Perineural dexamethasone does not enhance the anaIgesic efficacy of uItrasound-guided subcostal transversus abdominis plane block during laparoscopic cholecystectomy
2016-11-14ShengHuiHuangJingLuHongYunGanYiLiYongGangPengandShuanKeWang
Sheng-Hui Huang, Jing Lu, Hong-Yun Gan, Yi Li, Yong-Gang Peng and Shuan-Ke Wang
Lanzhou, China
Perineural dexamethasone does not enhance the anaIgesic efficacy of uItrasound-guided subcostal transversus abdominis plane block during laparoscopic cholecystectomy
Sheng-Hui Huang, Jing Lu, Hong-Yun Gan, Yi Li, Yong-Gang Peng and Shuan-Ke Wang
Lanzhou, China
BACKGROUND: Ultrasound-guided transversus abdominis plane (TAP) block is an adjunct therapy to provide effective postoperative analgesia in abdominal surgical procedures. Dexamethasone is a supplement agent that can improve the efficacy of local anesthesia. However, information about its additive effect is limited. This study aimed to compare the analgesic efficiency using ultrasound-guided TAP block with and without perineural dexamethasone for patients who underwent laparoscopic cholecystectomy.
METHODS: Sixty patients who underwent laparoscopic cholecystectomy were randomly divided into three groups: group I, controls; group II, TAP; and group III, TAP+perineural dexamethasone supplement. The requirement of additional analgesia and the first-time request of rescue-analgesia were recorded after operation and the numerical rating scale was evaluated at specific intervals.
RESULTS: Compared to group I, the first-time requirement of rescue-analgesia in groups II and III was significantly delayed(403.0±230.9, 436.0±225.3 vs 152.3±124.7, P<0.01). Compared with those in group I, patients in groups II and III were associated with lower numerical rating scale pain scores (P<0.01)and less postoperative analgesic consumption (P<0.01). There
was no significant difference in the variables mentioned above between groups II and III (P>0.05).
CONCLUSION: Perineural dexamethasone has no additive/ synergistic effect with subcostal TAP block on analgesic efficacy for the patients undergoing laparoscopic cholecystectomy.
(Hepatobiliary Pancreat Dis Int 2016;15:540-545)
ultrasonography;
local analgesic;
laparoscopic cholecystectomy;
dexamethasone
Introduction
Laparoscopic cholecystectomy (LC) is a less invasive surgical procedure compared with laparotomy. However, many patients complain of mild to moderate pain after operation[1,2]and analgesic management is necessary. Multimodal approaches[3]including patientcontrolled intravenous analgesia, epidural analgesia, and intraperitoneal injection of local anesthetics are applied in these patients. In the recent years, ultrasound-guided transversus abdominis plane (TAP) block has been used as a satisfactory approach that provides postoperative analgesia for patients underwent laparoscopic surgeries.[4-6]TAP block is safe; it diminishes or replaces the use of opioids; and it has a lower incidence of adverse effects including postoperative nausea and vomiting (PONV). Many clinicians are currently pursuing the accuracy of local anesthetic deposition under the guidance of ultrasonography.[4-7]This new technique has elucidated the analgesic efficiency in laparotomic and laparoscopic procedures.[8]However, clinical studies[9,10]showed negative results in TAP block. Hence, analgesic quality, duration of analgesia,patient satisfaction, and different operational approachesmust be further evaluated.
In addition, many local anesthetic adjuvants, such as clonidine, epinephrine, dexmedetomidine, and dexamethasone, have been used to enhance the analgesic effect and duration of similar blocks. Among them, dexamethasone is a more effective adjuvant for peripheral nerve block.[11,12]Because the data on the combination of perineural dexamethasone administration and TAP block are limited, we in this prospective, randomized and controlled study added dexamethasone to ropivacaine to determine whether it is able to enhance the analgesic efficacy of ultrasound-guided TAP block.
Methods
Patients
Sixty patients with American Society of Anesthesiologists (ASA) physical status I and II who had undergone LC from November 2013 to April 2014 were enrolled in the study. The study was approved by the local Clinical Research Ethics Committee and a written informed consent was obtained from all participating patients. Patients with systemic cardiovascular or endocrine diseases,coagulopathy, needle puncture site infection, body mass index (BMI) greater than 30, history of chronic pain, and hypersensitive or allergic to any medicine were excluded from the study. All of the patients were trained with the numeric rating scale (NRS) to evaluate pain intensity and diversity as well as with the Ramsay sedation scale to assess the degree of sedation.[13]
Grouping criteria
Before surgery, the patients were randomly divided into three groups. Group I received general anesthesia without TAP block (control group, n=20); group II underwent bilateral ultrasound-guided TAP block with 30 mL of 0.375% ropivacaine after tracheal intubation, with 15 mL on each side (group TAP, n=20); group III underwent bilateral ultrasound-guided TAP block with 30 mL of 0.375% ropivacaine and 2 mL of dexamethasone (10 mg), with 16 mL on each side (group TAP plus dexamethasone, n=20).
Anesthetic technique
While a lactated Ringer's solution was administered through the peripheral vein, all patients received pretreatment with penehyclidine hydrochloride. Noninvasive blood pressure, electrocardiography, pulse oximetry,and biospectral index (BIS) were monitored. Anesthesia was induced through intravenous injection of 0.05 mg/kg midazolam as well as 2% propofol and remifentanil with a target controlled infusion system (TCI, Beijing Slgo Medical Technology Co., Ltd., Beijing, China). After the plasma effective concentration of propofol and remifentanil reached 3.0 μg/mL and 2.5 ng/mL, respectively,the unconscious patients were given rocuronium (0.6 mg/kg). Endotracheal intubation was performed after mask ventilation for 90 seconds. Mechanical ventilation was regulated by volume-controlled ventilation mode,tidal volume at 6-7 mL/kg, respiration rate at 10-12 per minute, and oxygen flow at 1.5 L per minute. During the operation, the effective concentration of propofol was maintained at 2 μg/mL and remifentanil at 2-4 ng/mL. Rocuronium was administered for muscle relaxation if needed. The TCI system was adjusted to target the blood pressure within the 20% range of baseline before the induction. The end-tidal carbon dioxide partial pressure(PETCO2) was maintained between 30-40 mmHg and BIS was targeted in the range of 40 to 60. All patients received 4 port laparoscopic procedures.
When the vital signs were stable after endotracheal intubation, bilateral TAP block was performed in patients in groups II and III. After draping the related area around the costal margin and xyphoid, the subcostal approach was applied under ultrasonographic guidance with a Sonosite ultrasound machine (Sonosite M-Turbo®,Sonosite, USA) and a linear 5-13 MHz ultrasound transducer. The probe was placed on the anterior abdominal wall and inferior to the costal margin. At this level, the transverse abdominis muscle (TAM) was identified underneath the rectus muscle (RM). A 100-mm pajunk 19G needle (Medizintechnologie, Geisingen, Germany) was inserted through the RM from an anterior and medial position, advancing inferolaterally and adjacent to the border of the costal margin, about 2 cm beside the probe. The advancement of the needle was maintained within the ultrasound beam by the in-plane technique. While the needle tip was visualized at the border between the RM and TAM, negative aspiration was done, followed by 2 mL of normal saline injection to confirm the needle position. Afterwards, 15 mL of 0.375% ropivacaine (ropivacaine hydrochloride injection, 7.5 mg/mL, Sodertalje,Sweden) or 16 mL of mixed solution (15 mL of 0.375% ropivacaine and 1 mL of dexamethasone) was injected. Local analgesic agent diffused through ultrasonography as a hypoechoic enlargement, and the peritoneum was pushed below by gravity derived from the local analgesic agent distributed around the thinner layer of TAM. The same procedure was performed to the contralateral site. A skilled anesthesiologist did the operation on all patients in groups II and III.
Postoperative care and pain assessment
All patients received total intravenous anesthesia(TIVA) throughout the operation. At the end of the surgery, the residual neuromuscular blockade was reversed with 50 μg/kg of intravenous neostigmine and 0.3-0.5 mg of atropine, whilst 0.1 mg/kg of intravenous ondansetron was given to all patients. Before entering the post-anesthesia care unit (PACU), all patients were provided with 40 mg of intravenous parecoxib sodium as a standard postoperative analgesic regimen, followed by repeated doses of 20-40 mg at intervals of 6-12 hours if needed. The total dose should not exceed more than 80 mg within 24 hours. If the pain was still not controlled,5-10 μg of intravenous sulfentanil was administered slowly. The first request for rescue-analgesics and the 24-hour consumption of parecoxib and sulfentanil were recorded. The values of NRS were defined as follows: 0 for no pain, and 10 for intolerable severest pain. Drug intervention was usually applied if the score was more than or equal to 4. Patients and postoperative assessors were blinded to the intervention protocol, while members of anesthesia and operation groups were not blinded. Time to arrival at PACU was set as time 0 for pain assessment and at 30 and 60 minutes, and 6, 12 and 24 hours after the operation. The presence of adverse effects such as PONV, abnormal sedation, shivering, and wound bleeding were also assessed. The Ramsay sedation scale was used to determine the sedation degrees.[13]Scores between 2 and 4 were recognized as satisfactory sedation degrees.
The primary end-point was evaluated by the effect of intervention on the time of the first request for additional analgesics. The second end-point consisted of pain scores, total analgesic consumption, and related adverse effects.
Statistical analysis
Measurement data were presented as mean±standard deviation or the numbers of patients. Statistical analysis was carried out using the Statistical Package for Social Sciences (version 20.0 for Windows; SPSS Inc., Chicago,IL, USA). Mean±standard deviation was calculated for all quantitative design variables. The Kolmogorov-Smirnov test of normality was used to check the normality of data. The mean was compared using one-way analysis of variance for normally distributed data, and between two groups by the least significant difference. The Mann-Whitney U test was applied for skewed data or for scores. A P<0.05 was considered statistically significant.
Results
In 65 patients initially enrolled, 5 were excluded: 2 patients in group I and 1 patient in group III were converted to open cholecystectomy, 1 in group II took significant longer operation due to intraoperative bile duct injury, and 1 in group III was subjected to laparoscopic appendectomy after LC (Fig.).
Analgesic duration, additional analgesic consumption and incidence of adverse effects
The patients in all groups were comparable in age,BMI and operative time (Table 1). The first request for rescue-analgesics, analgesic consumption, and the occurrence of adverse effects in the three groups are shown in Table 2. Compared with the patients in group I, those in groups II and III exhibited a longer interval between operation and first request for rescue-analgesics (403.0 ± 230.9, 436.0±225.3 vs 152.3±124.7, P<0.01); this interval was not significantly different between groups II and III. Total parecoxib consumption was significantly reduced for patients in groups II and III within the postoperative 24-hour period (46.0±14.7, 44.0±12.3 vs 66.0 ± 19.6, P<0.01). For additional use of opioids, 5 patients in group I, 1 in group II, and 2 in group III were given 5 μg of sulfentanil (P>0.05). For adverse effects, nausea,vomiting, and abnormal sedation were observed in thethree groups but shivering and wound bleeding did not occur in all patients. Groups II and III showed a lower incidence of adverse effects in comparison with group I.
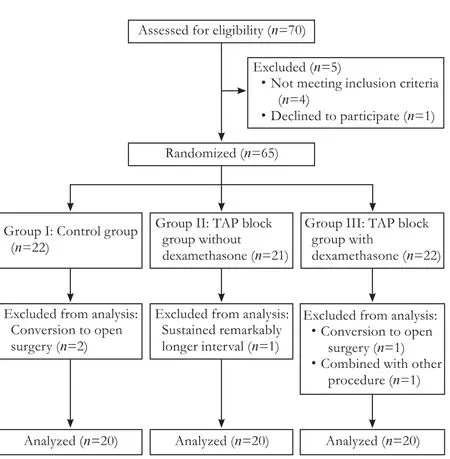
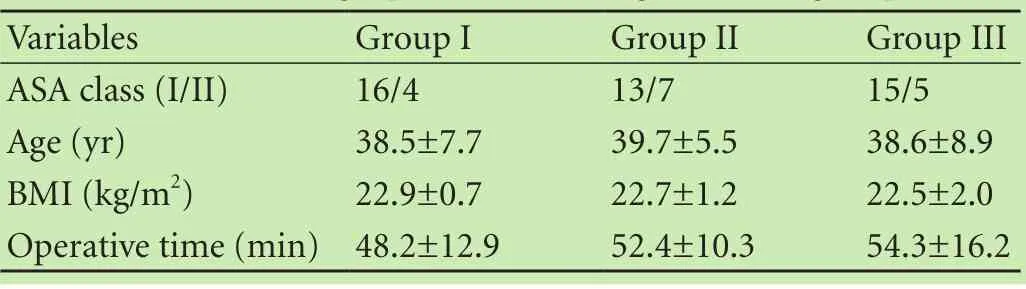
Table 1. Demographic data among the three groups
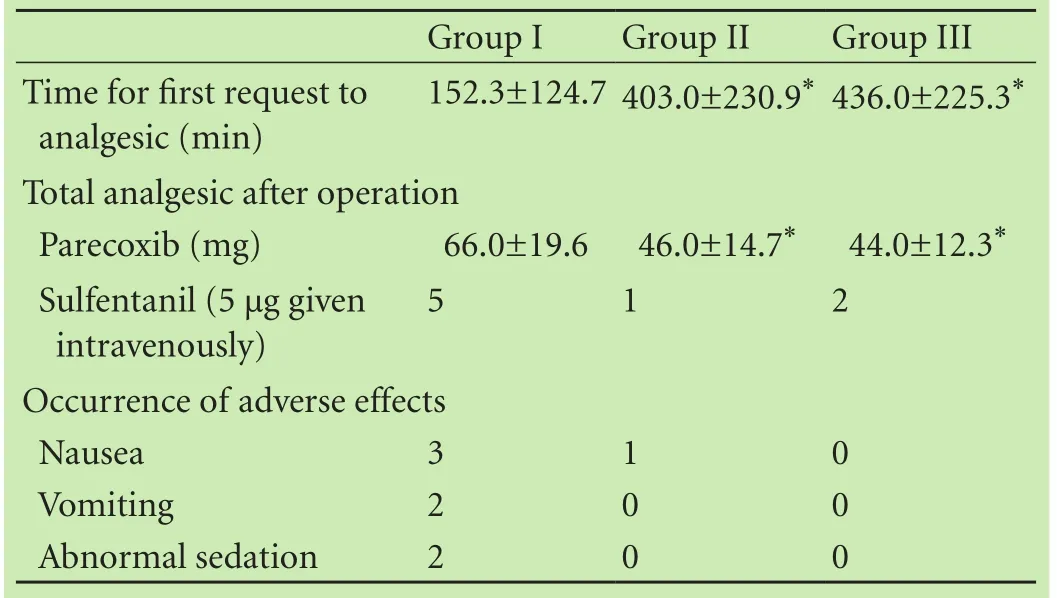
Table 2. Twenty-four hour rescue-analgesic requirement, time to first request for analgesia, and the incidence of adverse effects among the three groups
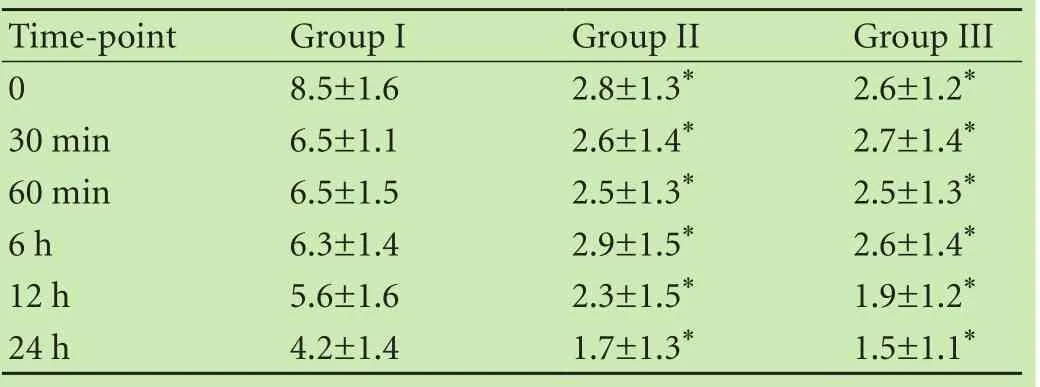
Table 3. Postoperative NRS among the three groups
Pain assessment
For NRS evaluation, the patients in groups II and III presented significantly lower pain scores than those in group I during the course of the observational period,and the pain scores in groups II and III were similar at different time point (Table 3).
Discussion
As a part of multimodal approach for postoperative analgesia, TAP block has been widely accepted in patients undergoing abdominal surgery, with reduced consumption of morphine and less occurrence of PONV within 24 hours postoperatively.[14]The distribution of local analgesic agent and the analgesic effect of TAP block are debatable because of different administration routes.[6,15-17]Subcostal TAP block was thought to exhibit the effective analgesia after surgery involving dermatome T6 to T10(for surgery involved in upper abdominal wall).[6]Based on this observation, we utilized the subcostal approach to perform TAP block.
Up to now, information is limited about dexamethasone as a local analgesic adjuvant with TAP block for postoperative analgesia. Dexamethasone has been reported to enhance the efficiency of peripheral nerve blocks, such as brachial plexus block and sciatic nerve block.[12,18]A recent meta-analysis[19]suggested that the optimal time for performance of TAP block should be preoperative, since preoperative TAP block was associated with reduced pain intensity and less opioid requirement in comparison with postoperative TAP block. Another study[20]concluded that administration of preoperative dexamethasone for patients undergoing LC could enhance the quality of recovery, including reduction of nausea, pain, and fatigue in the early postoperative period. Holte et al[21]revealed that administration of steroids at least 60 minutes before surgery could effectively reduce pain and inflammatory reactions. Based on the previous studies, we performed TAP block immediately after intubation with or without supplementation of dexamethasone.
In this study, the first request for rescue-analgesic requirement was significantly delayed in groups II and III in comparison with that in group I, also there was a lower NRS. However, no significant difference was found between groups II and III.
Several factors may contribute to the results, as the actual mechanism of dexamethasone has not yet been investigated, and its analgesic quality and prolonging effect remain an important criterion for the current study. First, we speculated that the anatomic characteristics of TAP can explain to some extent our results. The duration of TAP block is associated with the slow clearance of local analgesia within TAP because of the paucity of local blood vessels.[22]Perineural dexamethasone might be mildly absorbed and accounted for similar outcomes between the experimental groups. Murphy et al[20]reported that the positive effect of perioperative dexamethasone in LC was seen after intravenous administration. Because of the lack of direct evidence of blood dexamethasone concentration, further studies are needed. Second, as the increase of the local efficiency of dexamethasone to local analgesic, Akkaya et al[11]found that perineural dexamethasone combined with posterior TAP block exhibited a longer analgesic time. We consider that the variability of administration approach is a leading cause for observational difference; local analgesic agents with adjuvants can demonstrate varied diffusion, speed of blood absorption, and degradation relevant to the block site. In addition, different approaches for TAP block are associated with different nerve involvements.[15,16]
Moreover, we cannot ignore the characteristics of pain after LC. Overall postoperative pain tolerance may be in a relatively wide range. The sources of pain can berelated to surgical incision, visceral pain, and referred pain; severe pain occurs at the initial 48 hours and subsequently declines to low levels.[23]Typically, the intensity and duration of pain after LC are significantly diverse and unpredictable among individuals.[23]In analgesic treatment after LC, different administrations may lead to various outcomes though preoperative steroids are considered beneficial as mentioned above.[20,21]In other words, subcostal TAP block was an essential factor for the achievement of analgesic effect after LC in the present study.
Total postoperative analgesic consumption was similar for patients in groups II and III and less compared with group I. The patients in groups II and III exhibited a lower incidence of adverse effects, which may be due to the less consumption of opioids.
The present study has several limitations. First, all patients were assessed for postoperative pain only in the initial 24 hours, which was similar to other studies.[5,6]According to the characteristics of pain after LC,[23]continuous assessment for a minimum of 48 hours should be objective and practical. Second, various pain scores were used for the assessment of postoperative pain; thus a golden standard evaluation protocol was not established. Bhatia et al[6]evaluated visual analog scores for LC patients at rest and on movement, and in another study they used NRS to assess the difference in somatical pain and visceral pain.[11]These references are worthy of being considered. Third, a study[5]found the less consumption of intraoperative analgesics by TAP block, we did not calculate the use of intraoperative remifentanil. This limitation is also shown by failure to identify the sensory level of TAP block after induction of anesthesia.[10]Finally, research into TAP block and efficiency of dexamethasone[11]is related to the safety of local analgesics and adjuvants. Their blood concentration, neurotoxicity,and interaction should be further investigated.
In summary, although subcostal TAP block is superior to conventionally intravenous analgesia, perineural dexamethasone does not increase the analgesic duration or enhance the analgesic quality of ultrasound-guided subcostal TAP block after LC. Dexamethasone as a local analgesic adjuvant must be further evaluated to reveal its actual action to TAP block and compare it with other adjuvants.
Acknowledgements: The authors would like to thank Dr. Sheng-Quan Mi for his assistance in statistical analysis of the data.
Contributors: HSH proposed the study and wrote the manuscript. WSK designed the study. HSH and LY performed research. LJ analyzed the data. GHY collected the data. PYG revised the drafts. All authors contributed to the design and interpretation of the study and to further drafts. HSH and LJ contributed equally
to the paper. HSH is the guarantor.
Funding: None.
Ethical approval: This study was approved by Clinical Research Ethics Committee of the Second Hospital of Lanzhou University(2013083).
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Barczyński M, Herman RM. A prospective randomized trial on comparison of low-pressure (LP) and standard-pressure (SP)pneumoperitoneum for laparoscopic cholecystectomy. Surg Endosc 2003;17:533-538.
2 Wills VL, Hunt DR. Pain after laparoscopic cholecystectomy. Br J Surg 2000;87:273-284.
3 Bisgaard T. Analgesic treatment after laparoscopic cholecystectomy: a critical assessment of the evidence. Anesthesiology 2006;104:835-846.
4 Wassef M, Lee DY, Levine JL, Ross RE, Guend H, Vandepitte C, et al. Feasibility and analgesic efficacy of the transversus abdominis plane block after single-port laparoscopy in patients having bariatric surgery. J Pain Res 2013;6:837-841.
5 Ra YS, Kim CH, Lee GY, Han JI. The analgesic effect of the ultrasound-guided transverse abdominis plane block after laparoscopic cholecystectomy. Korean J Anesthesiol 2010;58:362-368.
6 Bhatia N, Arora S, Jyotsna W, Kaur G. Comparison of posterior and subcostal approaches to ultrasound-guided transverse abdominis plane block for postoperative analgesia in laparoscopic cholecystectomy. J Clin Anesth 2014;26:294-299.
7 Abrahams MS, Horn JL, Noles LM, Aziz MF. Evidence-based medicine: ultrasound guidance for truncal blocks. Reg Anesth Pain Med 2010;35:S36-42.
8 Urigel S, Molter J. Transversus abdominis plane (TAP) blocks. AANA J 2014;82:73-79.
9 Petersen PL, Stjernholm P, Kristiansen VB, Torup H, Hansen EG, Mitchell AU, et al. The beneficial effect of transversus abdominis plane block after laparoscopic cholecystectomy in day-case surgery: a randomized clinical trial. Anesth Analg 2012;115:527-533.
10 Ortiz J, Suliburk JW, Wu K, Bailard NS, Mason C, Minard CG,et al. Bilateral transversus abdominis plane block does not decrease postoperative pain after laparoscopic cholecystectomy when compared with local anesthetic infiltration of trocar insertion sites. Reg Anesth Pain Med 2012;37:188-192.
11 Akkaya A, Yildiz I, Tekelioglu UY, Demirhan A, Bayir H, Ozlu T, et al. Dexamethasone added to levobupivacaine in ultrasound-guided tranversus abdominis plain block increased the duration of postoperative analgesia after caesarean section: a randomized, double blind, controlled trial. Eur Rev Med Pharmacol Sci 2014;18:717-722.
12 Choi S, Rodseth R, McCartney CJ. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: a systematic review and meta-analysis of randomized trials. Br J Anaesth 2014;112:427-439.
13 Ramsay MA, Newman KB, Jacobson RM, Richardson CT, Rogers L, Brown BJ, et al. Sedation levels during propofol administration for outpatient colonoscopies. Proc (Bayl Univ MedCent) 2014;27:12-15.
14 Johns N, O'Neill S, Ventham NT, Barron F, Brady RR, Daniel T. Clinical effectiveness of transversus abdominis plane (TAP)block in abdominal surgery: a systematic review and metaanalysis. Colorectal Dis 2012;14:e635-642.
15 Milan Z, Tabor D, McConnell P, Pickering J, Kocarev M, du Feu F, et al. Three different approaches to Transversus abdominis planeblock: a cadaveric study. Med Glas (Zenica) 2011;8:181-184.
16 Abdallah FW, Laffey JG, Halpern SH, Brull R. Duration of analgesic effectiveness after the posterior and lateral transversus abdominis plane block techniques for transverse lower abdominal incisions: a meta-analysis. Br J Anaesth 2013;111:721-735.
17 Shin HJ, Oh AY, Baik JS, Kim JH, Han SH, Hwang JW. Ultrasound-guided oblique subcostal transversus abdominis plane block for analgesia after laparoscopic cholecystectomy: a randomized, controlled, observer-blinded study. Minerva Anestesiol 2014;80:185-193.
18 Rahangdale R, Kendall MC, McCarthy RJ, Tureanu L, Doty R Jr, Weingart A, et al. The effects of perineural versus intravenous dexamethasone on sciatic nerve blockade outcomes: a randomized, double-blind, placebo-controlled study. Anesth Analg 2014;118:1113-1119.
19 De Oliveira GS Jr, Castro-Alves LJ, Nader A, Kendall MC, Mc-Carthy RJ. Transversus abdominis plane block to ameliorate postoperative pain outcomes after laparoscopic surgery: a meta-analysis of randomized controlled trials. Anesth Analg 2014;118:454-463.
20 Murphy GS, Szokol JW, Greenberg SB, Avram MJ, Vender JS,Nisman M, et al. Preoperative dexamethasone enhances quality of recovery after laparoscopic cholecystectomy: effect on inhospital and postdischarge recovery outcomes. Anesthesiology 2011;114:882-890.
21 Holte K, Kehlet H. Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg 2002;195:694-712.
22 McDonnell JG, Curley G, Carney J, Benton A, Costello J, Maharaj CH, et al. The analgesic efficacy of transversus abdominis plane block after cesarean delivery: a randomized controlled trial. Anesth Analg 2008;106:186-191.
23 Bisgaard T, Klarskov B, Rosenberg J, Kehlet H. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain 2001;90:261-269.
Accepted after revision February 18, 2016
Whatever you can do, or dream you can, begin it. Boldness has genius, power and magic in it.
—Johann Wolfgang von Goethe
April 2, 2015
Author Affiliations: Department of Anesthesiology (Huang SH and Li Y)and Department of Orthopedics (Wang SK), Second Hospital of Lanzhou University, Lanzhou 730030, China; Department of Anesthesiology, Sichuan Provincial People's Hospital and Sichuan Academy of Medical Sciences, Chengdu 610072, China (Lu J); Morphology Teaching and Research Section, Medical School, Northwest University for Nationalities, Lanzhou 730000, China (Gan HY); Department of Anesthesiology, Shands Hospital at the University of Florida, Florida, USA (Peng YG)
Shuan-Ke Wang, MD, Department of Orthopedics, Second Hospital of Lanzhou University, Lanzhou 730030, China(Email: wsk2zzy@126.com)
© 2016, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(16)60086-3
Published online April 20, 2016.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Serum tumor markers not useful in screening patients with pancreatic mucinous cysticlesions associated with malignant changes
- Impact of centraIization of pancreaticoduodenectomy coupIed with fast track recovery protocoI:a comparative study from India
- CIinicaI significance of isoIated biIiary candidiasis in patients with unresectabIe choIangiocarcinoma
- Open versus laparoscopic cholecystectomies in patients with or without type 2 diabetes mellitus in Spain from 2003 to 2013
- Oleanolic acid attenuates liver ischemia reperfusion injury by HO-1/Sesn2 signaling pathway
- Human hepatocytes loaded in 3D bioprinting generate mini-liver
