盐酸硫胺(VB1)催化合成四氢咔唑类化合物
2016-09-18杨雅琴葛松兰
杨雅琴, 葛松兰, 马 磊
(华东理工大学药学院,上海市新药设计重点实验室,上海 200237)
盐酸硫胺(VB1)催化合成四氢咔唑类化合物
杨雅琴,葛松兰,马磊
(华东理工大学药学院,上海市新药设计重点实验室,上海 200237)
1,2,3,4-四氢咔唑类衍生物广泛应用于医药领域。在盐酸硫胺(VB1)催化作用下,苯肼盐酸盐和环己酮在乙醇中反应,可以简便、高产率地得到四氢咔唑类化合物。将不同基团取代的环己酮和含有吸电子或供电子基团的苯肼用VB1进行底物扩展,大部分目标产物的产率在90%以上,证实在合成四氢咔唑的反应中,VB1是一种温和、高效、环保型的催化剂。对VB1进行回收再利用,催化效率没有明显降低。
盐酸硫胺(VB1); 四氢咔唑; 苯肼; 环己酮
1,2,3,4-四氢咔唑类衍生物具有多种生物活性,在医药领域被广泛应用。据报道,1,2,3,4-四氢咔唑类衍生物可作为5-HT6受体拮抗剂[1],也被用作止吐药物的分子骨架[2]。同时,这类化合物对人乳头瘤病毒(HPVs)具有潜在的抵抗作用[3],也是很好的脂肪型脂肪酸结合蛋白(A-FABP)抑制剂[4]。有研究发现,四氢咔唑骨架在抗氧化方面具有显著效果[5]。当骨架上有不饱和羰基取代时,这类化合物是一种新型的神经肽Y-1(NPY-1)和G蛋白偶联受体(GPCR)拮抗剂[6-7];当骨架上被特定的烷基取代时,此类分子对某些癌细胞具有抑制作用[8]。
随着有机合成方法学的发展,有多种方法合成四氢咔唑类化合物。Siddalingamurthy等[9]以苯肼盐酸盐和环己酮为原料,通过三聚氯氰(TCT)催化,得到四氢咔唑。也可在超声波辐射条件下,通过Fischer吲哚合成方法,得到目标产物[10-11]。Yedukondalu等[12]发现,苯肼盐酸盐和环己酮在聚乙二醇(PEG-400)中反应,生成四氢咔唑。这类化合物也可通过还原[13]、交叉耦合[14]等其他反应[15-16]得到。近年来,酸性离子液体催化四氢咔唑类化合物的合成也有相关报道[17-20]。传统的合成方法,有反应温度高,时间过长,所用的催化剂缺乏经济性和环保性等缺点。因此,研究高效环保型的合成四氢咔唑类化合物具有重要意义。
VB1无毒无公害,价格经济,可以回收再利用,是一种环保型催化剂。VB1及其类似物在许多碳-碳偶联和碳-杂原子偶联的反应中,可以作为高效催化剂[21-23]。本文将盐酸硫胺(VB1)作为催化剂,以取代的盐酸苯肼和环己酮为底物,在非常温和的条件下,合成了一系列四氢咔唑类化合物,拓展了VB1在有机合成中的应用。
1 实验部分
1.1仪器和试剂
1H-NMR,13C-NMR:以DMSO-d6,CDCl3或CD3OD 为溶剂,由Bruker AVANCE 400 核磁共振仪测得; 熔点:SGWX4显微熔点仪; ESI低分辨质谱:Bruker Esquire 3000 plus spectrometer型质谱仪; ESI高分辨质谱:Bruker Atex III spectrometer型质谱仪。所有试剂均为分析纯,安耐吉化学公司。柱层析色谱使用200~300目(48~75 μm)的硅胶。
1.2VB1催化四氢咔唑类化合物的合成
图1所示为四氢咔唑类化合物的合成路线。在25 mL双口圆底烧瓶中,依次加入取代的苯肼盐酸盐1(2 mmol)、乙醇(15 mL)、环己酮类化合物2(2 mmol)。搅拌均匀后,加入催化剂VB1(0.2 mmol),在50 ℃下反应。薄层色谱(TLC)检测至反应完全。减压蒸出溶剂,分别用二氯甲烷(2×20 mL)、饱和食盐水(2×15 mL)萃取,无水硫酸钠干燥后除去溶剂,得粗品。柱层析分离提纯,得到产物3。
图2所示为化合物3b的合成路线。在双口烧瓶中分别加入对甲基苯肼盐酸盐(2 mmol)、溶剂(15 mL)、对甲基环己酮(2 mmol),搅拌10 min后,加入催化剂。分别在室温、50 ℃、回流状态下反应。TLC检测反应情况。待完全反应后,减压蒸出溶剂,分别用二氯甲烷(2×20 mL)、饱和食盐水(2×15 mL)萃取,无水硫酸钠干燥后减压蒸出溶剂,得粗品。柱层析分离提纯(石油醚和乙酸乙酯的体积比为40∶1),得产物3b。

图1 VB1催化下的苯肼盐酸盐和环己酮类的反应Fig.1 Reaction of phenylhydrazine with cyclohexanone in the presence of VB1
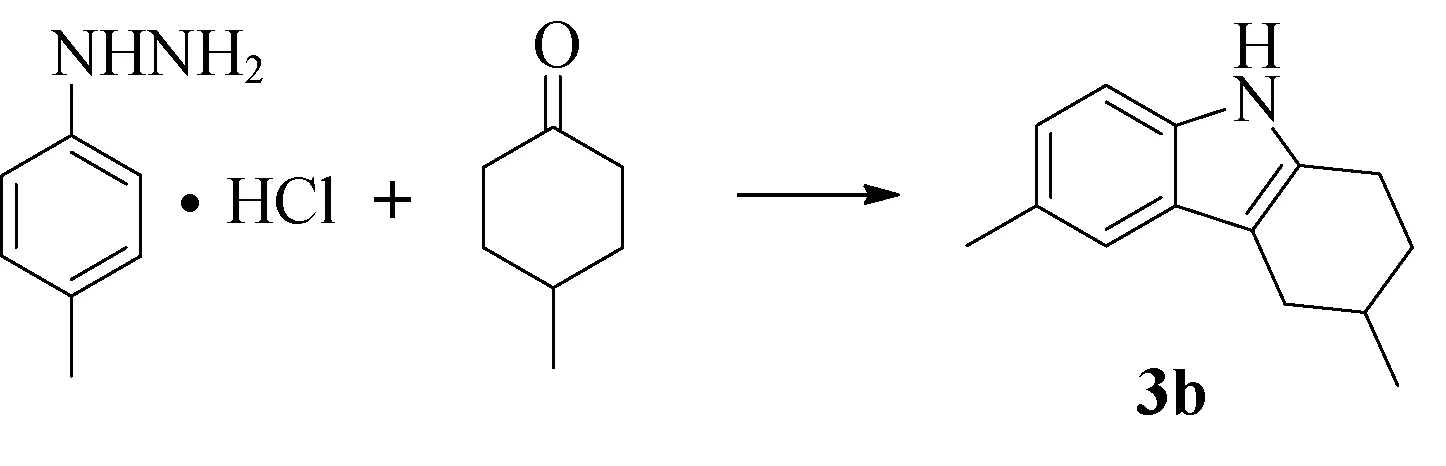
图2 3,6-二甲基-1,2,3,4-四氢咔唑(3b)的合成Fig.2 Synthesis of 3,6- dimethyl-1,2,3,4- tetrahydrocarbazole (3b)
1.3催化剂的回收利用
反应结束后,减压蒸出溶剂,浓缩物用二氯甲烷(20 mL)和水(15 mL)萃取。将萃取液(水层)pH调至3,减压浓缩萃取液。将浓缩物冷却至室温,加入乙醇(25 mL),搅拌30 min后放入冰箱过夜,析出结晶。过滤,真空干燥2 h,得到VB1。将回收的VB1重复使用,催化苯肼盐酸盐和环己酮类化合物的反应。重复4次,催化效果没有明显降低。
2 结果与讨论
2.1反应条件筛选
使用对甲基苯肼盐酸盐和对甲基环己酮为反应底物进行条件的优化。优化的条件包括反应温度、催化剂用量、溶剂种类,结果见表1。首先,讨论温度对反应的影响。在室温时,反应较慢(Entry 1),而回流状态下,副产物多且不易分离(Entry 3),因此选用50 ℃作为反应温度。接下来,研究催化剂摩尔分数对反应的影响。结果表明VB1摩尔分数为10%是最佳催化剂用量(Entry 5)。增加VB1摩尔分数,反应产率没有明显提升(Entry 2,4); 当VB1摩尔分数低于10%时,产率降低(Entry 6,7); 当用盐酸的乙醇饱和溶液作催化剂时,产率没有明显提升(Entry 8)。在探索溶剂对反应的影响中发现(Entry 9~Entry 13),以水作为反应溶剂时,产率较低且反应时间长; 以甲醇、DCM(二氯甲烷)作为反应溶剂时,反应较慢; 以DMF(N,N-二甲基甲酰胺)和DMSO(二甲基亚砜)作反应溶剂时,杂点较多,后处理不易。因此,选用乙醇作为反应溶剂。
综上所述,在VB1(摩尔分数10%)的催化下,等物质的量的4-甲基苯肼盐酸盐和4-甲基环己酮在乙醇中、50 ℃下反应,能简单、高效地得到四氢咔唑类化合物。
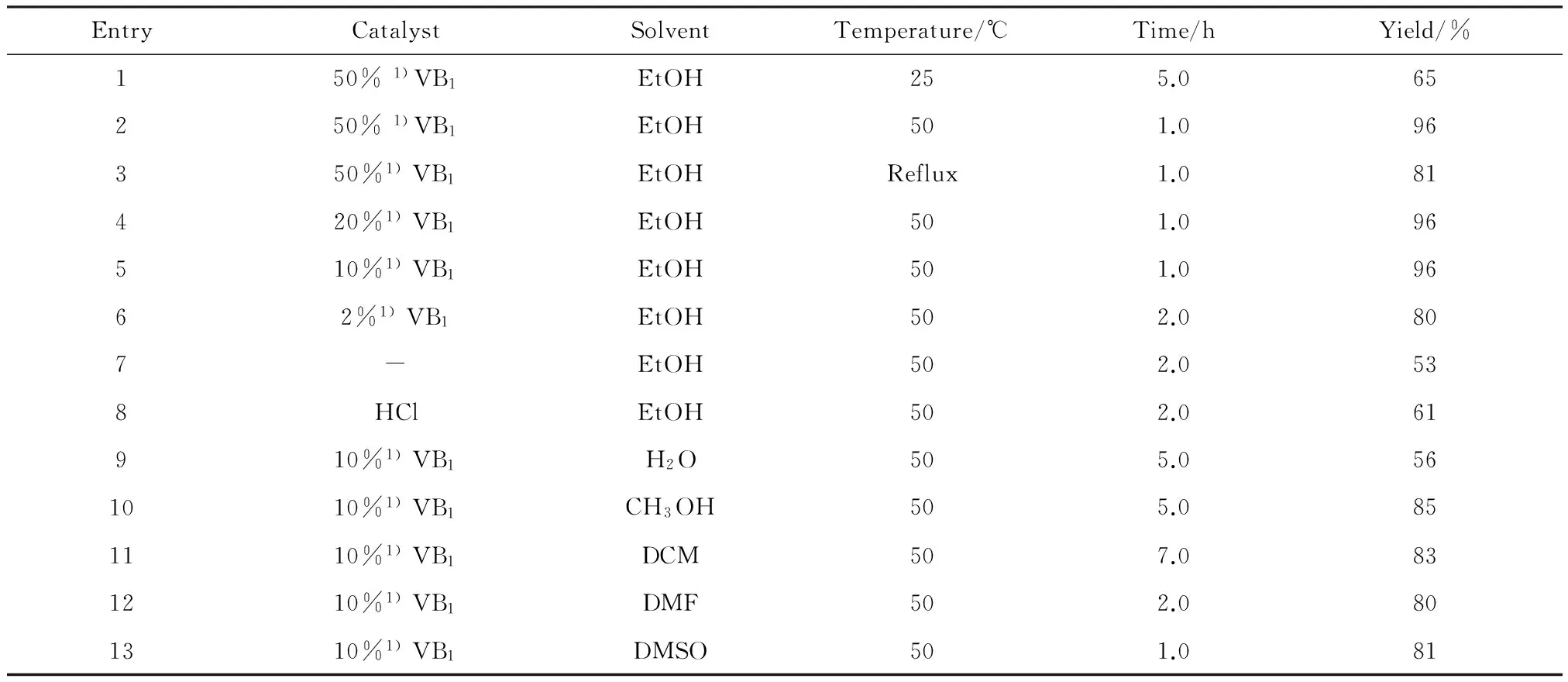
表1 反应条件的优化
1)mol fraction of VB1
2.2底物扩展
在获得最佳反应条件之后,用VB1催化不同的苯肼盐酸盐和环己酮类化合物,得到一系列化合物(表2)。总体而言,用VB1催化合成四氢咔唑的方法具有较好的底物适应性和官能团耐受性。当底物环己酮邻位上含有较大基团,如环己烯基时,反应较慢,产率也相对降低(Entry 23); 当底物环己酮邻位上含有乙氧羰基时,反应产率低于其他反应(Entry 24),这可能因为乙氧羰基(—CO2Et)中的—C=O和环己酮上的—C=O竞争性和苯肼结合,影响反应的进行。底物苯肼上含有吸电子或供电子小基团如羧基、三氟甲基、甲氧基、甲基等时,反应在较短的时间内(0.8~2 h)具有很高的产率,说明VB1催化合成四氢咔唑的方法具有较好的底物适用性和官能团耐受性。
3-羧基-6,8-二氟-1,2,3,4-四氢咔唑(3a):黄色固体,产率92%,熔点183~184 ℃。1H-NMR(400 MHz,DMSO-d6,δ):11.28(s,1H),7.03(dd,J=9.4,1.6 Hz,1H),6.84(t,J=10.6 Hz,1H),2.88(q,J=9.6 Hz,1H),2.82~2.64(m,4H),2.18(d,J=14.1 Hz,1H),1.96~1.77(m,1H);13C-NMR(100 MHz,DMSO-d6,δ):176.2,156.7,154.2,137.2,119.9,108.5,98.7,95.4,94.8,38.8,25.2,23.4,21.8; ESI-MS[M+H]+m/z:252; HRMS(ESI)m/z:[M+H]+分子式为C13H12F2NO2,相对分子质量计算值为252.083 1,测量值为252.083 5。
3,6-二甲基-1,2,3,4-四氢咔唑(3b):浅棕色固体,产率96%,熔点112~113 ℃(文献值112~113 ℃[24])。1H-NMR(400 MHz,CDCl3,δ):7.57(s,1H),7.23(s,1H),7.15(d,J= 8.2 Hz,1H),6.92(d,J= 8.0 Hz,1H),2.85~2.68(m,3H),2.43(s,3H),2.25(dd,J=15.3,9.3 Hz,1H),1.99~1.88(m,2H),1.56~1.50(m,1H),1.13(d,J=6.5Hz,3H);13C-NMR(100 MHz,DMSO-d6,δ):134.2,134.1,127.4,126.1,121.4,116.8,110.2,107.4,31.1,29.2,29.2,22.4,21.7,21.2; ESI-MS[M+H]+m/z:200; HRMS(ESI)m/z:[M+H]+分子式为C14H18N,相对分子质量计算值为200.143 4,测量值为200.143 0。
3-乙酰氨基-8-氟-1,2,3,4-四氢咔唑(3c):棕色固体,产率97%,熔点207~208 ℃。1H-NMR(400 MHz,CDCl3,δ):8.21(s,1H),7.18(d,J=7.8 Hz,1H),6.98(td,J=7.8,4.8 Hz,1H),6.85(dd,J=11.2,7.9 Hz,1H),5.69(d,J=7.0 Hz,1H),4.51~4.36(m,1H),3.07(dd,J=15.4,5.1 Hz,1H),2.93~2.73(m,2H),2.60(dd,J=15.5,6.4 Hz,1H),1.98(s,3H),1.79(d,J=17.7 Hz,2H);13C-NMR(100 MHz,CDCl3,δ):169.8,148.0,133.6,119.7,113.5,108.2,106.7,106.6,99.8,45.0,27.9,27.8,23.6,20.4; ESI-MS[M+H]+m/z:247; HRMS(ESI)m/z:[M+H]+的分子式为C14H16FN2O,相对分子质量计算值为247.124 2,测量值为247.123 9。
1,2,3,4-四氢咔唑(3d):米白色固体,产率93%,熔点118~120 ℃(文献值118~120 ℃[25])。1H-NMR(400 MHz,CDCl3,δ):7.63(s,1H),7.45(d,J=7.5 Hz,1H),7.25(t,J= 5.4 Hz,1H),7.15~7.01(m,2H),2.71(dd,J=7.0,5.2 Hz,4H),1.97~1.81(m,4H);13C-NMR(100 MHz,CDCl3,δ):135.7,134.2,127.8,121.0,119.1,117.8,110.4,110.1,23.3,23.3,21.0; ESI-MS[M+H]+m/z:172; HRMS(ESI)m/z:[M+H]+分子式为C12H14N,相对分子质量计算值为172.112 1,测量值为172.112 4。
3-乙酰氨基-6,8-二甲基-1,2,3,4-四氢咔唑(3e):棕色固体,产率93%,熔点78~79 ℃。1H-NMR(400 MHz,CDCl3,δ):7.67(s,1H),7.07(s,1H),6.80(s,1H),5.62(d,J=6.9 Hz,1H),4.45(m,1H),3.04(dd,J=15.5,5.0 Hz,1H),2.89~2.75(m,2H),2.60(dd,J=15.5,5.8 Hz,1H),2.42(d,J=6.9 Hz,6H),2.09~2.02(m,2H),1.96(s,3H);13C-NMR(100 MHz,CDCl3,δ):169.7,134.3,133.2,129.0,123.9,119.4,115.2,107.2,99.0,55.0,29.7,27.7,23.6,21.4,20.3,16.6; ESI-MS[M+H]+m/z:257; HRMS(ESI)m/z:[M+H]+分子式为C16H21N2O,相对分子质量计算值为257.164 9,测量值为257.165 0。
3-苯基-1,2,3,4-四氢咔唑(3f):黄色固体,产率83%,熔点220~221 ℃(文献值220~221 ℃[26])。1H-NMR(400 MHz,CDCl3,δ):7.76(s,1H),7.45(d,J=7.7 Hz,1H),7.36~7.29(m,5H),7.24(d,J= 5.6 Hz,1H),7.11(dt,J=14.8,7.1 Hz,2H),3.14~3.03(m,2H),2.98~2.77(m,3H),2.27~2.09(m,2H);13C-NMR(100 MHz,CDCl3,δ):146.7,136.0,133.6,128.5,127.5,127.1,126.2,121.2,119.3,117.8,110.4,110.2,41.2,30.3,29.3,23.4; ESI-MS[M+H]+m/z:248; HRMS(ESI)m/z:[M+H]+分子式C18H18N,相对分子质量计算值为248.143 4,测量值为248.143 0。
3-苯基-6-羧基-1,2,3,4-四氢咔唑(3g):黄色固体,产率82%,熔点155~156 ℃。1H-NMR(400 MHz,DMSO-d6,δ):11.72(s,1H),8.11(dd,J= 7.6,5.8 Hz,2H),7.80(d,J= 8.5 Hz,1H),7.38~7.16(m,5H),2.65(td,J= 11.5,4.5 Hz,1H),2.08(dd,J= 26.8,7.4 Hz,2H),1.87~1.72(m,4H);13C-NMR(100 MHz,DMSO-d6,δ):210.2,145.3,132.7,130.5,129.2,128.5,128.4,126.7,126.6,126.2,123.9,122.1,122.0,41.6,40.7,33.3,30.4; ESI-MS[M+H]+m/z:292; HRMS(ESI)m/z:[M+H]+分子式为C19H18NO2,相对分子质量计算值为292.133 3,测量值292.133 1。
3-乙酰氨基-7-甲基-1,2,3,4-四氢咔唑(3h):黄色固体,产率99%,熔点91~92 ℃。1H-NMR(400 MHz,CDCl3,δ):7.73(s,1H),7.31(d,J=8.0 Hz,1H),7.10(d,J=6.1 Hz,1H),6.92(d,J=8.0 Hz,1H),5.64(d,J=6.8 Hz,1H),4.43(m,1H),3.05(dd,J=15.5,5.1 Hz,1H),2.95~2.68(m,3H),2.65~2.55(m,2H),2.44(s,3H),1.96(s,3H);13C-NMR(100 MHz,DMSO-d6,δ):168.6,136.5,132.7,129.0,125.1,119.7,116.8,110.6,106.2,45.1,28.7,27.4,22.8,21.6,21.3; ESI-MS[M+H]+m/z:243; HRMS(ESI)m/z:[M+H]+分子式为C15H19N2O,相对分子质量计算值为243.149 2,测量值为243.149 0。
3-乙酰氨基-6-甲基-1,2,3,4-四氢咔唑(3i):浅黄色固体,产率99%,熔点182~183 ℃。1H-NMR(400 MHz,DMSO-d6,δ):10.54(s,1H),7.94(d,J=7.6 Hz,1H),7.20~7.03(m,2H),6.81(d,J=8.1 Hz,1H),4.03(d,J=6.8 Hz,1H),2.90~2.69(m,3H),2.44(dd,J=15.1,8.6 Hz,1H),2.34(s,3H),2.01~1.91(m,1H),1.83(s,3H),1.78~1.67(m,1H);13C-NMR(100 MHz,DMSO-d6,δ):168.6,134.4,133.5,127.3,126.4,121.7,116.9,110.3,105.9,45.2,28.7,27.3,22.7,21.2,21.2;ESI-MS[M+H]+m/z:243; HRMS(ESI)m/z:[M+H]+分子式为C15H19N2O,相对分子质量计算值为243.149 2,测量值为243.149 0。
(1)确保PCL控制系统运用环境干燥。虽然PCL控制系统的环境适应能力较强,但是由于其为电气设备,因此应当保证应用PCL控制系统的环境的干燥,确保PCL控制技术在金矿山电气设备中的安全稳定性。
3-乙酰氨基-1,2,3,4-四氢咔唑(3j):黄色固体,产率91%,熔点123~125 ℃(文献值123~125 ℃[27])。1H-NMR(400 MHz,CDCl3,δ):7.83(s,1H),7.44(d,J=7.7 Hz,1H),7.31(d,J=7.9 Hz,1H),7.12(dt,J=23.0,7.2 Hz,2H),5.62(d,J=7.3 Hz,1H),4.53~4.39(m,1H),3.09(dd,J=15.5,5.1 Hz,1H),2.94~2.73(m,2H),2.63(dd,J=15.5,6.1 Hz,1H),2.20~2.00(m,2H),1.97(s,3H);13C-NMR(100 MHz,CDCl3,δ):169.8,136.2,132.9,127.6,121.5,119.4,117.7,110.6,107.2,58.4,27.9,27.7,23.6,20.4; ESI-MS[M+H]+m/z:229; HRMS(ESI)m/z:[M+H]+分子式为C14H17N2O,相对分子质量计算值为229.133 6,测量值为229.133 2。
3-乙酰氨基-6-甲氧基-1,2,3,4-四氢咔唑(3k):浅棕色固体,产率95%,熔点93~94 ℃(文献值93~94 ℃[27])。1H-NMR(400 MHz,CDCl3,δ):7.73(s,1H),7.18(t,J=12.9 Hz,1H),6.89(s,1H),6.79(t,J=9.1 Hz,1H),5.65(d,J=7.0 Hz,1H),4.45(m,1H),3.85(s,3H),3.06(dd,J=15.3,4.8 Hz,1H),2.80(qd,J=16.7,8.4 Hz,2H),2.60(dd,J=15.4,5.9 Hz,1H),2.03(dd,J=12.3,5.7 Hz,2H),1.98(s,3H);13C-NMR(100 MHz,DMSO-d6,δ):168.6,152.9,134.2,131.1,127.5,111.1,109.7,106.3,99.6,55.3,45.1,28.6,27.4,22.8,21.2; ESI-MS[M+H]+m/z:259; HRMS(ESI)m/z:[M+H]+分子式为C15H19N2O2,相对分子质量计算值为259.144 2,测量值为259.144 5。
3-乙酰氨基-8-三氟甲基-1,2,3,4-四氢咔唑(3l):黄色固体,产率93%,熔点129~130 ℃。1H-NMR(400 MHz,CDCl3,δ):7.66(d,J=8.4 Hz,1H),7.58(s,1H),7.44(t,J=9.5 Hz,2H),6.85(t,J=7.4 Hz,1H),4.09(d,J=8.0 Hz,1H),2.70(d,J=15.1 Hz,1H),2.60(d,J=15.2 Hz,1H),2.46~2.35(m,1H),2.24~2.05(m,3H),2.00(s,3H);13C-NMR(100 MHz,CD3OD,δ):172.9,136.7,134.3,130.7,126.8,122.4,119.6,118.9,115.7,108.5,47.3,31.7,29.7,24.2,22.7; ESI-MS[M+H]+m/z:297; HRMS(ESI)m/z:[M+H]+分子式为C15H16F3N2O,相对分子质量计算值为297.121 0,测量值为297.121 3。
2-甲基-6-羧基-1,2,3,4-四氢咔唑(3m):黄色固体,产率94%,熔点96~97 ℃。1H-NMR(400 MHz,DMSO-d6,δ):8.00~7.92(m,1H),7.64(dd,J=13.3,5.9 Hz,1H),7.51(t,J=7.7 Hz,1H),2.35~2.10(m,3H),1.97~1.87(m,1H),1.86~1.72(m,2H),1.57(tdd,J=16.7,8.3,4.7 Hz,1H),0.95(d,J=6.5 Hz,3H);13C-NMR(100 MHz,DMSO-d6,δ):167.4,150.2,130.9,130.4,122.0,120.7,119.1,118.0,111.0,34.4,32.2,25.6,24.4,21.9; ESI-MS[M+H]+m/z:230; HRMS(ESI)m/z:[M+H]+分子式为C14H16NO2,相对分子质量计算值为230.117 6,测量值为230.118 0。
3-羧基-8-氟-1,2,3,4-四氢咔唑(3n):棕色固体,产率96%,熔点141~142 ℃。1H-NMR(400 MHz,DMSO-d6,δ):12.31(s,1H),11.15(s,1H),7.19(d,J=7.7 Hz,1H),6.94~6.75(m,2H),2.91(dd,J=19.3,9.1 Hz,1H),2.81~2.67(m,4H),2.19(d,J=13.8 Hz,1H),1.85(dd,J=20.8,9.1 Hz,1H);13C-NMR(100 MHz,DMSO-d6,δ):176.3,147.5,135.2,131.0,123.7,118.5,113.5,107.9,105.3,38.9,25.3,23.5,21.7; ESI-MS[M+H]+m/z:234; HRMS(ESI)m/z:[M+H]+分子式为C13H13FNO2,相对分子质量计算值为234.092 5,测量值为234.092 5。
3-乙酰氨基-6-三氟甲基-1,2,3,4-四氢咔唑(3o):棕色固体,产率95%,熔点76~77 ℃。1H-NMR(400 MHz,CDCl3,δ):7.86(d,J=8.3 Hz,1H),7.76(d,J=8.4 Hz,1H),7.56~7.45(m,1H),5.66(s,1H),4.26(ddt,J=11.1,7.7,5.2 Hz,1H),2.53~2.35(m,3H),2.30~2.20(m,2H),2.02(t,J=3.3 Hz,3H),1.75~1.68(m,1H);13C-NMR(100 MHz,CD3OD,δ):172.6,135.0,132.3,129.9,127.4,123.9,118.1,116.2,112.6,100.4,46.9,31.7,29.7,28.3,23.6; ESI-MS[M+H]+m/z:297; HRMS(ESI)m/z:[M+H]+分子式为C15H16F3N2O,相对分子质量计算值为297.121 0,测量值为297.121 3。
3-羧基-6-甲氧基-1,2,3,4-四氢咔唑(3p):浅黄色固体,产率97%,熔点205~206 ℃。1H-NMR(400 MHz,CD3OD,δ):7.14(d,J=8.7 Hz,1H),6.90(d,J=2.4 Hz,1H),6.69(dd,J=8.7,2.4 Hz,1H),3.82(s,3H),2.98(t,J=9.7 Hz,1H),2.92~2.70(m,4H),2.40~2.18(m,1H),2.14~1.84(m,1H);13C-NMR(100 MHz,DMSO-d6,δ):176.5,152.9,134.5,130.9,127.4,111.1,109.7,106.7,99.6,55.3,38.8,25.5,23.7,21.9; ESI-MS[M+H]+m/z:246; HRMS(ESI)m/z:[M+H]+分子式为C14H16NO3,相对分子质量计算值为246.112 5,测量值为246.112 3。
3-羧基-1,2,3,4-四氢咔唑(3q):白色固体,产率95%,熔点199~200 ℃(文献值199~200 ℃[28])。1H-NMR(400 MHz,CD3OD,δ):7.37(d,J=7.6 Hz,1H),7.26(d,J=7.9 Hz,1H),7.00(dt,J=25.8,7.2 Hz,2H),3.00(d,J=11.2 Hz,1H),2.92~2.72(m,4H),2.28(d,J=12.5 Hz,1H),2.12~1.89(m,1H);13C-NMR(100 MHz,DMSO-d6,δ):176.5,135.9,133.7,127.0,120.2,118.1,117.1,110.5,106.7,38.8,25.5,23.5,21.8; ESI-MS[M+H]+m/z:216; HRMS(ESI)m/z:[M+H]+分子式为C13H14NO2,相对分子质量计算值为216.102 0,测量值为216.101 5。
3-羧基-6-三氟甲基-1,2,3,4-四氢咔唑(3r):浅黄色固体,产率96%,熔点298~299 ℃。1H-NMR(400 MHz,CD3OD,δ):7.14(d,J=8.7 Hz,1H),6.90(d,J= 2.4 Hz,1H),6.69(dd,J=8.7,2.4 Hz,1H),2.98(t,J=9.7 Hz,1H),2.92~2.70(m,4H),2.40~2.18(m,1H),2.14~1.84(m,1H);13C-NMR(100 MHz,CD3OD,δ):171.8,140.7,136.6,128.1,125.5,123.5,121.4,119.9,111.1,109.8,41.4,27.0,24.8,23.0; ESI-MS[M+H]+m/z:284; HRMS(ESI)m/z:[M+H]+分子式为C14H13F3NO2,相对分子质量计算值为284.089 3,测量值为284.089 0。
3,6-二羧基-1,2,3,4-四氢咔唑(3s):浅黄色固体,产率97%,熔点291~293 ℃。1H-NMR(400 MHz,CD3OD,δ):7.14(d,J=8.7 Hz,1H),6.90(d,J=2.4 Hz,1H),6.69(dd,J=8.7,2.4 Hz,1H),2.98(t,J=9.7 Hz,1H),2.92~2.70(m,4H),2.40~2.18(m,1H),2.14~1.84(m,1H);13C-NMR(100 MHz,DMSO-d6,δ):176.3,168.5,138.4,135.6,126.6,121.8,120.6,119.7,110.2,108.2,38.9,25.3,23.2,21.7; ESI-MS[M+H]+m/z:260; HRMS(ESI)m/z:[M+H]+分子式为C14H14NO4,相对分子质量计算值为260.091 8,测量值为260.091 5。
6-三氟甲基-1,2,3,4-四氢咔唑(3t):黄色固体,产率94%,熔点83~84 ℃(文献值83~84 ℃[29])。1H-NMR(400 MHz,CDCl3,δ):7.84(s,1H),7.73(s,1H),7.34(dd,J=8.5,1.5 Hz,2H),2.81~2.63(m,4H),2.01~1.80(m,4H);13C-NMR(100 MHz,CDCl3,δ):140.5,129.6,124.6,121.3,119.9,117.6,112.2,108.4,106.1,25.0,24.4,22.7; ESI-MS[M+H]+m/z:240; HRMS(ESI)m/z:[M+H]+分子式为C13H13F3N,相对分子质量计算值为240.099 5,测量值为240.099 2。
3-甲基-6-甲氧基-1,2,3,4-四氢咔唑(3u):棕色固体,产率94%,熔点112~113 ℃(文献值112 ℃[30])。1H-NMR(400 MHz,CDCl3,δ):7.36(d,J=8.3 Hz,1H),6.94(s,1H),6.84(d,J=8.4 Hz,1H),3.79(d,J=1.4 Hz,3H),2.78(ddd,J=15.0,10.9,6.2 Hz,4H),2.41(dt,J=14.6,3.0 Hz,1H),1.64~1.55(m,2H),0.93(d,J=6.6 Hz,3H);13C-NMR(100 MHz,CDCl3,δ):153.8,134.9,131.1,128.1,111.0,110.5,110.0,100.3,56.0,31.4,29.7,29.5,23.0,21.8; ESI-MS[M+H]+m/z:216; HRMS(ESI)m/z:[M+H]+分子式为C14H18NO,相对分子质量计算值为216.138 3,测量值为216.138 0。
3-乙酰氨基-7-氟-1,2,3,4-四氢咔唑(3v):棕色固体,产率98%,熔点188~189 ℃。1H-NMR(400 MHz,CDCl3,δ):7.38~7.27(m,1H),7.17~7.01(m,2H),6.30(s,1H),4.29~4.18(m,1H),2.48~2.38(m,4H),2.22(dt,J=20.8,7.2 Hz,2H),2.02(s,3H);13C-NMR(100 MHz,CDCl3,δ):169.9,138.8,133.0,130.5,124.2,121.8,118.2,107.9,107.0,45.2,29.7,27.7,23.6,20.5; ESI-MS[M+H]+m/z:247; HRMS(ESI)m/z:[M+H]+分子式为C14H16FN2O,相对分子质量计算值为247.124 2,测量值为247.124 5。
1-环己烯基-6-甲基-1,2,3,4-四氢咔唑(3w):黄色固体,产率83%,熔点102~104 ℃。1H-NMR(400 MHz,CDCl3,δ):7.45(d,J=7.8 Hz,1H),7.10(d,J=7.8 Hz,1H),7.01(s,1H),5.79(t,J=3.7 Hz,1H),2.90~2.79(m,1H),2.77~2.68(m,1H),2.54~2.41(m,1H),2.36(s,3H),2.18~2.01(m,4H),1.84~1.67(m,2H),1.46(m,6H);13C-NMR(100 MHz,CDCl3,δ):146.1,134.6,134.0,130.0,128.1,122.9,122.4,119.5,115.3,108.4,35.2,30.9,29.1,25.6,24.7,22.8,22.3,21.8,21.5; ESI-MS[M+H]+m/z:266; HRMS(ESI)m/z:[M+H]+分子式为C19H24N,相对分子质量计算值为266.190 4,测量值为266.190 8。
1-乙氧羰基-1,2,3,4-四氢咔唑(3x):米白色固体,产率66%,熔点81~82 ℃。1H-NMR(400 MHz,CDCl3,δ):7.67(d,J=8.1 Hz,2H),7.39(t,J=7.8 Hz,2H),4.17(q,J=7.0 Hz,2H),3.48(m,1H),2.68(t,J=6.0 Hz,2H),2.60(t,J=5.9 Hz,2H),1.85~1.78(m,2H),1.32(t,J= 7.0 Hz,3H);13C-NMR(100 MHz,DMSO-d6,δ):164.8,148.8,138.8,128.8,125.6,121.4,118.4,113.6,99.6,68.1,40.1,23.7,22.7,20.0,15.1; ESI-MS[M+H]+m/z:244;HRMS(ESI)m/z:[M+H]+分子式为C15H18NO2,相对分子质量计算值为244.133 3,测量值为244.133 7。

表2 VB1催化合成化合物31)
1)Reaction conditions:phenylhydrazine hydrochloride 1 (2 mmol),cyclohexanone 2 (2 mmol) and VB1(0.2 mmol) were mixed in 15 mL of EtOH at 50 ℃;2) Isolated yields
2.3催化剂的回收再利用
考察催化剂的回收利用情况。将萃取液(水层)pH调至3,减压浓缩后,将浓缩物冷却至室温,加入乙醇,搅拌并放至冰箱过夜,析出结晶。VB1重复使用4次,产率没有明显下降(表3)。因此,用VB1催化合成四氢咔唑类化合物的方法在工业生产上具有潜在的应用价值。

表3 VB1 的循环使用1)
1) Reaction conditions:p-tolylhydrazine hydrochloride (2 mmol),4-methylcyclohexanone (2 mmol) and VB1(0.2 mmol) were mixed in 15 mL of EtOH at 50 ℃;2)Recrystallized in ethanol
3 结 论
本文以VB1为催化剂,以取代的苯肼盐酸盐和环己酮为底物,在非常温和的条件下,简单、高效地得到一系列四氢咔唑类化合物,拓展了VB1在有机合成中的应用。VB1无毒无公害,价格经济,可以回收再利用,同时具有较好的底物适用性。用VB1催化合成四氢咔唑类化合物,在绿色制药工艺领域具有潜在的应用价值。
[1]CHANG-Fong J,RANGISETTY J B,DUKAT M,etal.1,2,3,4-Tetrahydrocarbazoles as 5-HT6serotonin receptor ligands[J].Bioorganic & Medicinal Chemistry Letters,2004,14(8):1961-1964.
[2]徐启贵,刘天渝,田睿,等.3-(4-取代-哌嗪-1-基甲基)-1,2,3,9-四氢咔唑-4-酮衍生物的合成与止吐活性研究[J].有机化学,2008,28(2):234-239.
[3]GUDMUNDSSON K S,BOGGS S D,SEBAHAR P R,etal.Tetrahydrocarbazole amides with potent activity against human papillomaviruses[J].Bioorganic & Medicinal Chemistry Letters,2009,19(15):4110-4114.
[4]BARF T,LEHMANN F,HAMMER K,etal.N-Benzyl-indolo carboxylic acids:Design and synthesis of potent and selective adipocyte fatty-acid binding protein (A-FABP) inhibitors[J].Bioorganic & Medicinal Chemistry Letters,2009,19(6):1745-1748.
[5]BROWN D W,GRAUPNER P R,SAINSBURY M,etal.New antioxidants incorporating indole and indoline chromophores[J].Tetrahedron,1991,47(25):4383-4408.
[6]FABIO R D,GIOVANNINI R,BERTANI B,etal.Synthesis and SAR of substituted tetrahydrocarbazole derivatives as new NPY-1 antagonists[J].Bioorganic & Medicinal Chemistry Letters,2006,37(21):1749-1752.
[7]KOPPITZ M,REINHARDT G,LINGEN A V.Solidphase synthesis of substituted 3-amino-3′-carboxy-tetrahydrocarbazoles[J].Tetrahedron Letters,2005,46(6):911-914.
[8]KUMAR T O S,MAHADEVAN K M,KUMARA M N.Synthesis and cytotoxic studies of 2,3-dimethylindoles and tetrahydrocarbazoles[J].International Journal of Pharmacy and Pharmaceutical Sciences,2014,6(2):137-140.
[9]SIDDALINGAMURTHY E,MAHADEVAN K M,MASAGALLI J N,etal.Mild,efficient Fischer indole synthesis using 2,4,6-trichloro-1,3,5-triazine (TCT)[J].Tetrahedron Letters,2013,54(41):5591-5596.
[10]DESROSES M,WIECKOWSKI K,STEVENS M,etal.A microwave-assisted,propylphosphonic anhydride (T3P©) mediated one-pot Fischer indole synthesis[J].Tetrahedron Letters,2011,52(34):4417-4420.
[11]DANDIA A,SAHA M,TANEJA H.ChemInform abstract:Synthesis of fluorinated ethyl 4-aryl-6-methyl-1,2,3,4-tetrahydropyrimidin-2-one/thione-5-carboxylates under microwave irradiation[J].ChemInform,2010,29(41):146-146.
[12]SHITOLE N V,SHITOLE B V,KAKDE G K,etal.PEG-400 as an efficient and recyclable reaction medium for the synthesis of polyhydroquinolines via Hantzsch reaction[J].Orbital the Electronic Journal of Chemistry,2012,4(4):245-252.
[13]MITSUDOME T,MIKAMI Y,EBATA K,etal.Copper nanoparticles on hydrotalcite as a heterogeneous catalyst for oxidant-free dehydrogenation of alcohols[J].Chemical Communications,2008,39(39):4804-4806.
[14]CREECH G S,KWON O.ChemInform abstract:Synthesis of nitrodienes,nitrostyrenes,and nitrobiaryls through Palladium-catalyzed couplings ofβ-nitrovinyl ando-nitroaryls thioethers[J].Chemical Science,2013,44(44):2670-2674.
[15]BYEONG-Yun L,BO-Eun J,CHEON-Gyu C.Ene-hydrazide from enol triflate for the regioselective Fischer indole synthesis[J].Organic Letters,2014,16(17):4492-4495.
[16]MIGUEL P A L,HELFRIED N,MATTHIAS B.Ruthenium-catalyzed synthesis of indoles from anilines and epoxides[J].Chemistry:A European Journal,2014,20(7):1818-1824.
[17]YADAV U N,SHANKARLING G S.Room temperature ionic liquid choline chloride-oxalic acid:A versatile catalyst for acid-catalyzed transformation in organic reactions[J].Journal of Molecular Liquids,2014,191(3):137-141.
[18]TAO Lili,JIANG Jing,PAN Yechu,etal.SO3H-functionalized ionic liquids-catalyzed facile and efficient procedure for Fischer indole synthesis under ultrasound irradiation[J].Advanced Materials Research,2013,661:150-153.
[19]JAIN R,SHARMA K,KUMAR D.ChemInform abstract:One-pot,three-component synthesis of novel spiro[3H-indole-3,2′-thiazolidine]-2,4′(1H)-diones in an ionic liquid as a reusable reaction media[J].ChemInform,2013,44(31):414-418.
[20]LI Bailin,XU Danqian,ZHONG Aiguo.Novel SO3H-functionalized ionic liquids catalyzed a simple,green and efficient procedure for Fischer indole synthesis in water under microwave irradiation[J].Journal of Fluorine Chemistry,2012,144(144):45-50.
[21]NOONAN C,BARAGWANATH L,CONNON S J.Nucleophilic carbene-catalysed oxidative esterification reactions[J].Tetrahedron Letters,2008,49(25):4003-4006.
[22]PASCAL D,DORIS K J,ADAM N,etal.Development of a donor-acceptor concept for enzymatic cross-coupling reactions of aldehydes:The first asymmetric cross-benzoin condensation[J].Journal of the American Chemical Society,2002,124(41):12084-12085.
[23]ORLANDI S,CAPORALE M,BENAGLIA M,etal.Synthesis of new enantiomerically pure C1- and C2- symmetricN-alkyl-benzimidazolium and thiazolium salts[J].Tetrahedron:Asymmetry,2003,14(24):3827-3830.
[24]LI Bailin.Synthesis of indole-like compounds in aqueous media using microwave radiation:102351773[P].2012-02-15.
[25]SAIDYKHAN A,AYRTON S T,GALLAGHER R T,etal.Novel formation of[2M-H]+species in positive electrospray mass spectra of indoles[J].Rapid Communications in Mass Spectrometry,2014,28(17):1948-1952.
[26]CAMPAIGNE E,LAKE R D.Synthesis of tetrahydrocarbazoles and carbazoles by the Bischler reaction[J].Journal of Organic Chemistry,1959,24(1):478-487.
[27]DAVIES D,GARRATT P,TOCHER D,etal.Mapping the melatonin receptor 5 melatonin agonists and antagonists derived from tetrahydrocyclopent(b) indoles,tetrahydrocarbazoles and hexahydrocyclohept(b) indoles[J].Journal of Medicinal Chemistry,1998,41(4):451-467.
[28]ERGUEN Y,PATIR S,OKAY G.A novel synthesis towards ellipticine and its derivatives:Synthesis of a new precursor compound[J].Synthetic Communications,2004,35(29):435-442.
[29]FORBES E J,STACEY M,TATLOW J C,etal.The synthesis of 1-,2- and 3-trifluoromethylcarbazoles by the fischer-indole method[J].Tetrahedron,1960,8(1-2):67-72.
[30]CHAKRABORTY D P,DAS K C,CHOWDHURY B K.Synthesis of glycozoline[J].Phytochemistry,1969,8(4):773-776.
Synthesis of Tetrahydrocarbazole Derivatives Using Thiamine Hydrochloride (VB1) as Efficient Catalyst
YANG Ya-qin,GE Song-lan,MA Lei
(Shanghai Key Laboratory of New Drug Design,School of Pharmacy,East China University of Science and Technology,Shanghai 200237,China)
The tetrahydrocarbazole derivatives are widely used in pharmaceutical chemistry.A simple and highly efficient method was developed for the construction of tetrahydrocarbazole derivatives from phenylhydrazine hydrochloride and cyclohexanone in the presence of thiamine hydrochloride (VB1) in ethanol.This protocol relies on the use of various cyclohexanone and phenylhydrazine hydrochloride with electron-donating and electron-withdrawing groups to access tetrahydrocarbazole scaffolds with a wide scope.Most of the target products catalyzed by VB1were obtained in good to excellent yields (> 90%).VB1acted as an efficient,mild and non-toxic catalyst in the reaction,and it could be reused without reducing the catalytic efficiency.
thiamine hydrochloride (VB1); tetrahydrocarbazole; phenylhydrazine; cyclohexanone
1006-3080(2016)04-0499-09
10.14135/j.cnki.1006-3080.2016.04.010
2015-11-27
上海自然科学基金(15ZR1408800);上海浦江人才计划(15PJD0122015)
杨雅琴(1991-),女,江西人,硕士生,研究方向为合成药物化学。E-mail:yangyaqin09@126.com
通信联系人:马磊,E-mail:malei@ecust.edu.cn
O621.3
A
