Optimization of Ethanol Extraction of Total Flavonoids from Suaeda salsa and Its Antioxidant, Hypoglycemic and Hypolipidemic Activities
2016-11-14GONGYanlingLIUFeiJINHong
GONG Yanling, LIU Fei, JIN Hong
(Department of Pharmacy, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao 266042, China)
Optimization of Ethanol Extraction of Total Flavonoids from Suaeda salsa and Its Antioxidant, Hypoglycemic and Hypolipidemic Activities
GONG Yanling, LIU Fei, JIN Hong
(Department of Pharmacy, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao 266042, China)
Suaeda salsa an annual herb of Chenopodiaceae Suaeda well accepted as both a folk medicinal and food plant. In the current study, the extraction of total flavonoids from S. salsa and the in vitro antioxidant, hypoglycemic and hypolipidemic activities of the extracts were investigated. The total flavonoids was extracted by refluxing with ethanol and the selection and optimization of main experimental parameters were carried out using single factor experiments combined with central composite design-response surface methodology (CCD-RSM). The total flavonoids extracted from S. salsa were tested for hydroxyl and oxygen radical scavenging activity, and inhibitory activities against α-amylase and lipase in vitro. Results revealed that the optimal conditions for the extraction of total flavonoids were as following: liquid-tosolid ratio, 31.40:1; ethanol concentration, 83.12%; reflux temperature, 90 ℃, and reflux time, 128.40 min. Under these optimized conditions, the predicted and actual values of total flavonoids yield were 0.896% and 0.932%, respectively. The in vitro antioxidant tests revealed that the total flavonoids could scavenge hydroxyl and oxygen radicals and showed inhibitory activity against α-amylase and lipase. The optimal extraction conditions obtained in this experiment are simple to perform and have good reproducibility. The results have demonstrated that the total flavonoids from S. salsa can be used as a potential source of natural antioxidant, hypoglycemic and hypolipidemic ingredients, which provides new pharmacological basis for the development of S. salsa.
Suaeda salsa; total flavonoids; antioxidant activity; hypoglycemic activity; hypolipidemic activity
Suaeda salsa, an annual herb of Chenopodiaceae Suaeda, is mainly distributed in Asia, Europe, northeast,northwest and coastal provinces of China, especially widely growing in sandy and saline alkali soil in coastal beaches. Suadeda salsa is well accepted as a medicinal and food plant in folks owning to its detoxification and digestion promoting activities[1]. The main constitutes of Suadeda salsa include flavonoids, fatty acids, terpenoids, polysaccharides, pigments,and fat soluble components etc.[2-3]. Modern pharmacological study revealed that Suadeda salsa has anti-inflammatory,antioxidant, lipid-lowering and immunity-improving activities[4-5]. An et al.[6]separated compounds from Suadeda salsa which are hepatoprotective against tacrine-induced cytotoxicity in human liver cancer HepG2cells. To date,the domestic and foreign researches mainly focused on the plant physiology and salt tolerance of Suadeda salsa. As a pioneer halophyte, Suadeda salsa possesses more efficient abilities over non-halophytic plants, such as a more efficient antioxidant system, synthesizing more osmo-protectants to keep a favorable water gradient potential and to maintain normal cellular function[7-8]. Similar to most halophytes,Suadeda salsa exhibits physiological mechanisms with heavy metal-tolerant activity[9-10]. Recent researches revealed that Suadeda salsa could tolerate several pollutants, including heavy metals and petroleum hydrocarbons[11-13], which made it possible to be a potential plant in pollution indication around heavy metal-contaminated coast. But few studies focused on the constituents, extraction technology and pharmacology activities to develop its medical values.
In the present study, we aimed to explore the ethanol extraction of total flavonoids from Suaeda salsa and extraction parameters including liquid-to-solid ratio,ethanol concentration, reflux time and reflux temperature. The optimal extraction technique was optimized by central composite design-response surface methodology(CCD-RSM). The in vitro antioxidant, hypoglycemic and hypolipidemic activities of Suaeda salsa extracts were also evaluated.
1 Materials and Methods
1.1Plant material
Suaeda salsa was collected from the gulf of Huanghai Sea in October 2013, washed with distilled water and dried for 72 h with a blowing drier at 60 ℃. Dried Suaeda salsa was then ground to powder with an herb grinder (FW135, Tianjin, China) and subsequently sieved to 20 mesh of particles. Suaeda salsa was identified as the over-ground part of Chenopodiaceae Suaeda (C. Suaeda) annual herb Suaeda glauca Bunge by Chinese medicine professor Jin Hong in our university and the specimen was stored in our laboratory.
1.2Instruments and equipments
Grinder (FW135, Tianjin Taisite Instrument Corporation); drying oven (GZX-9240MBE, Shanghai Boxun Medical Biological Instrument Corporation); multi-use of recycled water vacuum pump (SHB-Ⅲ, Zhengzhou Great Wall Branch Industry and Trade Corporation); rotary evaporators (RE-52C, Shanghai Yarong Biochemistry Instrument Factory).
1.3Methods
1.3.1Extraction process of total flavonoids from Suaeda salsa
The dried Suaeda salsa powder was weighed 5.0 g and heating to reflux in ethanol. The extract was filtered, ethanol concentrated to a certain volume by rotary evaporator for measurement of the total flavonoids.
1.3.2Determination of the total flavonoids and extraction yield
The total flavonoid content in the extracts was determined by aluminum chloride colorimetric method reported by Sultana et al.[14]. Quercetin was used to make the calibration curve. 1 mL of extract solution was diluted in 30% ethanol and 0.3 mL of 5% sodium nitrite was added. After incubation at room temperature for 5 min, 10% aluminum chloride was added and after 6 min 2 mL of 1.0 mol/L sodium hydroxide was added. Absorbance of the solution was measured at 430 nm. The total flavonoid concentration in the extracts was expressed in terms of quercetin (mg/mL) using the following equation Y=0.842 85X-0.000 4, R2=0.998 7. The quality of the total flavonoids was calculated according to the concentration and the total flavonoids yield was calculated as follows: the quality of the total flavonoids in the extracts.
1.3.3Simple-factor experiment
The factors that may influence ethanol extraction include liquid-to-solid ratio, reflux time, ethanol concentration, and reflux temperature. In this part of experiment, the above factors were monitored.
1.3.4Optimization of extraction conditions by CCD-RSM
According to the results of single factor experiment,CCD-RSM was conducted to optimize extraction technique using the total flavonoids extraction ratio as the response value based on three factors, including liquid-to-solid ratio, reflux time and ethanol concentration. A fixed refluxtemperature of 90 ℃ was chosen. The coded and actual levels of the three variables were shown in Table 1.

Table 1 Variables and their coded levels used in CCD
1.3.5Sample preparation for activity determination
Suaeda salsa was extracted using the conditions optimized by CCD-RSM. The extracts were filtered, ethanol recovered, concentrated and dried. The dried extracts were weighed accurately and dissolved in distilled water to a certain concentration for activity determination.
1.3.6Evaluation of antioxidant activity in vitro
1.3.6.1Determination of hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity was measured based on Fenton's reaction, as described previously[15], with a few minor modifications. Hydroxyl radicals were generated using 150 mmol/L of sodium phosphate buffer pH 7.4 containing 10 mmol/L of ferrous sulphate, 10 mmol/L ethylene diamine tetraacetic acid (EDTA), 2 mmol/L sodium salicylate and 30% hydrogen peroxide with different concentrations of samples. In control tubes, hydrogen peroxide was replaced by sodium phosphate buffer. The solutions were incubated at 37 ℃ for 60 min, and the absorbance was measured at 510 nm. Vitamin C was used as positive control. The scavenging activity was calculated as the following equation (1):
Hydroxyl radical scavenging percentage/%=[(A0-Ai)/ A0]×100 (1)
Where A0is the absorbance of the measure tubes; Aiis of the control tubes.
1.3.6.2Determination of oxygen radical scavenging activity
Oxygen radical scavenging activity was measured based on the pyrogallol autoxidation method described by Li Xican et al.[16]. Briefly, the different concentrations of samples was mixed with Tris-HCl buffer (0.05 mol/L, pH 8.2) containing EDTA (1 mmol/L) and pyrogallol (80 µL, 6 mmol/L), and then shaken rapidly at room temperature. The absorbance of the mixture was measured at 325 nm against the Tris-HCl buffer as blank per 30 s for 5 min. The reaction mixture without a sample was used as the negative control. The slope of the correlation of absorbance with time was calculated. The oxygen radical scavenging ability was calculated as equation (2):

1.3.7Evaluation of hypoglycemic activity in vitro
The hypoglycemic activity in vitro was evaluated by inhibition of α-amylase activity using enzyme-starch system[17]. A sample was mixed by stirring with 25 mL potato starch (4%) in a beaker, then 100 mg of α-amylase was added,stirred vigorously and incubated at 37 ℃ for 60 min. After the incubation period, 2 mL of 0.1 mol/L NaOH was added to terminate enzyme activity. The mixture was centrifuged at 3 000×g for 15 min and glucose content was measured in the supernatant. A control test was also run without the addition of test sample. Inhibition of α-amylase activity was calculated as equation (3):

Where Asampleis the absorbance of sample; Acontrolis the absorbance of the control.
1.3.8Evaluation of hypolipidemic activity in vitro
Hypolipidemic activity in vitro was evaluated by inhibition of lipase activity which was determined by the rate of oleic acid released from triolein[18]. The substrate emulsion was prepared by sonicating triolein (160 mg) with 3 mL of TCA-Na (0.83 mg/mL) in a 0.1 mol/L Tris-HCl buffer (pH 7.4) for 60 min. Different concentrations of samples were mixed with the diluted substrate emulsion and sonicated for 60 min. The mixture was incubated with 0.1 mg/mL of pancreatic lipase (0.2 mL/0.108 U) for 30 min at 37 ℃. The enzyme reaction was terminated by incubation in boiling water for 2 min. The amount of oleic acid released in the suspension was measured by a commercially available kit. Inhibition of lipase activity was calculated as equation (4):

1.4Statistical analysis
Each experiment was performed in triplicate. The results were presented asThe CCD-RSM design was analyzed by Design-Expert 8.05b, and the results of pharmaceutical activity experiments were analyzed using t-test by SPSS 17.0. P < 0.05 was considered as statistically significant.
2 Results and Analysis
2.1Influence of single factor on the total flavonoids extraction from Suaeda salsa
2.1.1Effect of liquid-to-solid ratio on the total flavonoids extraction from Suaeda salsa

Fig.1 Effect of liquid-to-solid ratio on the total flavonoids yield from Suaeda salsa
Extraction was carried out at different liquid-to-solid ratio while other extraction parameters were kept constant(ethanol concentration 60%, reflux temperature 60 ℃ and time 30 min). The effect of liquid-to-solid ratios on the total flavonoids extraction was shown in Fig.1. The total flavonoids yield was increased significantly when liquidto-solid ratio was changed from 10:1 to 30:1, and reached the maximum at 30:1, then decreased from 30:1 to 50:1. We chose 30:1 as the centeral condition of CCD.
2.1.2Effect of ethanol concentration on the total flavonoids extraction from Suaeda salsa

Fig.2 Effect of ethanol concentration on the total flavonoids yield from Suaeda salsa
Extraction was carried out at different ethanol concentration while other extraction parameters were kept constant (liquid-to-solid ratio 15:1, reflux temperature 60 ℃and time 30 min). The effect of ethanol concentration on the total flavonoids extraction was shown in Fig.2. The extraction rate of the total flavonoids was increased with the increase of ethanol concentration from 50% to 80% and decreased at 90%. We chose 80% as the center condition of CCD.
2.1.3Effect of reflux temperature on the total flavonoids extraction from Suaeda salsa
Extraction was carried out at different reflux temperature while other extraction parameters were kept constant (liquidto-solid ratio 15:1, ethanol concentration 60% and reflux time 30 min). The effect of reflux temperature on the total flavonoids extraction was shown in Fig.3. The extraction rate of the total flavonoids was increased significantly with the increase of reflux temperature from 60 ℃ to 90 ℃ and showed a slight decrease at 100 ℃. We chose 90 ℃ as the center condition of CCD.

Fig.3 Effect of reflux temperature on the total flavonoids yield from Suaeda salsa
2.1.4Effect of reflux time on the total flavonoids extraction from Suaeda salsa
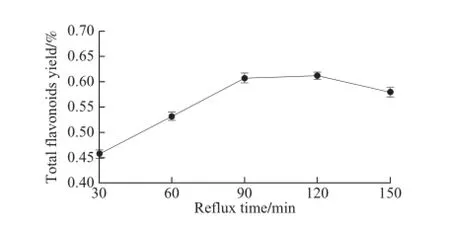
Fig.4 Effect of reflux time on the total flavonoids yield from Suaeda salsa
Extraction was carried out at different reflux time while other extraction parameters were kept constant (liquid-to-solid ratio 15:1, ethanol concentration 60% and reflux temperature 60 ℃). The effect of reflux time on the total flavonoids extraction was shown in Fig.4. The extraction rate of the total flavonoids was increased firstly and then decreased with the maximum value at 120 min. We chose 120 min as the center condition of CCD.
2.2Optimization of the total flavonoids extraction from Suaeda salsa by CCD-RSM
The experiments were arranged and the results obtained as shown in Table 2.
Using the software of Design-Expert 8.05b for multiple regression analysis on the experimental data, the response variable (the total flavonoids yield) and the test variables are related by the following second-order polynomial equation:
Y=0.88+0.016X1+0.038X2+0.015X3+0.011X1X2-8.125×10-3X1X3+9.125×10-3X2X3-0.038X12-0.043-0.019(R2=0.986 6)
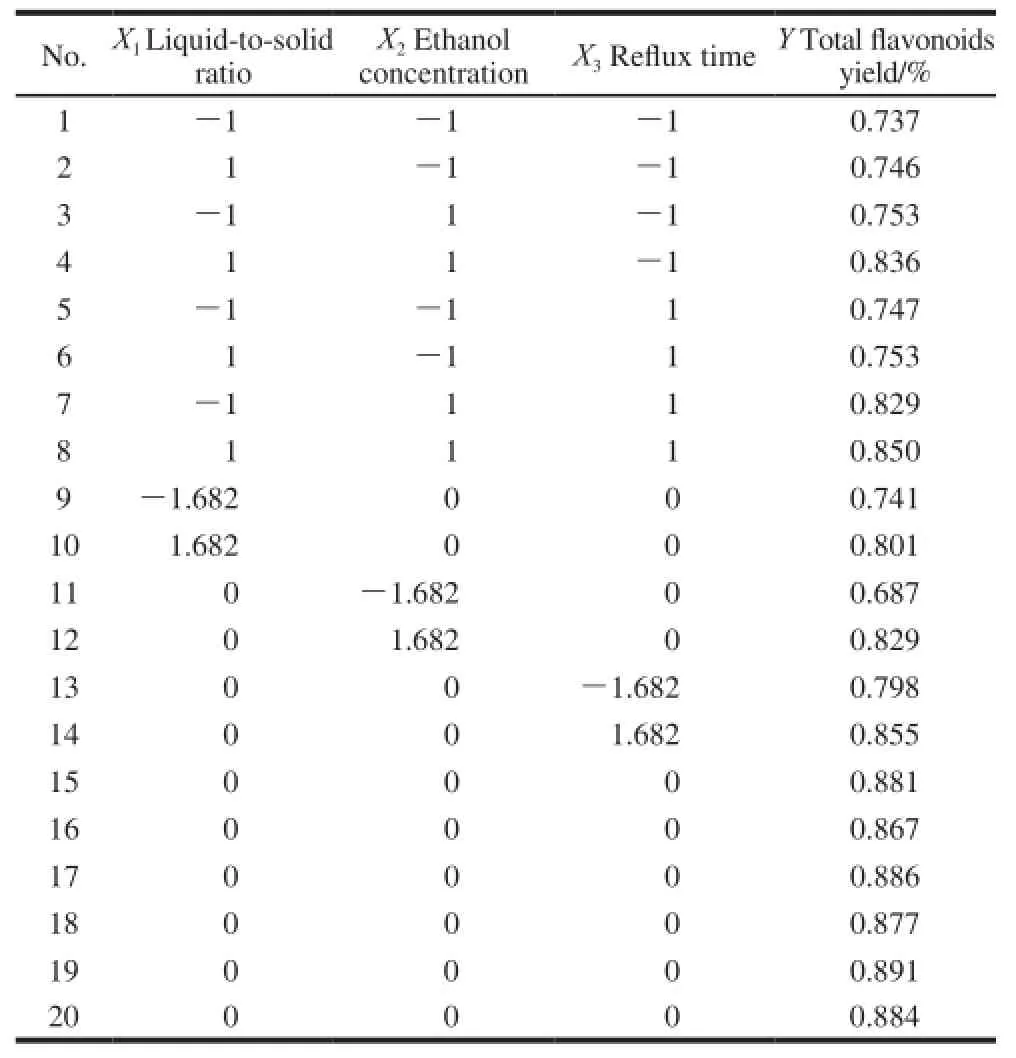
Table 2 Experimental designs by CCD-RSM with experimental results
The analysis of variance (ANOVA) for the regression equation was shown in Table 3.

Table 3 Analysis of variance of quadratic regression model
P value of model was less than 0.000 1, indicating a very high degree of precision and reliability of the experimental values. The linear term, quadratic term and the interaction were significant, indicating that the relationship between the total flavonoids and the test variables was not simply a linear one.

Fig.5 Response surface plots for total flavonoids yield from Suaeda salsa as affected by liquid-to-solid ratio, ethanol concentration and reflux time
Three-dimensional response surface plots were presented in Fig.5. It was estimated that there was a high yield of the total flavonoids when liquid-to-solid ratio in the range of 24.06:1-35.94:1, ethanol concentration 74.06%-85.94% and reflux time 102.18-137.82 min. The optimal conditions of the total flavonoids extraction were as follows: liquid-to-solid ratio 31.40:1, ethanol concentration 83.12%,reflux temperature 90 ℃ and reflux time 128.40 min. The predicted total flavonoids yield was 0.896%. To confirm these above results, three triplicate tests were performed under optimal conditions. The total flavonoids yield value was (0.932±0.02)% (n=3). The average deviation was 0.65% and the RSD value was 0.88%, which clearly showed that the model fitted the experimental data and the optimal extraction procedure had good reproducibility.
2.3Antioxidant activity of the total flavonoids extracts from Suaeda salsa
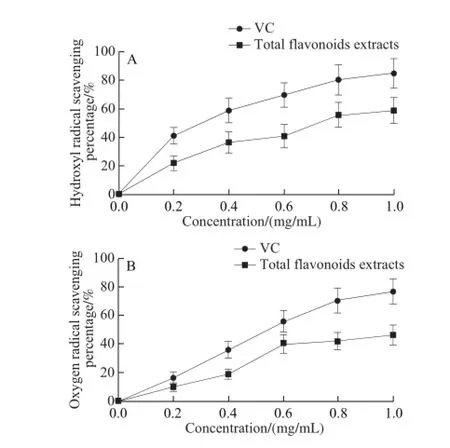
Fig.6 Scavenging effects of total flavonoids from Suaeda salsa on hydroxyl (A) and oxygen (B) radicals
Oxidative stress is the consequence of an imbalance between production and scavenging of free radicals leading to cell damage and tissue injury[19]. Oxidative stress is common in tissues and organs with high energy and metabolic demands, such as skeletal and heart muscle,liver and blood cells, participating in various pathological processes. One of the reasons for the occurrence of oxidative stress is the exhaustion of antioxidant systems, which results in accumulation of reactive oxygen species (ROS) or free radicals[20]. Therefore, screening of safe and efficient free radical scavenger gradually becomes the research hotspot. In this experiment the effects of the total flavonoids extracts from Suaeda salsa on free radical scavenging was measured. Results revealed that the total flavonoids extracts could scavenge the hydroxyl and oxygen radicals in vitro in a dosedependent manner, although the scavenging percentages were lower than that of VC at the same concentration (Fig.6). Total flavonoids are the most widely distributed constituents known as the most potent antioxidants from herbs. The antioxidant activity of flavonoids is related to the presence of a number of phenolic hydroxyl groups which are attached to the phenyl ring structures[21]. The total flavonoids from Suaeda salsa showed an antioxidant potential with a high development value.
2.4Hypoglycemic and hypolipidemic activity of the total flavonoids extracts from Suaeda salsa
α-Amylase and α-glucosidase are two important carbohydrate-hydrolyzing enzymes which participate in carbohydrate digestion. The inhibition of these two enzymes can delay carbohydrate digestion, resulting in a reduction in the glucose absorption rate and consequently delay the postprandial plasma glucose elevation. The inhibitors of these two enzymes may possess the potential to control postprandial hyperglycemia in diabetes mellitus[22-23]. In this experiment we preliminarily observed the inhibition of α-amylase by the total flavonoids from Suaeda salsa. Results revealed that the total flavonoids exhibited α-amylase inhibitory activity in a concentration-dependent manner(Fig.7A). At the concentration of 5 mg/mL, the inhibition reached 46.39%, lower than that of acarbose (70.62%). Since the total flavonoids are a mixture of various ingredients,the inhibition activity can be improved by increasing the concentration or purify the mixture.

Fig.7 Inhibitory effects of total flavonoids from Suaeda salsa on α-amylase (A) and pancreatic lipase (B) activities
Pancreatic lipase is an important enzyme involved in the hydrolysis of triacylglycerols because it is responsible for the hydrolysis of 50% to 70% of total dietary fats[24]. Pancreatic lipase mainly functions in the digestion and absorption of triglycerides (TG) into monoglycerides and fatty acids[25]. Inhibition of pancreatic lipase might be a valuable pathway for the treatment of diet-induced hyperglycemia in humans. As shown in Fig.7B, the total flavonoids from Suaeda salsa inhibited the pancreatic lipase activity in a dose-dependent manner at the concentrations ranging from 0.2 mg/mL to 1.0 mg/mL, lower than simvastatin, exhibiting its hypolipidemic potential.
3 Conclusion
The present study investigates the extraction technique of the total flavonoids from Suaeda salsa and its antioxidation,hypoglycemic and hypolipidemic activities. The extraction technique is optimized to be 83.12% ethanol, with a liquidto-solid ratio of 31.40:1 (mL/g), a reflux temperature of 90 ℃and a reflux time of 128.40 min, which gives the yield of the total flavonoids up to 0.932% of dry weight. Our investigation indicates that the total flavonoids from Suaeda salsa can scavenge hydroxyl and oxygen radicals and inhibit α-amylase and pancreatic lipase activities in vitro, exhibiting a potential for antioxidation, hypoglycemic and hypolipidemic values. Undoubtedly, further in vivo studies are required to confirm these results and the active ingredients need to be isolated and identified.
References:
[1] SHAO Qiuling, LI Yujuan. The development of Suaeda salsa[J]. Plants, 1999, 53: 43-48.
[2] ZHANG Zesheng, WANG Li, YANG Jianbo, et al. Chemical constituents of Suaeda salsa[J]. Natural Product Research, 2012,24(6): 775-776.
[3] ZHOU Dongsheng, WANG Qizhi, WANG Ming, et al. Reaserch progress on the chemical constituents of Suaeda salsa and their development and utilization[J]. Chinese Wild Plants Resources, 2011,30(1): 6-9.
[4] ZHENG Weifa, CHEN Caifa, LI Wei. Chemical composition and anti inflammation of methanol/chloroform extracts from seeds and seedlings of Suaeda salsa (L.) Pall[J]. Chinese Traditional Patent Medicine, 2003, 25(12): 997-1002.
[5] WANG Zhaojing. Studies on separation purification and antioxidant activity of a water-soluble polysaccharide SPA from Suaeda Spp.[J]. Journal of Liaoning University of TCM, 2009, 11(9): 168-169.
[6] AN R B, SOHN D H, JEONG G S, et al. In vitro hepatoprotective compounds from Suaeda glauca[J]. Archives of Pharmacal Research, 2008, 31(5): 594-597. DOI:10.1007/s12272-001-1198-1.
[7] ZHU Zhujun, WEI Guoqiang, LI Juan, et al. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of saltstressed cucumber (Cucumis sativus L.)[J]. Plant Science, 2004, 167: 527-533. DOI:10.1016/j.plantsci.2004.04.020.
[8] LEFÈVRE I, MARCHAL G, MEERTS P, et al. Chloride salinity reduces cadmium accumulation by the Mediterranean halophyte species Atriplex halimus L.[J]. Environmental and Experimental Botany, 2009, 65: 142-152. DOI:10.1016/j.envexpbot.2008.07.005.
[9] THOMAS J C, MALICK F K, ENDRESZL C, et al. Distinct responses to copper stress in the halophyte Mesembryanthemum crystallinum[J]. Physiologia Plantarum, 1998, 102: 360-368. DOI:10.1016/ j.physiolo.1998.10.036.
[10] PRZYMUSINSKI R, RUCINSKA R, GWÓZDZ E A. Increased accumulation of pathogenesis-related proteins in response of lupine roots to various abiotic stresses[J]. Environmental and Experimental Botany, 2004, 52: 53-61. DOI:10.1016/j.envexpbot.2004.01.006.
[11] ZHU Minghe, DING Yongsheng, ZHENG Daochang, et al. Accumulation and tolerance of Cu, Zn, Pb and Cd in plant Suaeda heteroptera Kitag in tideland[J]. Marine Environmental Research,2005, 24(2): 13-16.
[12] LIU Xianbin, XU Chongyan. Remediation effect of Suaeda salsa planting on the petroleum hydrocarbon polluted coastal zones[C]// The 2nd International Conference on Bioinformatics and Biomedical Engineering. Shanghai: IEEE, 2008: 4158-4161. DOI:10.1109/ ICBBE.2008.540.
[13] XU Chongyan, LIU Xianbin, LIU Zhanguang, et al. Remedial effect of Suaeda salsa (L.) Pall. planting on the oil-polluted coastal zones[J]. Journal of Safety and Environment, 2007, 7: 37-39.
[14] SULTANA B, ANWAR F, ASHRAF M. Effect of extraction solvent technique on the antioxidant activity of selected medicinal plant extracts[J]. Molecules, 2009, 114(6): 2167-2180. DOI:10.3390/ molecules14062167.
[15] SMIRNOFF N, CUMBES Q J. Hydroxyl radical scavenging activity of compatible solutes[J]. Phytochemistry, 1989, 28: 1057-1060. DOI:10.1016/0031-9422(89)80182-7.
[16] LI Xican, WU Xiaoting, HUANG Ling. Correlation between antioxidant activities and phenolic contents of Radix Angelica sinensis (Danggui)[J]. Molecules, 2009, 14: 5349-5361. DOI:10.3390/ molecules14125349.
[17] OU S, KWOK K C, LI Y, et al. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose[J]. Journal of Agricultural and Food Chemistry, 2001, 49: 1026-1029. DOI:10.1021/ jf000574n.
[18] IWATA E, HOTTA H, GOTO M. Hypolipidemic and bifidogenic potentials in the dietary fiber prepared from Mikan (Japanese mandarin orange: Citrus unshiu) albedo[J]. Journal of Nutritional Science and Vitaminology (Tokyo), 2012, 58(3): 175-180.
[19] PIETRO C. Biomarkers of oxidative stress in ruminant medicine[J]. Immunopharmacology and Immunotoxicol, 2011, 33(2): 233-240. DOI:10.3109/08923973.2010.514917.
[20] PUPPEL K, KAPUSTA A, KUCZYŃSKA B. The etiology of oxidative stress in the various species of animals: a review[J]. Journal of the Science of Food and Agriculture, 2015, 95(11): 2179-2184. DOI:10.1002/jsfa.7015.
[21] PRIYANKA C, KADAM D A, KADAM A S, et al. Free radical scavenging (DPPH) and ferric reducing ability (FRAP) of some gymnosperm species[J]. International Research Journal of Botany,2013, 3: 34-36.
[22] BHANDARI M R, NILUBON J A, HONG G, et al. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed(Bergenia ciliata, Haw.)[J]. Food Chemistry, 2008, 106: 247-252. DOI:10.1016/j.foodchem.2007.05.077.
[23] KIM J S, HYUN T K, KIM M J. The inhibitory effects of ethanol extracts from sorghum, foxtail millet and prosomilleton α-glucosidase and α-amylase activities[J]. Food Chemistry, 2011, 124: 1647-1651. DOI:10.1016/j.foodchem.2010.08.020.
[24] BIRARI R B, BHUTANI K K. Pancreatic lipase inhibitors from natural sources: unexplored potential[J]. Drug Discovery Today, 2007,12: 879-889. DOI:10.1016/j.drudis.2007.07.024.
[25] KIM T H, KIM J K, ITO H, et al. Enhancement of pancreatic lipase inhibitory activity of curcumin by radiolytic transformation[J]. Bioorganic and Medicinal Chemistry Letters, 2011, 21: 1512-1514. DOI:10.1016/j.bmcl.2010.12.122.
响应面试验优化盐地碱蓬总黄酮乙醇萃取工艺及其抗氧化、降糖、降脂活性
公衍玲,刘 菲,金 宏
(青岛科技大学化工学院药学系,山东 青岛 266042)
盐地碱蓬系藜科碱蓬属1a生草本植物,既可作药物也可作为食物被人们广泛接受。本研究优化了从碱蓬中提取总黄酮的工艺及条件,并研究了其体外抗氧化、降血糖、降血脂活性。通过乙醇回流提取法进行总黄酮提取,使用单因素试验结合中心组合设计-响应面法确定了最优的工艺参数。并且将提取的总黄酮进行体外清除羟自由基和氧自由基、抑制α-淀粉酶和脂肪酶实验。总黄酮的乙醇提取工艺优化条件为液固比31.40∶1、乙醇体积分数83.12%、回流温度90 ℃、回流时间128.40 min。预测和实际的总黄酮产量分别为0.896%和0.932%。体外实验表明,总黄酮可以清除羟自由基和氧自由基,并且可以抑制α-淀粉酶和脂肪酶的活性。本实验中最优提取条件容易实现并且有良好的重复性。结果表明,总黄酮作为一种潜在的抗氧化、降糖、降血脂的自然资源的成分,可为碱蓬的发展提供新的药理学依据。
盐地碱蓬;总黄酮;抗氧化活性;降糖活性;降血脂活性
10.7506/spkx1002-6630-201608001
R932
A
1002-6630(2016)08-0001-07
GONG Yanling, LIU Fei, JIN Hong. Optimization of ethanol extraction of total flavonoids from Suaeda salsa and its antioxidant, hypoglycemic and hypolipidemic activities[J]. Food Science, 2016, 37(8): 1-7. DOI:10.7506/spkx1002-6630-201608001. http://www.spkx.net.cn
公衍玲, 刘菲, 金宏. 响应面试验优化盐地碱蓬总黄酮乙醇萃取工艺及其抗氧化、降糖、降脂活性[J]. 食品科学, 2016,37(8): 1-7. DOI:10.7506/spkx1002-6630-201608001. http://www.spkx.net.cn
2015-07-31
国家自然科学基金青年科学基金项目(81300281);山东省高校科技计划项目(J15LK12)
公衍玲(1975—),女,副教授,博士,研究方向为药物活性筛选和药效学。E-mail:hanyu_ma@126.com
引文格式:
