紫杉醇脂质体联合卡培他滨治疗老年晚期三阴乳腺癌临床疗效观察
2016-11-05阮寒光周学亮程晓华邬蒙
阮寒光,周学亮,程晓华,邬蒙
(1.南昌市第三医院乳腺肿瘤诊治中心乳腺肿瘤内科,江西330003;2.南昌大学江西医学院第一附属医院乳腺外科,江西南昌330015;3.南昌大学江西医学院第一附属医院药理研究所,江西南昌330015;4.江西省肿瘤医院乳腺肿瘤放射治疗科,南昌330029;5.南昌大学江西医学院研究生院医学部,江西南昌330006)
紫杉醇脂质体联合卡培他滨治疗老年晚期三阴乳腺癌临床疗效观察
阮寒光1,5,周学亮2△,程晓华3,邬蒙4
(1.南昌市第三医院乳腺肿瘤诊治中心乳腺肿瘤内科,江西330003;2.南昌大学江西医学院第一附属医院乳腺外科,江西南昌330015;3.南昌大学江西医学院第一附属医院药理研究所,江西南昌330015;4.江西省肿瘤医院乳腺肿瘤放射治疗科,南昌330029;5.南昌大学江西医学院研究生院医学部,江西南昌330006)
目的观察紫杉醇脂质体联合卡培他滨(TX)治疗老年晚期三阴乳腺癌(TNBC)的疗效及发生不良反应情况。方法回顾性分析该院2013年1月至2015年1月收治的54例临床分期为ⅢA~C、Ⅳ期的原发性老年晚期TNBC患者的临床资料,将其分为TX组和紫杉醇脂质体联合顺铂(TP)组各27例。TX组:紫杉醇脂质体150 mg/m2,第1天静脉滴注,卡培他滨1 000 mg/m2,第1~14天口服,每天2次,同时给予维生素B630 mg口服,每天3次,预防手足综合征,21 d为1个周期;TP组:紫杉醇脂质体150 mg/m2,第1天静脉滴注,顺铂25 mg/m2,第1~3天静脉滴注,21 d为1个周期。2个周期后评价两组疗效,最多不超过6个周期。待所有患者完成化疗后对比分析两组近期疗效及不良反应。结果54例老年晚期TNBC辅助化疗中,总有效率为68.5%(37/54)。TP组有效率[63.0%(17/27)]高于TX组[74.1%(20/27)],但差异无统计学意义(χ2=0.773,P=0.379);TX组手指足综合征发生情况明显高于TP组,差异有统计学意义(χ2=44.220,P=0.000);且TP组恶心、呕吐反应发生情况明显高于TX组,差异有统计学意义(χ2=14.172,P=0.001);两组脱发,肝、肾功能异常和血液学毒性发生情况比较,差异均无统计学意义(P>0.05)。结论在临床分期为ⅢA~C、Ⅳ期老年晚期TNBC患者的辅助化疗中,TX方案与TP方案近期疗效相当。TX方案减轻了老年晚期TNBC患者胃肠道反应的发生情况,但手足综合征的发生情况增加;因此,在提前预防手足综合征的情况下,TX方案可以作为老年晚期TNBC辅助化疗方式之一。
乳腺肿瘤;脂质体;紫杉酚;卡培他滨;不良反应
三阴乳腺癌(TNBC)是指雌激素受体(ER)、孕激素受体和人类表皮生长因子受体-2(HER-2)均为阴性的乳腺癌,此类乳腺癌患者不能从内分泌治疗和赫赛汀(注射用曲妥珠单抗)靶向治疗中获益。TNBC的治疗目前尚无统一的标准方案,有研究结果显示,在辅助治疗中含铂方案具有一定优势[1-2]。由于含铂方案对肾功能的损伤及胃肠道的影响,尤其对于老年患者更为明显,部分老年患者不能耐受。目前,临床针对老年晚期TNBC患者的辅助治疗报道较少。本研究回顾性分析了52例老年晚期TNBC的临床资料,观察紫杉醇脂质体联合卡培他滨(TX)对老年晚期TNBC的疗效与不良反应,以便为今后临床治疗方案的制订提供依据。
1 资料与方法
1.1一般资料收集南昌市第三医院2013年1月至2015年1月临床分期为ⅢA~C、Ⅳ期[依据2003版美国肿瘤联合会(AJCC)乳腺癌分期标准进行分期]的原发性TNBC老年患者54例的临床资料,且均为女性,年龄60~78岁,中位年龄67岁;辅助化疗前均经粗针活检病理结果确诊及免疫组织化学明确ER、孕激素受体、HER-2表达水平;卡氏评分(KPS评分)为70分以上;临床具有可测量的病灶。化疗前所有患者均经肝脏、肺部CT片和全身核素骨显像检查是否远处转移及转移的部位数量明确M分期。患者区域淋巴结肿大经粗针活检病理结果确诊,明确N分期,其中TX组27例,浸润性导管癌12例,浸润性小叶癌10例,其他5例;ⅢA~C期15例,Ⅳ期12例。紫杉醇联合顺铂(TP)组27例,浸润性导管癌15例,浸润性小叶癌8例,其他4例;ⅢA~C期13例,Ⅳ期14例。所有患者在化疗前均行血常规,肝、肾功能,心电图,心脏彩色多普勒超声检查,排除心、肝、肾功能严重损害。两组患者在年龄、病理类型、临床分期及KPS评分等方面比较,差异均无统计学意义(P>0.05)。见表1。
1.2方法
1.2.1治疗方法TX组:于化疗前30min给予地塞米松10 mg静脉注射及苯海拉明20 mg肌内注射预防过敏反应,紫杉醇脂质体150 mg/m2,第1天静脉滴注,卡培他滨1 000 mg/m2,第1~14天口服,每天2次,同时给予维生素B630 mg口服,每天3次,预防手足综合征,21 d为1个周期。TP组:紫杉醇脂质体150 mg/m2,第1天静脉滴注,顺铂25 mg/m2,第1~3天,静脉滴注,21d为1个周期。每2个周期对两组患者进行疗效评价,治疗有效化疗持续至第6个周期。如疾病进展(PD),则停止该方案治疗。化疗期间监测两组患者血常规及肝、肾功能。若临床出现Ⅲ度以上不良反应,化疗药物剂量减量20%。
1.2.2疗效判断标准采用实体瘤治疗疗效评价(RECIST)标准,乳腺肿瘤原发病灶及转移病灶最大直径缩小大于或等于30%为部分缓解(PR),增大大于或等于20%为PD。所有患者均由同一组医生对肿瘤原发病灶及转移病灶进行评价,化疗后出现PD时终止化疗。疗效分为临床完全缓解(CR)、PR、疾病稳定(SD)及PD,临床有效率(RR)=CR+PR。毒性反应按WHO分级标准分为0、Ⅰ、Ⅱ、Ⅲ、Ⅳ级进行评价。
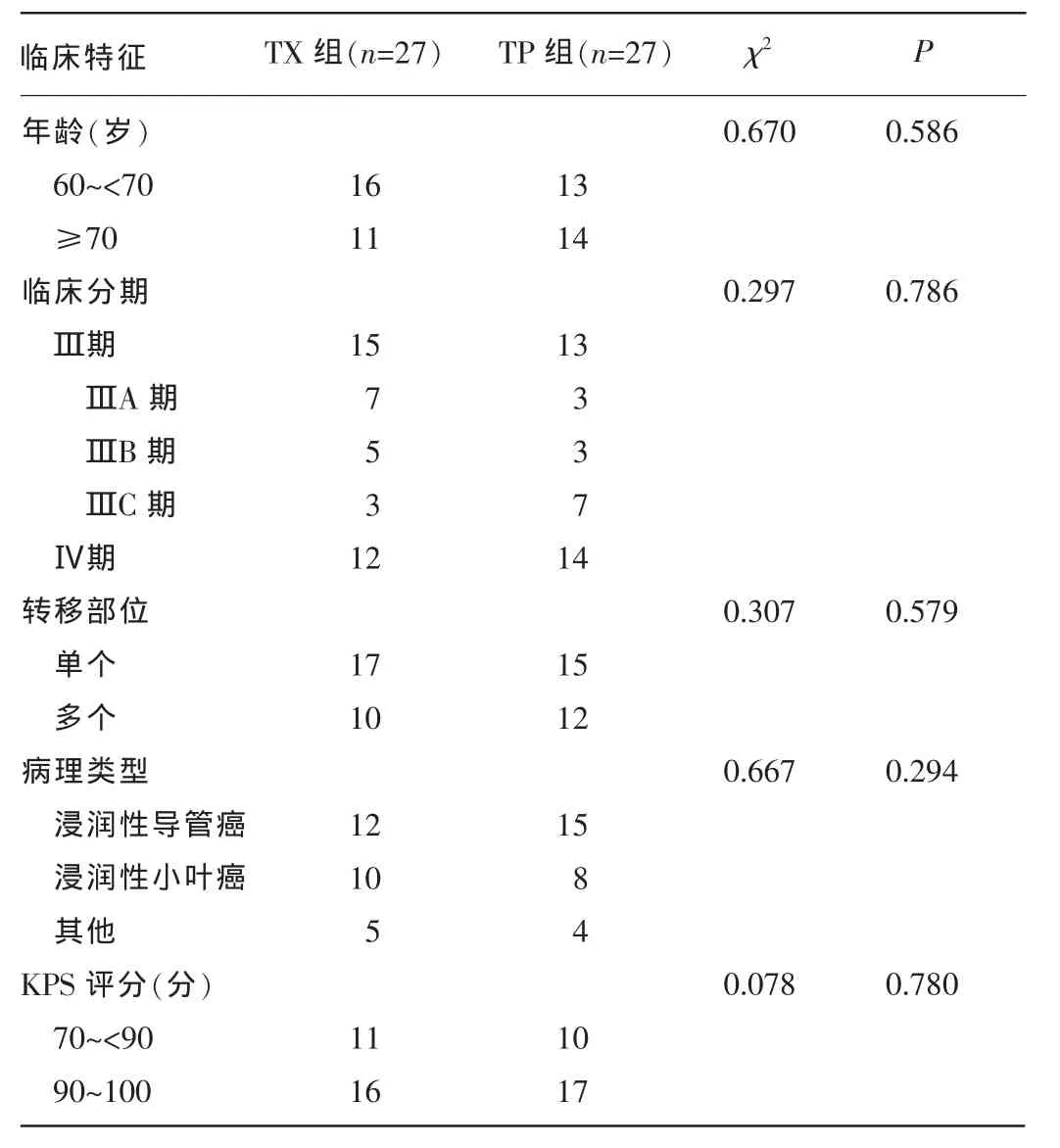
表1 两组患者临床特征比较
1.3统计学处理应用SPSS17.0统计软件进行数据分析,采用Pearson χ2检验或Fisher′s精确检验。P<0.05为差异有统计学意义。
2 结果
2.1两组患者近期疗效比较所有患者行2个及2个以上周期化疗,最多不超过6个周期,共完成278个周期化疗,平均5.15个周期。其中TX组完成143个周期,TP组完成135个周期。TX组2例患者因Ⅲ度手足综合征经2个周期化疗后终止治疗,而TP组1例患者出现Ⅳ度恶心、呕吐后终止治疗;TX组3例患者完成4个周期后出现PD改用其他方案治疗,TP组2例患者4周期化疗后因疾病进展更改方案。54例老年晚期TNBC辅助化疗中,总有效率为68.5%(37/54),TP组有效率[74.1%(20/27)]高于TP组[63.0%(17/27)],但差异无统计学意义(χ2=0.773,P=0.379)。见表2。未完成6个周期化疗的患者评估其体力状况及心、肺、肝、肾功能,决定是否采用继续化疗或最佳支持治疗。而完成6个周期化疗患者选择继续口服卡培他滨维持治疗或观察等待处理。
2.2两组患者不良反应发生情况比较两组患者均出现不同程度骨髓抑制,如中性粒细胞、血小板及血红蛋白减少,TX组Ⅲ+Ⅳ度骨髓抑制稍高于TP组,但差异均无统计学意义(P>0.05)。在肾功能损伤方面,两组患者不良反应均较轻,可能与患者年龄均偏大、化疗前及化疗中预先给予相应的处理措施有关。TX组发生手足综合征明显高于TP组,因发生Ⅲ度手足综合征2个周期后终止治疗的患者2例,两组比较,差异有统计学意义(χ2=44.220,P=0.000);而TP组发生恶心、呕吐明显高于TX组,差异有统计学意义(χ2=14.172,P=0.001),且TP组1例患者出现Ⅳ度恶心、呕吐后终止治疗,两组患者发生腹泻均较低,差异均无统计学意义(P>0.05)。两组患者脱发,肝、肾功能异常发生情况比较,差异均无统计学意义(P>0.05)。见表3。
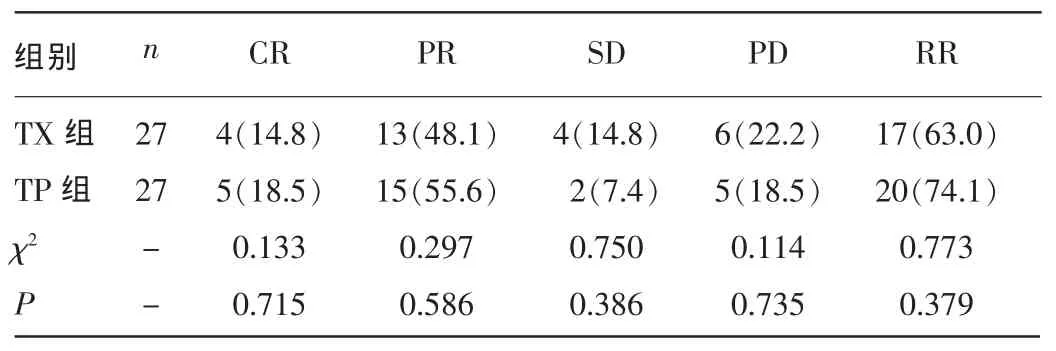
表2 两组患者近期疗效比较
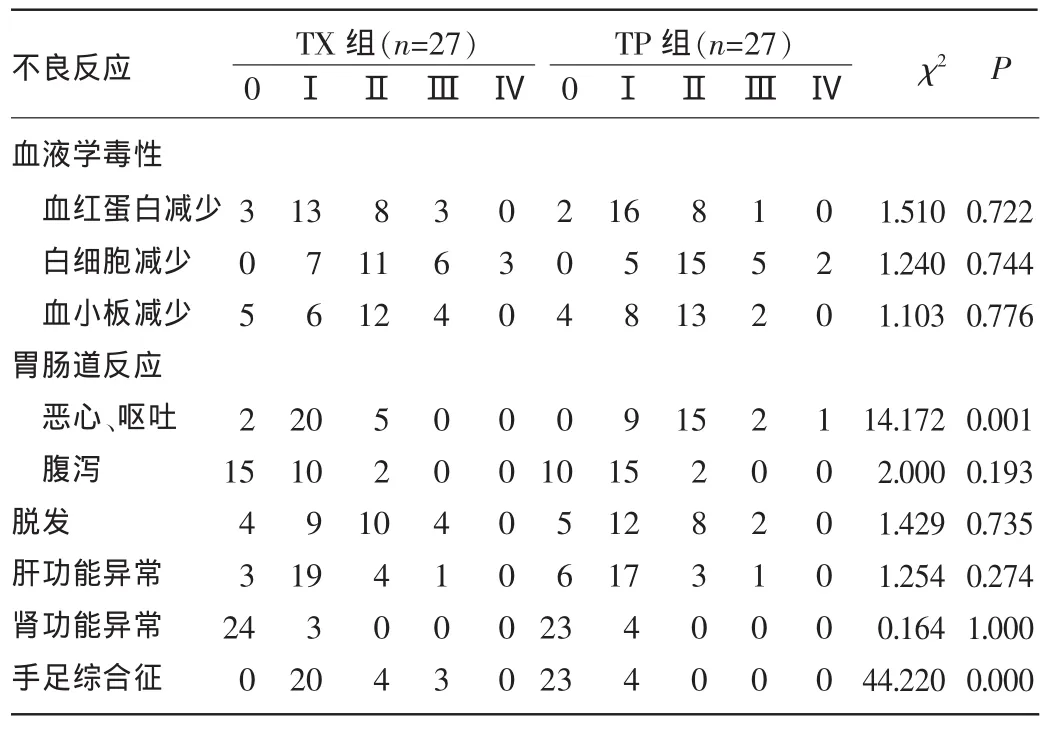
表3 两组患者化疗不良反应发生情况比较(n)
3 讨论
临床根据乳腺癌患者病理组织的免疫组织化学、肿瘤细胞形态及基因表达产物的不同,把乳腺癌分为4种亚型:Luminal A型、Luminal B型、HER-2过表达型和其他型。TNBC是指ER、孕激素受体和HER-2均为阴性乳腺癌。TNBC患者和“基底样”患者有近80%的重合,但前者还包含一些特殊组织学类型,如低危(典型)髓样癌及腺样囊性癌。此类乳腺癌患者不能从内分泌治疗和赫赛汀靶向治疗中获益[3-5]。晚期TNBC包括局部晚期及转移性乳腺癌,目前临床治疗手段仍是以化疗为主的综合治疗为指导。已有大量研究证实,紫杉醇类药物及卡培他滨在乳腺癌的辅助治疗中有效[6-12]。紫杉醇药物加入脂蛋白后患者外周血液和肝脏毒性反应明显减弱,且显著减弱对骨髓的抑制作用,其对血压的影响及相关不良反应发生率也明显低于单纯紫杉醇注射液,明显提高了机体对紫杉醇的耐受性。本品在部分脏器中水平明显增高,且呈靶向性。卡培他滨是新一代氟尿嘧啶类口服治疗药物,具有应用方便、生物利用度高等优点。卡培他滨作用于细胞S期,其特点是选择性肿瘤内活化,所以肿瘤组织中氟尿嘧啶浓度高而全身氟尿嘧啶浓度低,降低了全身毒性。而主要不良反应为手足综合征、恶心、呕吐等[13]。本研究结果显示,二者联合治疗不良反应轻、疗效肯定,且与蒽环类药无交叉耐药性。
本研究将TX方案应用于老年晚期TNBC辅助治疗中,取得了较好的效果,TP组有效率稍高于TX组,但差异无统计学意义(χ2=0.773,P=0.379)。一项临床Ⅱ期研究结果显示,卡培他滨联合紫杉醇类药物用于晚期乳腺癌的新辅助治疗中,患者CR发生率为19.0%~22.0%。且总有效率达72.0%~97.6%[14-16]。而有研究结果显示,卡培他滨单药用于晚期乳腺癌的一线治疗是安全、有效的[17-18]。与其他药物联合使用,总有效率达30.0%~70.0%[19],与本研究结果相似。在不良反应方面,TX组主要表现为手足综合征,3例患者发生Ⅲ度以上手足综合征,且发生率为11.1%(3/27),其中2例患者因该不良反应放弃治疗,1例患者通过治疗后坚持治疗完成整个疗程。而TP组主要不良反应为肾功能损伤,胃肠道中的恶心、呕吐不适。Ⅲ度以上恶心、呕吐发生率明显高于TX组。Smorenburg等[20]进行了一项临床Ⅲ期试验,将单药脂质体阿霉素或卡培他滨应用于老年晚期乳腺癌的一线治疗,两组患者中位生存期分别为5.6、7.7个月,手足综合征发生率分别为10.0%、16.0%,该结果与本研究报道的不良反应发生情况相似。在老年乳腺癌患者中大多数年龄偏大,胃肠道反应不适影响其进食及营养吸收,从而导致该类患者体质量下降,耽误治疗。而手足综合征大多数表现为表面皮肤颜色改变及瘙痒、皲裂等,通常不影响患者营养状态及内脏功能。作者认为,通过适当的对症治疗,大部分发生手足综合征的患者仍可以克服其不良反应,从而继续治疗。
本研究表明,TX方案对老年晚期TNBC有较好疗效,且不良反应通过相应的对症治疗后可以克服,且药物使用方便,因此,该方法可成为老年晚期TNBC的治疗方案。
[1]Berrada N,Delaloge S,André F.Treatment of triple-negative metastatic breastcancer:towardindividualizedtargetedtreatmentsorchemosensitization?[J].Ann Oncol,2010,21 Suppl 7:vii30-vii35.
[2]Kim HR,Jung KH,Im SA,et al.Multicentre phaseⅡtrial of bevacizumab combined with docetaxel-carboplatin for the neoadjuvant treatment of triple-negative breast cancer(KCSG BR-0905)[J].Ann Oncol,2013,24(6):1485-1490.
[3]Silver DP,Richardson AL,Eklund AC,et al.Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer[J].J Clin Oncol,2010,28(7):1145-1153.
[4]Venkitaraman R.Triple-negative/basal-like breast cancer:clinical,pathologicand molecular features[J].Expert Rev Anticancer Ther,2010,10(2):199-207.
[5]Isakoff SJ,Mayer EL,He L,et,al.TBCRC009:a Multicenter phaseⅡclinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer[J].J Clin Oncol,2015,33(17):1902-1909.
[6]O′Shaughnessy J,Schwartzberg L,Danso MA,et al.PhaseⅢstudy of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer[J]. J Clin Oncol,2014,32(34):3840-3847.
[7]Biganzoli L,Coleman R,Minisini A,et al.A joined analysis of two European Organization for the Research and Treatment of Cancer(EORTC)studies to evaluate the role of pegylated liposomal doxorubicin(Caelyx)in the treatment of elderly patients with metastatic breast cancer[J].Crit Rev Oncol Hematol,2007,61(1):84-89.
[8]Pierga JY,Fumoleau P,Brewer Y,et al.Efficacy and safety of single agent capecitabine in pretreated metastatic breast cancer patients from the French compassionate use program[J].Breast Cancer Res Treat,2004,88(2):117-129.
[9]Wildiers H,Mauer M,Pallis A,et al.End points and trial design in geriatric oncology research:a joint European organisation for research and treatment of cancer——Alliance for Clinical Trials in Oncology——International Society Of Geriatric Oncology position article[J].J Clin Oncol,2013,31(29):3711-3718.
[10]Perez EA,Hillman DW,Dentchev T,et al.North central cancer treatment group(NCCTG)N0432:phase II trial of docetaxel with capecitabine and bevacizumab as first-line chemotherapy for patients with metastatic breast cancer[J].Ann Oncol,2010,21(2):269-274.
[11]Gerritse FL,Meulenbeld HJ,Roodhart JM,et al.Analysis of docetaxel therapyinelderly(≥70years)castrationresistantprostatecancerpatients enrolled in the Netherlands Prostate Study[J].Eur J Cancer,2013,49(15):3176-3183.
[12]Hamaker ME,Seynaeve C,Nortier JW,et al.Slow accrual of elderly patients with metastatic breast cancer in the Dutch multicentre OMEGA study[J].Breast,2013,22(4):556-559.
[13]PerezEA,Hillman DW,Dentchev T,et al.North Central Cancer Treatment Group(NCCTG)N0432:phaseⅡtrial of docetaxel with capecitabine and bevacizumab as first-line chemotherapy for patients with metastatic Breast cancer[J].Ann of Oncol,2010,21(2):269-274.
[14]Lebowitz PF,Eng-Wong J,Swain SM,et al.A phaseⅡtrial of neoadjuvant docetaxel and capecitabine for locally advanced breast cancer[J].Clin Cancer Res,2004,10(20):6764-6769.
[15]Telli ML,Jensen KC,Vinayak S,et al.PhaseⅡstudy of gemcitabine,carboplatin,and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer with assessment of a tumorbased measure of genomic instability[J].J Clin Oncol,2015,33(17):1895-1901.
[16]Jinno H,Sakata M,Hayashida T,et al.A phaseⅡtrial of capecitabine and docetaxel followed by 5-fluorouracil/epirubicin/cyclophosphamide(FEC)as preoperative treatment in women with stageⅡ/Ⅲbreast cancer[J].Ann Oncol,2010,21(6):1262-1266.
[17]Ershler WB.Capecitabine monotherapy:safe and effective treatment for metastatic breast cancer[J].Oncologist,2006,11(4):325-335.
[18]Lee SH,Lee J,Park J,et al.Capecitabine monotherapy in patients with anthracycline-and taxane-pretreated metastatic breast cancer[J].Med Oncol,2004,21(3):223-231.
[19]Arowolo OA,Njiaju UO,Ogundiran TO,et al.Neo-adjuvant capecitabine chemotherapy in women with newly diagnosed locally advanced breast cancer in a resource-poor setting(Nigeria):efficacy and safety in a phaseⅡfeasibility study[J].Breast J,2013,19(5):470-477.
[20]Smorenburg CH,de Groot SM,van Leeuwen-Stok AE,et al.A randomized phaseⅢstudy comparing pegylated liposomal doxorubicin with capecitabine as first-line chemotherapy in elderly patients with metastatic breast cancer:results of the OMEGA study of the Dutch Breast Cancer Research Group BOOG[J].Ann Oncol,2014,25(3):599-605.
Observation on clinical efficacy of docetaxel liposo me combined with capecitabine in treating elderly advanced 3-negative breast cancer
Ruan Hanguang1,5,Zhou Xueliang2△,Chen Xiaohua3,Wu Meng4(1.Department of Breast Oncology,DiagnosisandTreatmentCenterofBreastTumor,NanchangMunicipalThirdHospital,Jiangxi330003,China;2.Department of Breast Surgery,First Affiliated Hospital of Jiangxi Medical College,Nanchang University,Nanchang,Jiangxi 330015,China;3.Research Institute of Pharmacology,First Affiliated Hospital of Jiangxi Medical College,Nanchang University,Nanchang,Jiangxi330015,China;4.Department of Breast Oncology Radiotherapy,Jiangxi Provincial Tumor Hospital,Nanchang 330029,China;5.Department of Medicine,Postgraduate School,Jiangxi Medical College,Nanchang University,Nanchang,Jiangxi330006,China)
ObjectiveToobservetheefficacyandadversereactionsofdocetaxelliposomecombinedwithcapecitabine(TX)in the treatment of elderly advanced 3-negative breast cancer(TNBC).MethodsThe clinical data in 54 cases of clinical stageⅢA~C,Ⅳof elderly advanced TNBC in this hospital from January 2013 to January 2015 were retrospectively analyzed.All patients were divided into the TX group and docetaxel liposome combined with cisplatin group(TP),27 cases in each group.TX group was given docetaxel liposome 150 mg/m2by intravenous drip on 1 d and oral TX 1 000 mg/m2on 1-14 d twice daily,meanwhile oral vitamin B630 mg,3 times daily,for preventing hand-foot syndrome,with 21 d as 1 cycle;the TP group was given docetaxel liposome 150 mg/m2by intravenous drip on 1 d and cisplatin 25 mg/m2by intravenous drip on 1-3 d,with 21 d as 1 cycle.The efficacy was evaluated after 2 cycles,not more than 6 cycles.The short-term efficacy and adverse reactions were analyzed and compared between the two groups after finishing chemotherapy in all the cases.ResultsIn the adjuvant chemotherapy of 54 cases of elderly advanced TNBC,the total effective rate was 68.5%(37/54),the effective rate in the TP group was higher than that in the TX group without statistical difference[63.0%(17/27)vs.74.1%(20/27),χ2=0.773,P=0.379];the occurrence rate of hand-foot syndrome in the TX group was significantly higher than that in the TP group,the difference was statistically significant(χ2=44.220,P=0.000),moreover the occurrence rates of nausea and vomiting in the TP group was significantly higher than that in the TX group,the difference was statistically significant(χ2=14.172,P=0.001);but the occurrence of hair loss,liver and kidney function abnormality and hematological toxicology had no statistically significant difference between the two groups(P>0.05).Conclusion
In the adjuvant chemotherapy of clinical stageⅢA-C andⅣof elderly advanced TNBC,the TX regimen and TP regimen have the same short-term effect.The TX regimen alleviates the occurrence of gastrointestinal reactions,but the occurrence of hand-foot syndrome is increased;therefore in that case of preventing this complication in advance,the TX regimen may serve as one of adjuvant chemotherapeutic regimens in elderly advanced TNBC.
Breast neoplasms;Liposomes;Paclitaxel;Capecitabine;Side effects
10.3969/j.issn.1009-5519.2016.19.006
A
1009-5519(2016)19-2954-03
阮寒光(1982-),博士研究生,主治医师,主要从事乳腺肿瘤放、化疗的研究。△
,E-mail:jxcancer@163.com。
(2016-05-16)
