers with good health and similar weight were divided into 3 groups with 6 replicates of 10 birds each. The chicks were fed control diet (CON group), containing 2% normal fish meal diet (FM group) and 2% oxidized fish meal diet (OFM group), respectively. The experiment lasted for 21 d. The results were demonstrated that: compared with CON group and FM group, 1) OFM group significantly reduced the body weight and average daily gain (P<0.05), significantly increased the ratio of feed to gain of broilers (P<0.05). 2) OFM group resulted in constantly diarrhea of broilers, significantly decreased the fecal pH (P<0.05), and significantly increased the moisture content of faeces of broilers at 14- and 21-day-old (P<0.05). 3) The villus height, crypt depth and ratio of villus height to crypt depth of ileum of 21-day-old broilers did not significantly differ across three groups (P>0.05). 4) There were no significant differences for contents of glutathione (GSH), oxidized glutathione (GSSG), lipid peroxide (LPO), malondialdehyde (MDA) and ratio of GSH to GSSG in crop among there groups (P>0.05), however, the contents of LPO and MDA were significantly increased in the OFM group (P<0.05). In conclusion, oxidized fish meal can not only induce the imbalance of redox states in ileum, but also induce the diarrhea and influence the gut functions, and significantly decrease the growth performance of broilers in early stage, however there is no significant effects on the ileum morphology.[ChineseJournalofAnimalNutrition, 2016, 28(10):3084-3092]
broilers; oxidized fish meal; gut; growth performance
10.3969/j.issn.1006-267x.2016.10.010
2016-04-05
国家肉鸡产业技术体系(CARS-42)
廖瑞波(1986—),男,河南洛阳人,博士研究生,家禽营养与饲料科学专业。E-mail: liao231@163.com
S831.5
A
1006-267X(2016)10-3084-09
*同等贡献作者
**通信作者:蔡辉益,研究员,博士生导师,E-mail: caihuiyi@caas.cn
