NADPH氧化酶在Ang II介导H9C2心肌细胞凋亡中的作用
2016-10-20何茜蔡超陈珍
何茜,蔡超,陈珍
· 论著 ·
NADPH氧化酶在Ang II介导H9C2心肌细胞凋亡中的作用
何茜1,蔡超1,陈珍1
目的 分析血管紧张素Ⅱ(Ang Ⅱ)介导的H9C2心肌细胞凋亡是否通过NADPH氧化酶/P38MAPK途径。方法 体外培养H9C2心肌细胞,分组干预:①Control组:仅加细胞培养液;②AngⅡ组:在Control组的基础上加入AngⅡ;③apocynin组:在Control组的基础上加apocynin;④AngⅡ+apocynin组:在Control组的基础上加入AngⅡ以及apocynin(n=6)。干预24 h后测定NADPH氧化酶活性,TUNEL法检测细胞凋亡,Western Blot 检测P38MARK及相关凋亡蛋白的表达水平。结果 Control组、apocynin组、AngⅡ组、Ang II+apocynin组凋亡比例分别为(12.20±1.18)%、(14.71±3.88)%、(62.33± 4.79)%、(13.67±2.59)%。与Control组比较,AngⅡ组凋亡比例明显增加,而AngⅡ+apocynin组凋亡比例较AngⅡ组下降,差异有统计学意义(P均<0.05)。与Control组比较,加入NADPH氧化酶抑制剂apocynin后细胞NADPH氧化酶活性降低;而仅加入AngⅡ的细胞NADPH氧化酶活性明显升高,差异有统计学意义(P<0.05)。AngⅡ+apocynin组的NADPH氧化酶活性较AngⅡ组降低,差异有统计学意义(P<0.05)。与正常细胞比较,AngⅡ作用后,P38MAPK表达增加,凋亡蛋白Bax表达增加,抗凋亡蛋白Bcl-2表达减少,差异有统计学意义(P均<0.05)。Ang II+apocynin组较AngⅡ组P38MAPK表达降低,凋亡蛋白Bax表达减少,抗凋亡蛋白Bcl-2表达增加,差异有统计学意义(P均<0.05)。结论 血管紧张素II通过NADPH氧化酶/P38MAPK途径介导H9C2心肌细胞凋亡。
凋亡;血管紧张素II;NADPH氧化酶;信号传导
NADPH氧化酶类是决定内皮细胞和血管平滑肌细胞氧化还原状态的关键因素[1,2]。NADPH氧化酶激活参与高血压和动脉粥样硬化的病理生理过程。血管紧张素Ⅱ(Ang Ⅱ)被证实激活内皮细胞、血管平滑肌细胞和成纤维细胞的NADPH氧化酶。另有研究表明[3],Ang II诱导心肌细胞凋亡,但NADPH氧化酶是否参与其中尚不明确。
apocynin是NADPH氧化酶的特异性抑制剂,抑制其亚基p47-phox活性,进而抑制其激活[4]。同时抑制NADPH的活性和氧自由基(ROS)的产生,能够抑制动脉粥样硬化的起始过程。本研究将明确Ang Ⅱ介导的H9C2心肌细胞凋亡是否通过NADPH氧化酶以及P38MAPK途径。
1 材料与方法
1.1主要试剂 H9C2心肌细胞购自中国科学院上海生命科学研究院,DMEM培养基、胎牛血清(FBS)、胰蛋白酶均购自Hyclone公司,TUNEL细胞凋亡原位检测试剂盒购自凯基生物科技发展有限公司,兔抗鼠P38 MAPK、Bcl-2及Bax 抗体购自Proteintech,其他试剂均购自上海生物工程有限公司。
1.2细胞培养 H9C2心肌细胞用含90% DMEM培养基、10%胎牛血清、青霉素(100μ/ml)和链霉素(100 μg /ml)的细胞培养液,在95%空气和5% CO2培养箱中37℃培养。培养基每2~3 d更换1次。待细胞生长至对数期时,接种至6孔培养板,4 ml/孔(细胞数4×105/孔),进行分组处理。
1.3实验分组和干预 H9C2心肌细胞分组:①Control组:仅加细胞培养液;②AngⅡ组:在Control组的基础上加AngⅡ,终浓度为100 nmol/L;③apocynin组:在Control组的基础上加apocynin,终浓度为100 μmol/L;④Ang Ⅱ+apocynin组:在Control组的基础上加终浓度100 nmol/L的AngⅡ以及100 μmol/L的apocynin。
1.4NADPH氧化酶活性测定 细胞经药物处理24 h后,除去培养基,PBS洗涤2次。 每孔加100 μl胰蛋白酶消化1min,吹打后将细胞悬液加入EP 管中, 每孔再加入100 μl PBS洗涤,在4℃下2500 g离心5 min,然后以PBS再悬浮,随后加入250 μmol/L NADPH孵育,在λ=340 nm下观察5 min,通过吸光率的减少来探测NADPH的消耗量。为分析NADPH氧化酶的活性,在检测之前30 min加入10 μmol/L DPI,来检测DPI抑制后的NADPH消耗率。为了标准化,取等量的细胞加入SDS溶解,浓缩蛋白后通过Lowry solution测定。用来计算NADPH消耗总量的吸收消光系数是6.22 mM-1 cm-1,结果以pmol NADPH / min·mg蛋白表示。
1.5TUNEL法检测细胞凋亡 ①细胞96孔板固定:各孔分别加入100μl预冷的4%多聚甲醛,室温下固定1h;②通透:吸出多聚甲醛后用PBS洗3次,各孔加入100μl的0.1%柠檬酸钠、0.1%TritonX-100,冰上通透8 min;③ 封闭:将液体吸出后PBS洗3次(3 min/次),并用3%H2O2/甲醇室温避光反应30 min;④ 制阳性片:每孔加100 μl含不同活性单位的DNaseⅠ反应液,室温反应30 min,然后PBS洗3次(3 min/次);⑤连接标记:吸干PBS后每孔加40μl Equilibration Buffer+1.0 μl FITC-12-dUTP和4.0 μl TdT Enzyme,加盖玻片37℃避光60 min(其中阴性对照不加TdT酶反应液),结束后PBS洗3次(3 min/次);⑥镜检:采用激发波长450~500 nm,发射波长515~565 nm荧光显微镜检测;⑦采集图片,统计凋亡率:每组设3个复孔,每个复孔取6个视野,统计每视野下TUNEL阳性细胞所占百分比,计算细胞凋亡率。
1.6Western Blot测定凋亡相关蛋白的表达 细胞经药物处理24 h后,除去培养基,PBS 洗涤2次。 每孔加1 ml 胰蛋白酶消化1 min,吹打后将细胞悬液加入EP 管中, 每孔再加入 200 μl PBS洗涤,4℃ 2500 g离心5 min,将EP管底部的细胞用冷 0.01 mol/L PBS 清洗3次后加150 μl 细胞裂解液和3 μl的蛋白酶抑制剂PMSF,提取总蛋白后通过BCA 法测定蛋白浓度。40 μg蛋白加入5 ×SDS凝胶加样缓冲液中,100℃加热5 min使蛋白变性。12% SDS-PAGE电泳分离蛋白,转移至PVDF膜,封闭液封闭1 h,按1:1000分别加入兔抗鼠P38 MAPK、Bcl-2及Bax 一抗,4℃孵育过夜。分别加入辣根过氧化物酶标记的山羊抗兔二抗(1:1000),室温孵育1 h,用超敏ECL化学发光显色液进行显色。用Labwork凝胶图像分析系统对胶片扫描,以Control组的面积灰度值为100与实验组进行比较和半定量分析。
1.7统计学分析 所有数据均采用SPSS 18.0统计软件处理。计量资料采用均数±标准差(±s)表示,多组间均数的比较采用方差分析。P<0.05为差异有统计学意义。
2 结果

图1 TUNEL染色结果(A:Control组;B:apocynin组;C:Ang II组;D:Ang II+apocynin组,n=3)
2.1TUNEL染色结果 采用荧光显微镜观察H9C2心肌细胞变化,绿色荧光标记的凋亡细胞的细胞核,AngⅡ组凋亡细胞较多(图1)。Control组、apocynin组、AngⅡ组、AngⅡ+apocynin组凋亡比例分别为(12.20±1.18)%、(14.71 ±3.88)%、(62.33±4.79)%、(13.67± 2.59)%。与Control组比较,AngⅡ组凋亡比例明显增加,而AngⅡ+apocynin组凋亡比例较AngⅡ组下降,差异有统计学意义(P均<0.05)。
2.2各组NADPH氧化酶活性比较 Control组、apocynin组、AngⅡ组、AngⅡ+apocynin组NADPH氧化酶活性分别为(3.34±0.89)pmol NADPH/ min·mg蛋白、(3.11±0.78)pmol NADPH/ min·mg蛋白、(11.03±3.41)pmol NADPH/ min·mg蛋白、(3.76±1.06)pmol NADPH/ min·mg蛋白。与Control组比较,加入NADPH氧化酶抑制剂apocynin后NADPH氧化酶活性降低;而仅加入Ang II的NADPH氧化酶活性明显升高,差异有统计学意义(P<0.05)。AngⅡ+apocynin组的NADPH氧化酶活性较AngⅡ组降低,差异有统计学意义(P<0.05)。
2.3各组凋亡相关蛋白的表达水平比较 与正常细胞比较,AngⅡ作用后,P38 MAPK表达增加,凋亡蛋白Bax表达增加,抗凋亡蛋白Bcl-2表达减少,差异有统计学意义(P<0.05)。AngⅡ+apocynin组较AngⅡ组,P38 MAPK表达降低,凋亡蛋白Bax表达减少,抗凋亡蛋白Bcl-2表达增加,差异有统计学意义(P均<0.05)(图2,表1)。
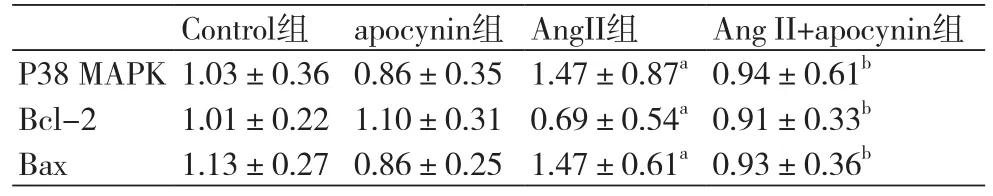
表1 各组凋亡相关蛋白的表达水平(n=6)
3 讨论
NADPH氧化酶在血管平滑肌细胞和内皮细胞均表达,AngⅡ激活NADPH氧化酶提高此类细胞的活性[5]。本研究进一步表明,AngⅡ提高NADPH氧化酶活性,这种变化能被NADPH氧化酶抑制剂apocynin逆转,与其他研究[6]结果一致。此外,NADPH氧化酶参与AngⅡ诱导的心肌肥大[7]。
Ma等[8]研究发现,AngⅡ可以诱导新生大鼠心肌细胞和成年大鼠心室细胞凋亡。这些发现表明,AngⅡ介导的心肌细胞凋亡是通过AT1受体。Komiya M等[9]证实在细胞中加入NADPH氧化酶抑制剂apocynin可抑制细胞凋亡。然而,该研究未提供关于NADPH氧化酶活性和表达的变化。
Bcl-2位于线粒体和细胞核膜,Bcl-2磷酸化和失活导致细胞凋亡。P38MAPK激活已被证明诱导线粒体Bcl-2失活,产生细胞色素C从线粒体释放细胞质,导致Caspase-3激活和凋亡[10]。本研究中,Ang II促使P38MAPK以及Bax蛋白表达增加,而抑制Bcl-2蛋白表达,与Zhao HR等[11]研究结果一致。而apocynin干预后P38 MAPK及Bax蛋白表达减少,Bcl-2蛋白表达增加,说明这些变化通过NADPH氧化酶介导。总之,Ang II通过NADPH氧化酶/P38 MAPK途径诱导H9C2心肌细胞凋亡。
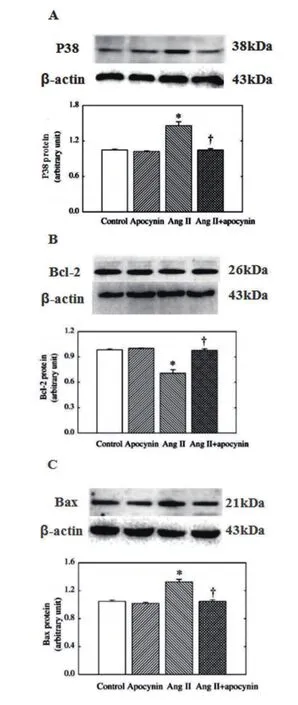
图2 凋亡相关蛋白的表达水平(与Control组比较,*P<0.05;与AngII组比较,+P<0.05,n=6)
[1] Bai YP,Hu CP,Yuan Q,et al. Role of VPO1, a newly identified heme-containing peroxidase, in ox-LDL induced endothelial cell apoptosis[J]. Free Radic Biol Med,2011,51(8):1492-500.
[2] Ma QL,Zhang GG,Peng J,et al. Vascular peroxidase 1: a novel enzyme in promoting oxidative stress in cardiovascular system[J]. Trends Cardiovasc Med,2013,23(5):179-83.
[3] Kim JI,Jung SW,Yang E,et al. Heat shock augments angiotensin II-induced vascular contraction through increased production of reactive oxygen species[J]. Biochem Biophys Res Commun,2010,399(3):452-7.
[4] Winiarska K,Focht D,Sierakowski B,et al. NADPH oxidase inhibitor,apocynin, improves renal glutathione status in Zucker diabetic fatty rats:a comparison with melatonin[J]. Chem Biol Interact,2014,218(2):12-9.
[5] Silva J,Pastorello M,Arzola J,et al. AT1receptor and NAD(P)H oxidase mediate angiotensin II-stimulated antioxidant enzymes and mitogen-activated protein kinase activity in the rat hypothalamus[J]. J Renin Angiotensin Aldosterone Syst,2010,11(4):234-42.
[6] Kim JI,Jung SW,Yang E,et al. Heat shock augments angiotensin II-induced vascular contraction through increased production of reactive oxygen species[J]. Biochem Biophys Res Commun,2010,399(3):452-7.
[7] Kaysen GA,Eiserich JP. The role of oxidative stress-altered lipoprotein structure and function and microinflammation on cardiovascular risk in patients with minor renal dysfunction[J]. J Am Soc Nephrol,2004,15(3):538-48.
[8] Ma Y,Kong L,Kai N,et al. Apolipoprotein-J prevents angiotensin II-induced apoptosis in neonatal rat ventricular cells[J]. Lipids Health Dis,2015,14(1):114.
[9] Komiya M,Fujii G,Miyamoto S,et al. Suppressive effects of the NADPH oxidase inhibitor apocynin on intestinal tumorigenesis in obese KK-A(y) and Apc mutant Min mice[J]. Cancer Sci,2015,106(11):1499-505.
[10] Shimizu S,Ishigamori R,Fujii G,et al. Involvement of NADPH oxidases in suppression of cyclooxygenase-2 promoterdependent transcriptional activities by sesamol[J]. J Clin Biochem Nutr,2015,56(2):118-22.
[11] Zhao HR,Jiang T,Tian YY,et al. Angiotensin II triggers apoptosis via enhancement of NADPH oxidase-dependent oxidative stress in a dopaminergic neuronal cell line[J]. Neurochem Res,2015,40(4):854-63.
本文编辑:姚雪莉
Effect of NADPH oxidase on Ang II-mediated apoptosis of H9C2 cardiomyocytes
HE Qian*, CAI Chao,CHEN Zhen.*Department of Cardiovasology, Taihe Hospital, Shiyan City, Shiyan 442000, China.
Objective To analyze the apoptosis of H9C2 cardiomyocytes induced by angiotensin II (Ang II)through NADPH oxidase/P38MAPK pathway. Methods H9C2 cardiomyocytes were cultured in vitro, and then divided into control group (added only nutrient fluid), Ang II group (added Ang II besides nutrient fluid), apocynin group (added apocynin besides nutrient fluid) and Ang II+apocynin group (added Ang II and apocynin besides nutrient fluid, each n=6). The activity of NADPH oxidase was detected after 24 h, apoptosis was detected by using terminal-deoxynucleoitidyl transferase mediated nick end labeling (TUNEL), and expressions of P38MARK and relevant apoptotic protein were detected by using Western Blot method. Results The apoptotic rate was (12.20± 1.18)% in control group, (14.71±3.88)% in apocynin group, (62.33±4.79)% in Ang II group and (13.67±2.59)% in Ang II+apocynin group. Compared with control group, the apoptotic rate increased significantly in Ang II group,and compared with Ang II group it decreased in Ang II+apocynin group (all P<0.05). Compared with control group,the activity of NADPH oxidase decreased in apocynin group and increased significantly in Ang II group (P<0.05). The activity of NADPH oxidase decreased in Ang II+apocynin group compared with Ang II group (P<0.05). Compared with normal cells, the expressions of P38MAPK and apoptotic protein Bax increased, and expression of anti-apoptotic protein Bcl-2 decreased after Ang II effecting (all P<0.05). The expressions of P38MAPK and apoptotic protein Bax decreased, and expression of anti-apoptotic protein Bcl-2 increased in Ang II+apocynin group compared with Ang II group (all P<0.05). Conclusion The apoptosis of H9C2 cardiomyocytes is induced by Ang II through NADPH oxidase/P38MAPK pathway.
Apoptosis; Angiotensin II; NADPH oxidase; Signal transduction
· 论著 ·
R541
A
1674-4055(2016)09-1093-03
1442000 十堰,十堰市太和医院心血管内科
10.3969/j.issn.1674-4055.2016.09.23
