Effects of Pesticide Exposure on Embryonic Development and Hatchling Traits of Turtles
2016-09-28BaofengWULiangLIANGLiangMAandWeiguoDU
Baofeng WU, Liang LIANG, Liang MA and Weiguo DU
Key Laboratory of Animal Ecology and Conservation Biology, Institute of Zoology, Chinese Academy of Sciences,Beijing 100101, People's Republic of China
Effects of Pesticide Exposure on Embryonic Development and Hatchling Traits of Turtles
Baofeng WU, Liang LIANG, Liang MA and Weiguo DU*
Key Laboratory of Animal Ecology and Conservation Biology, Institute of Zoology, Chinese Academy of Sciences,Beijing 100101, People's Republic of China
Deltamethrin is a widespread environmental hormone with endocrine-disrupting properties, but its effect on embryonic development of reptiles is largely unexplored. We investigated the effects of deltamethrin on embryonic development and offspring traits in two turtle species, one with parchment-shelled eggs and the other with rigidshelled eggs. Deltamethrin exposure during egg incubation did not affect hatching success and hatchling body size in either species. However, embryonic exposure to deltamethrin resulted in reduced hatchling locomotor performance in the red-eared slider turtle (Trachemys scripta) with parchment-shelled eggs, but not in the Chinese three-keeled pond turtle (Chinemys reevesii) with rigid-shelled eggs. These results suggest that parchment-shelled eggs are likely more vulnerable to deltamethrin than rigid-shelled eggs.
Parchment-shelled eggs, Rigid-shelled eggs, Red-eared slider turtle, Trachemys scripta, Chinese threekeeled pond turtle, Chinemys reevesii, Embryo, Environmental hormone
1. Introduction
Pyrethroids are widely applied to control insect pests of agriculture (particularly on crops), public health, home,and garden (Amweg et al., 2005; Oros and Werner, 2005;U.S. Department of Agriculture, 2007). In spite of the relatively low mammalian toxicity and biodegradability(Leahay, 1985), environmental pollution and food safety problems are concomitant with the increase in pyrethroid pesticide application amounts. Moreover, pyrethroids have been found to occur in waters (0.04 to 24 μg/L) and animals (3 to 50ng/g) (Pawlisz et al., 1998), and their biological effects have received increasing scientific attention (Mubarak et al., 2006; Righi, et al., 2009). Endocrine disruption by pesticides has raised concerns about their potential health hazard. The majority of pyrethroid pesticides belong to the “environmental hormones” group, and their long-term exposure can cause chronic disease and even have teratogenic, carcinogenic,and mutagenic effects (Sinha et al., 2004).
Deltamethrin, an important synthetic pyrethroid and a highly effective pesticide, has been widely applied in public health and agricultural programs (Moretti et al., 1997; McKinlay et al., 2008). Deltamethrin has been reported to be ecotoxic to various animals such as copepods (Tidou et al., 1992), mussels (Thybaud, 1990;Kontreczky et al., 1997), freshwater fi sh (Delistraty, 2000;Datta and Kaviraj, 2003; Svobodova et al., 2003), and the leopard frog (Bridges, 2000). Deltamethrin has already been reported to affect behavior (Kontreczky et al.,1997; Lazarini et al., 2001), growth (Datta and Kaviraj,2003), reproduction (Presibella et al., 2005), and the nervous system of animals (Aziz et al., 2001). However,few studies have reported the effect of deltamethrin on the growth and development of reptiles, and especially embryos.
Reptile embryos are extremely sensitive to environmental conditions. The environment experienced by embryos can signifi cantly infl uence the developmental rate and hatching success of embryos as well as the phenotype, functional performance, and fitness of offspring (Deeming 2004). Since most oviparous reptiles deposit their eggs in underground nests, embryonicdevelopment is affected by environmental contaminants,including pesticides (Deeming, 2004; Sparling et al.,2006; Hopkins and Winne, 2006). Nonetheless, the effects of deltamethrin on embryonic development and offspring traits of reptiles remain unexplored.
In this study, we investigated the effect of deltamethrin on egg incubation in two turtle species, Trachemys scripta and Chinemys reevesii. T. scripta produces parchmentshelled eggs, whereas C. reevesii lays rigid-shelled eggs. Given the fact that the rigid shell is heavily mineralized and displays highly reduced permeability (liquid,vapor, and gas) than the parchment shell (Deeming and Thompson, 1991; Deeming and Unwin, 2004), we hypothesized that parchment-shelled eggs would be more vulnerable to deltamethrin than rigid-shelled eggs in turtles.
2. Materials and Methods
2.1 Egg collection and incubation Eggs of T. scripta and C. reevesii were purchased from a commercial supplier and transported to the laboratory. We assigned eggs in groups of 10 to plastic boxes (16 × 11.5 × 4 cm3)containing a 2 cm layer of moist vermiculite (at water potentials of approximately -220 kPa). Eggs were incubated in an air-conditioned room under constant temperature of 28°C, which is the optimal temperature for egg incubation in this species and produces hatchlings with a balanced sex ratio (Du et al., 2007). We weighed boxes with moist vermiculite to evaluate water evaporation once a week, and added water to maintain relatively constant vermiculite moisture. We changed the position of boxes daily to avoid any potential shelf effects from temperature gradients.
Eggs were assigned randomly to three deltamethrin treatment groups (0.1, 0.02, and 0.004 mg/L of deltamethrin dissolved in ethanol) and one control group(ethanol). Sample sizes were 174 (forty-two clutches) of T. scripta, and 170 (forty-two clutches) of C. reevesii. Sample sizes of T. scripta for the treatments of 0, 0.004,0.02, and 0.1 mg/L were 42, 42, 45, and 45, respectively. Sample sizes of C. reevesii for the same treatments were 40, 43, 44, and 43, respectively. Eggs were spotted with 5 μL of different doses of deltamethrin (treatment)or ethanol (control) to the eggshell using a pipettor,which was applied once at approximately stage 17 of development (Crews and Bergeron, 1994; Wibbels et al.,1994). The deltamethrin dosage is based on governmental limits of permissible levels in accordance with Chinese national standards for drinking water quality (GB 5749-2006) and Chinese national environmental quality standards for surface water (GB 3838-2002), in which the water quality standard limit value is 0.02 mg/L. Therefore, we selected the standard value as the dosage of the moderate concentration treatment group (0.02 mg/L). The dosages of the other two (high and low concentration)treatment groups were 5 and 1/5 times the standard value,respectively (0.1 and 0.004 mg/L).
2.2 Embryonic development and hatchling traits Hatching success was calculated as the percentage of successfully-hatched eggs. After hatching, each turtle was weighed and placed in a separate cup labeled with an individual number. Hatchling mass (0.001 g),carapace length, and width (0.01 mm) were measured. We examined each hatchling for morphological deformities(e.g. underdeveloped head and tail, carapace and plastron abnormalities, extra or missing scutes). The locomotor performance (swimming speed) of hatchlings was tested at 28 °C in the fi rst week after hatching. We gently placed a hatchling turtle into a 1-m-long glassed sink. The sink was marked at 250 mm intervals, and fi lled with 10 cm height of water. We recorded the time that a turtle swam through the 1 m sink. Each hatchling was tested twice and the average swimming speed through the 1 m sink was calculated. The hatchlings were kept in cups individually at room temperature. An excess amount of commercial food were provided each day. Four months later, We dissected hatchlings to identify the sex of each individual by checking gonads. Female gonads are long and thin,whereas male gonads are short and well vascularized(Yntema, 1976; Crews et al., 1991).
2.3 Statistical analysis Mixed-model ANOVA was used to test the effect of treatments on heart rate of embryos,with clutch as the random factor. Hatching success,morphological deformity and sex ratio were analyzed by using chi-square tests. Hatchling mass was compared using mixed-model ANCOVA with initial egg mass as the covariate, and clutch as the random factor. When significant differences were found among treatments, a posterior Tukey's Honestly Signifi cant Difference (HSD)test for multiple comparisons of means was carried out(Zar, 1999). For all tests, differences at α = 0.05 level of confi dence were considered signifi cant. All statistical analyses were performed using SPSS software (version 17.0, SPSS Inc., Chicago. IL, USA).
3. Results
Deltamethrin exposure did not affect hatching successin both species (C. reevesii: X2= 4.40, df = 3, P = 0.22;T. scripta: X2= 0.81, df = 3, P = 0.85) (Figure 1a). Deltamethrin exposure did not affect morphological deformities of hatchlings in T. scripta (X2= 4.18, df = 3,P = 0.24), nor in C. reevesii (X2= 3.74, df = 3, P = 0.29)(Figure 1b), nor hatchling size and mass in both species(Table 1). The sex ratio of hatchlings was not affected by deltamethrin exposure in both species (C. reevesii:X2= 1.87, df = 3, P = 0.60; T. scripta: X2= 1.12, df = 3,P = 0.77) (Figure 1c). However, deltamethrin exposure during embryonic development significantly decreased the swimming speed of T. scripta hatchlings, but not of C. reevesii hatchlings (Table 1).
4. Discussion
Animal embryos are extremely sensitive to pesticides. Some studies have shown that pesticides or herbicides might significantly affect hatching success of embryos in oviparous species. For example, treatments containing high concentrations of Glypro induce more frequent embryonic fatalities (Sparling et al., 2006). Different concentrations of cypermethrin significantly affected hatching success of amphibians (Greulich et al., 2003). Carbaryl significantly reduced survival of bullfrog(Rana catesbeiana) tadpoles in the laboratory (Puglis and boone, 2007). Our study indicated that deltamethrin exposure did not affect hatching success of turtle eggs. This result is inconsistent with other previous studies on other oviporous species. For example, Kenan et al. (2004) found that deltamethrin could significantly influence the hatching success of common carp (Cyprinus carpio)embryos and larvae. The relative insensitivity of the hatching success of turtle embryos to environmental contaminants has also been found in other species. For example, dichlorodiphenyldichloroethylene (DDE) failed to influence the hatching success in the marine turtle(Chelonia mydas) (Podreka et al., 1998), and the toxicity of pesticides (chlorothalonil, S-metolachlor, metribuzin,and chlorpyrifos) did not infl uence the hatching success of the common snapping turtle (Chelydra serpentina)(Solla et al., 2014).
Endocrine disruptor treatments during embryonic development could have profound impacts on the morphological deformity, offspring size, and offspring performance. For example, high application rate of the pesticide tefl uthrin increased deformity rates of exposed snapping turtle embryos and hatchlings (Solla et al.,2011); 17α-Ethinylestradiol, genistein, and fadrozole could induce malformations in zebrafish (Santos et al.,2014); cadmium and EC50 caused lower hatchling weight in snail species (Coeurdassier et al., 2003, Schirling et al., 2006); Hopkins and Winne (2006) reported that high concentrations of carbaryl significantly reduced swimming performance of four species of natricine snakes. Our study indicated that exposure to deltamethrin did not affect hatchling morphology including carapace size and body mass, but reduced hatchling locomotor performance (average swimming speed) of T. scripta. This suggests that deltamethrin may have long-termeffects on offspring fitness-related phenotypes, and therefore, presumably have a negative impact on offspring fi tness. Such effects of environmental hormones on reptile embryos and offspring are largely unexplored and warrant further investigation.
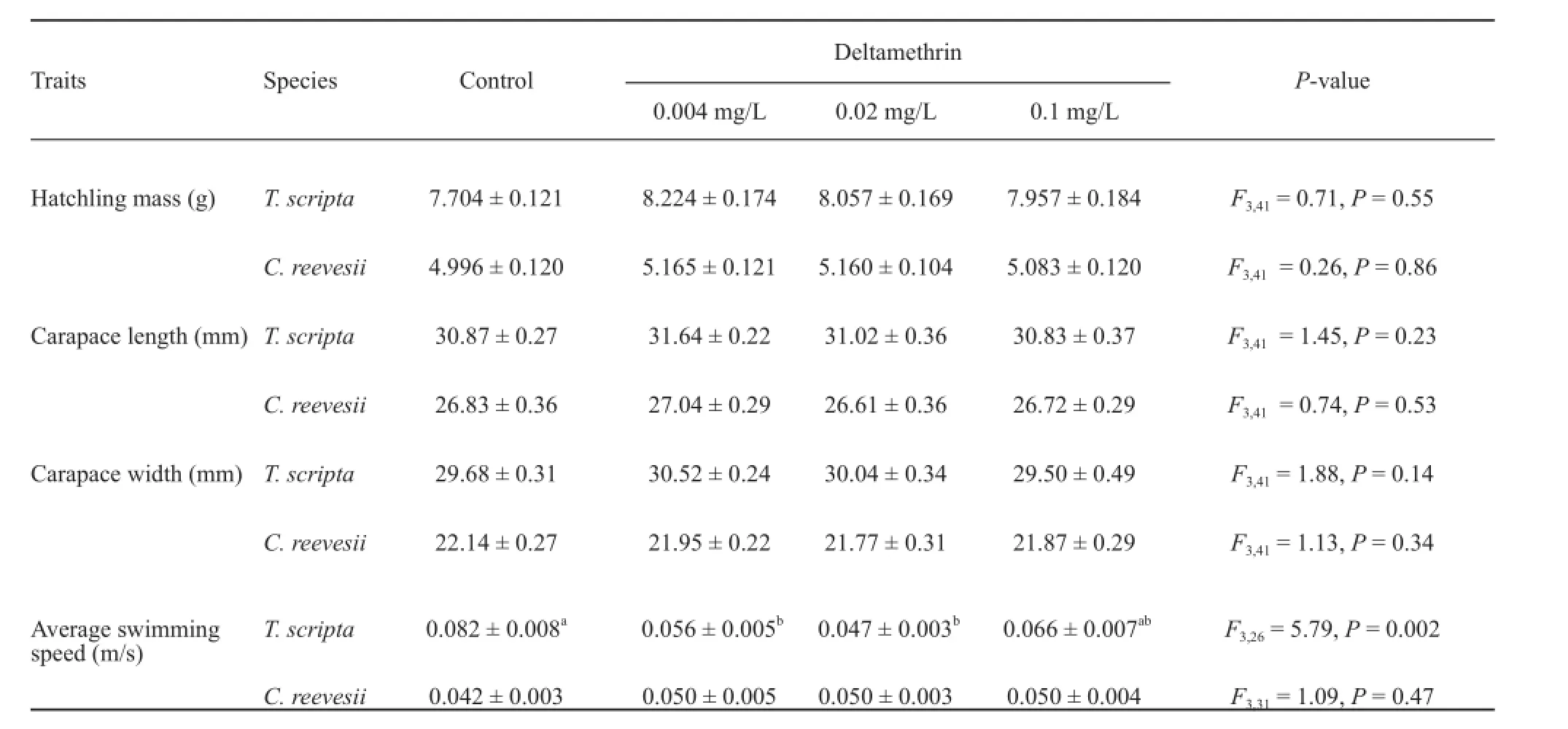
Table 1 Effect of deltamethrin exposure on hatchling morphometrics (hatchling mass, carapace length and width) and swimming speed of Trachemys scripta and Chinemys reevesii.
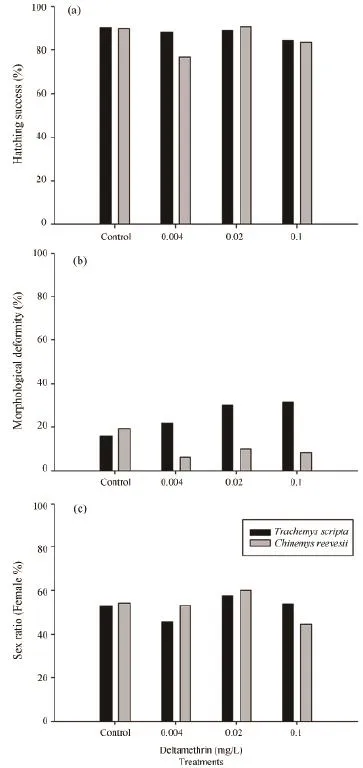
Figure 1 Effect of deltamethrin exposure on hatching success(a), morphological deformities (b) and Sex ratio (c) of Trachemys scripta and Chinemys reevesii. Sample sizes of T. scripta for the treatments of 0, 0.004, 0.02, and 0.1 mg/L were 42, 42, 45, and 45,respectively. Sample sizes of C. reevesii for the same treatments were 40, 43, 44, and 43, respectively.
The between-species difference in the effect of deltamethrin on embryonic development and offspring traits may be attributable to two aspects: species-specifi c sensitivity of embryos in response to deltamethrin and different types of eggshell (thickness and permeability). First, the sensitivity of reptile embryos to environmental stress differs among species. For example, reptile embryos have different optimal temperatures for developments (Du and Shine, 2015). Analogously, the response of reptile embryos to pesticides may also differ among species (Bishop et al., 1998; Sparling et al., 2006). Pesticide (e.g. atrazine) exposure could negatively affect embryonic development in T. scripta (Willingham, 2005),but not in the snapping turtle (Chelydra serpentina)(de Solla et al., 2011). Second, parchment- and rigidshelled eggs may have different responses to pesticides. Most reptiles lay eggs in beach sand or land soil, where the eggs are exposed to residual pesticides. Reptile eggs are able to absorb pesticides from soil because their eggshells are permeable and polyporous (Solla et al.,2011). The shell of parchment-shelled eggs is thinner and more permeable to environmental substances like water, gas, and contaminants than that of rigid-shelled eggs (Deeming and Thompson, 1991; Oftedal, 2002;Belinsky et al., 2004). As a result, the effect of pesticides on embryonic development and offspring traits is likely stronger in parchment-shelled eggs than in rigid-shelled eggs. In the two species we studied, the eggshell of C. reevesii (0.18 ± 0.02mm) is thicker than that of T. scripta (0.14 ± 0.01mm) (Kusuda et al., 2013), and deltamethrin exposure during embryonic development affected functional performance of T. scripta hatchlings,but not C. reevesii. This result supports the hypothesis that parchment-shelled eggs are more vulnerable to deltamethrin than rigid-shelled eggs. Similarly, embryonic development in other turtle species having parchmentshelled eggs is also sensitive to pesticides (Solla et al.,2011). Nonetheless, further verifi cation of this hypothesis requires more data comparing parchment-shelled and rigid-shelled eggs across a wide range of reptile species.
References
Amweg E. L., Weston D. P., Ureda N. M. 2005. Use and toxicity of pyrethroid pesticides in the Central Valley, California, USA. Environ Toxicol Chem, 24: 966-972
Aziz M. H., Agrawal A. K., Adhami V. M., Shukla Y., Seth P. K. 2001. Neurodevelopmental consequences of gestational exposure(GD14-GD20) to low dose deltamethrin in rats. Neurosci Lett,300: 161-165
Belinsky A., Ackerman R. A., Dmi'el R., Ar A. 2004. Water in reptilian eggs and hatchlings. In: Deeming D. C., editor. Reptilian incubation: environment, evolution and behaviour.Nottingham, UK: Nottingham University Press. pp. 125-41
Bishop C. A, Ng P., Pettit K. E., Kennedy S., Stegerman J. J., Norstrom R. J., Brooks R. J. 1998. Environmental contamination and developmental abnormalities in eggs and hatchlings of the common snapping turtle (Chelydra serpentina serpentina) from the Great Lakes-St Lawrence River basin(1989-1991). Environ Pollut, 99: 1-14
Bridges C. M. 2000. Long-term effects of pesticide exposure at various life stages of the southern leopard frog (Rana sphenocephala). Arch Environ Con Tox, 39: 91-96
Coeurdassier M., De Vaufleury A., Badot P. M. 2003. Bioconcentration of cadmium and toxic effects on life-history traits of pond snails (Lymnaea palustris and Lymnaea stagnalis)in laboratory bioassays. Arch Environ Con Tox, 45: 102-109
Crews D., Bergeron J. M. 1994. Role of reductase and aromatase in sex determination in the red-eared slider turtle, a turtle with temperature-dependent sex determination. J Endocrinol, 143:279-289
Crews D., Bull J. J., Wibbels T. 1991. Estrogen and sex reversal in turtles: A dose-dependent phenomenon. Gen Comp Endocr, 81:357-364
Datta M., Kaviraj A. 2003. Acute toxicity of the synthetic pyrethroid deltamethrin to freshwater catfi sh Clarias gariepinus. B Erviron Contam Tox, 70: 296-299
Deeming D. C., Thompson M. B. 1991. Gas exchange across reptilian eggs. In: Deeming D. C., Ferguson M. W. J. (Eds). Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge, UK: Cambridge University Press. pp 277-284
Deeming D. C., Unwin D. M. 2004. Reptilian incubation: evolution and the fossil record. In: Deeming D. C., editors. Reptilian incubation: environment, evolution and behaviour. Nottingham University Press, Nottingham, pp. 1-14
Delistraty D. 2000. Acute toxicity to rats and trout with a focus on inhalation and aquatic exposures. Ecotox Environ Safe, 46:225-233
de Solla S. R., Martin P. A., Mikoda P. 2011. Toxicity of pesticide and fertilizer mixtures simulating corn production to eggs of snapping turtles (Chelydra serpentina). Sci Total Environ, 409:4306-4311
Du W. G., Hu L. J., Lu J. L., Zhu L. G. 2007. Effects of incubation temperature on embryonic development rate, sex ratio and post-hatching growth in the Chinese three-keeled pond turtle, Chinemys reevesii. Aquaculture, 272:747-753
Du W. G., Shine R. 2015. The behavioural and physiological strategies of bird and reptile embryos in response to unpredictable variation in nest temperature. Biol Rev, 90: 19-30
Greulich K., Pfluqmacher S. 2003. Differences in susceptibility of various life stages of amphibians to pesticide exposure. Aquat toxicol, 65: 329-336
Hopkins W. A., Winne C. T. 2006. Influence of body size on swimming performance of four species of neonatal natricine snakes acutely exposed to a cholinesterase-inhibiting pesticide. Environ Toxicol Chem, 25: 1208-1213
Kenan K., Rahmi A. 2004. The toxic effects of pyrethroid deltamethrin on the common carp (Cyprinus carpio L.) embryos and larvae. Pestic Biochem Phys, 80: 47-53
Kontreczky C., Farkas A., Nemcsok J., Salanki J. 1997. Shortand long-term effects of deltamethrin on filtering activity of freshwater mussel (Anodonta cygnea L.). Ecotox Environ Safe,38: 195-199
Lazarini C. A., Florio J. C., Lemonica I. P., Bernardi M. M. 2001. Effects of prenatal exposure to deltamethrin on forced swimming behavior, motor activity, and striatal dopamine levels in male and female rats. Neurotoxicol Teratol, 23: 665-673
McKinlay R., Plant J. A., Bell J. N., Voulvoulis N. 2008. Endocrine disrupting pesticides: implications for risk assessment. Environ Int, 34: 168-183
Moretti M., Villarini M., Scassellati-Sforzolini G., Pasquini R., Monarca S. 1997. Applicability of aspecific noninvasive methods for biomonitoring of occupational exposure to deltamethrin: preliminary study using an animal model. Arch Environ Con Tox, 33: 323-328
Mubarak H. M., Suzuki T., Sato N., Sato I., Takewaki T., Suzuki K., Tachikawa E., Kobayashi H. 2006. Differential effects of pyrethroid insecticides on extracellular dopamine in the striatum of freely moving rats. Toxicol Appl Pharm, 217: 25-34
Oftedal O. T. 2002. The origin of lactation as a water source for parchment-shelled eggs. J. Mammary Gland Biol, 7: 253-266
Oros D. R., Werner I. 2005. Pyrethroid insecticides: an analysis of use patterns, distributions, potential toxicity and fate in the Sacramento-San Joaquin delta and central valley. White paper for the Interagency Ecological Program. SEFI Contribution 415. San Francisco Estuary Institute, Oakland, CA
Pawlisz J., Busnarda J., McLauchlin A., Caux P. Y., Kent R. A. 1998. Canadian water quality guidelines for deltamethrin. Environ Toxicol Water Qual, 13: 175-210
Podreka S., Georges A., Maher B., Limpus C. J. 1998. The environmental contaminant DDE fails to infl uence the outcome of sexual differentiation in the marine turtle Chelonia mydas. Environ Health Persp, 106: 185-188
Presibella K. M., Kita D. H., Carneiro C. B., Andrade A. J.,Dalsenter P. R. 2005. Reproductive evaluation of two pesticides combined (deltamethrin and endosulfan) in female rats. Reprod Toxicol, 20: 95-101
Puglis H. J., Boone M. D. 2007. Effects of a fertilizer, an insecticide, and a pathogenic fungus on hatching and survival of bullfrog (Rana catesbeiana) tadpoles. Environ Toxicol and Chem, 26: 2198-2201
Righi D. A., Xavier F. G., Palermo N. J. 2009. Effects of type II pyrethroid cyhalothrin on rat innate immunity: a fl ow cytometric study. Int Immunopharmacol, 9: 148-152
Santos D., Matos M., Coimbra A. M. 2014. Developmental toxicity of endocrine disruptors in early life stages of zebrafi sh,a genetic and embryogenesis study. Neurotoxicol Teratol, 46:18-25
Schirling M., Bohlen A., Triebskorn R., Koehler H. R. 2006. An invertebrate embryo test with the apple snail Marisa cornuarietis to assess effects of potential developmental and endocrine disruptors. Chemosphere, 64: 1730-1738
Sinha C. Agrawal A. K., Islam F., Seth K., Chaturvedi R. K.,Shukla S., Seth P. K. 2004. Mosquito repellent (pyrethroidbased) induced dysfunction of blood-brain barrier permeabilityin developing brain. Int J Dev Neurosci, 22: 31-37
Solla S. R. D., Martin P. A., Mikoda P. 2011. Toxicity of pesticide and fertilizer mixtures simulating corn production to eggs of snapping turtles (Chelydra serpentina). Sci Total Environ, 409:4306-4311
Solla, S. R. D., Palonen, K. E., Martin, P. A. 2014. Toxicity of pesticides associated with potato production, including soil fumigants, to snapping turtle eggs (Chelydra serpentina). Environ Toxicol Chem, 33: 102-106
Sparling D. W., Matson C., Bickham J., Doelling-Brown P. 2006. Toxicity of glyphosate as Glypro an LI700 to red-eared slider(Trachemys scripta elegans) embryos and early hatchlings. Environ Toxicol Chem, 25: 2768-2774
Svobodova Z., Luskova V., Drastichova M. J., Svoboda M.,Zlabek V. 2003. Effect of deltamethrin on haematological indices of common carp (Cyprinus carpio L.). Acta Vet Brno,72: 79-85
Thybaud E. 1990. Toxicité aiguë et bioconcentration du lindane et de la deltaméthrine par les têtards de Rana temporaria et les gambusies (Gambusia affi nis). Hydrobiologia, 190: 137-145
Tidou A. S., Moreteau J. C., Ramade F. 1992. Effects of lindane and deltamethrin on zooplankton communities of experimental ponds. Hydrobiologia, 232: 157-168
U.S. Department of Agriculture. Agricultural Resource Management Survey Tool. 2007. http://www.ers.usda.gov/Data/ ARMS/app/Crop.aspx
Wibbels T, Bull J. J., Crews D. 1994. Temperature-dependent sex determination: a mechanistic approach. J Exp Zool, 270: 71-78
Willingham E. J. 2005. The effects of atrazine and temperature on turtle hatchling size and sex ratios. Front. Ecol. Environ. 3:309-313
Yntema C. L. 1976. Effects of incubation temperatures on sexual differentiation in the turtle, Chelydra serpentina. J Morphol,150: 453-461
Zar J. H. 1999. Biostatistical analysis. Prentice Hall, Upper Saddle River, N. J.
Kusuda S., Yasukawa Y., Shibata H., Saito T., O., Ohya Y. Yoshizaki N. 2013. Diversity in the matrix structure of eggshells in the Testudines (Reptilia). Zool Sci, 30: 366-374
*
Prof. Weiguo DU, from the Institute of Zoology,Chinese Academy of Sciences, Beijing, China, with his research focusing on ecological adaptation of reptile.
Email: duweiguo@ioz.ac.cn
8 July 2015 Accepted: 11 November 2015
杂志排行
Asian Herpetological Research的其它文章
- Allelic Polymorphism, Gene Duplication and Balancing Selection of MHC Class IIB Genes in the Omei Treefrog (Rhacophorus omeimontis)
- Expression of HIF-1α and Its Target Genes in the Nanorana parkeri Heart: Implications for High Altitude Adaptation
- The Expression Plasticity of Hypoxia Related Genes in High-Altitude and Plains Nanorana parkeri Populations
- Soundscape Dynamics at Anuran Reproductive Sites in Pannonian Biogeographical Region: Effects of Road Noise on Vocal Activity
- No Evidence for Signifi cant Effect of Body Size and Age on Male Mating Success in the Spot-legged Treefrog
- Effects of Predation by Invasive Western Mosquitofi sh (Gambusia affi nis) on Survival of Eggs, Embryos and Tadpoles of Pelophylax nigromaculatus and Duttaphrynus melanostictus in South China
