儿茶酚抑素在大鼠肾性高血压中的作用及可能机制*
2016-09-15郑青青陈宣颖王雪瑞龚永生范小芳
丁 露,郑青青,李 洋,陈宣颖,陈 然,王雪瑞,龚永生,范小芳
儿茶酚抑素在大鼠肾性高血压中的作用及可能机制*
丁 露,郑青青,李 洋,陈宣颖,陈 然,王雪瑞,龚永生,范小芳△
(温州医科大学低氧医学研究所 ,浙江温州325035)
目的:观察儿茶酚抑素(CST)在两肾一夹(2K1C)肾性高血压大鼠中的表达改变,并初步探讨其对肾性高血压的影响及作用机制。方法:36只SD大鼠随机分为假手术组(Sham)(n=15)和肾性高血压模型组(Model组)(n=21)。Model组采用两肾一夹(2K1C)手术法建立肾性高血压模型,Sham组手术操作同Model组,但不结扎左肾动脉,每周动态监测大鼠尾动脉血压。6周后各组大鼠行颈总动脉插管测定动脉压,Model组再随机分为2K1C组(n=15)与2K1C+CST组(n=6)。2K1C+CST组经颈外静脉一次性给予CST(80μg/100 g·BW),Sham组与2K1C组给予等容积的生理盐水。各组动物经测血压、采集血标本后被处死,称取左心室加室间隔(LV+S)重量,计算(左心室+室间隔)/体重【(LV+S)/BW】;高效液相色谱-电化学方法测定血浆中去甲肾上腺素(NE)含量,ELISA法测定血浆CST含量,硝酸还原酶法测定血浆及心室肌一氧化氮(NO)浓度;Western blot法检测延髓、肾上腺髓质、左心室和肾脏的嗜铬蛋白A(Chga)及左心室内皮型一氧化氮合酶(eNOS)、诱导型一氧化氮合酶(iNOS)蛋白表达量。结果:①与Sham组相比,2K1C组大鼠尾动脉压显著升高,左心室明显肥厚(P<0.01);血浆NE含量增高246%(P<0.01),CST水平降低56%(P<0.05);延髓Chga含量增高108%,左心室和肾脏分别降低60%和30%(P<0.05);左心室NO含量增高46%,血浆NO含量增高24%(P<0.05);左心室eNOS、iNOS蛋白表达分别增高66%和40%(P<0.05);②外源性CST显著降低2K1C大鼠颈总动脉压(P<0.05);③与2K1C组相比,2K1C+CST组左心室和血浆NO含量分别增高35%和19%(P<0.05);左心室eNOS蛋白表达高50%(P<0.05),而iNOS表达无显著统计学差异。结论:两肾肾性高血压时大鼠CST表达下调,外源性CST可能通过NO/NOS系统降低肾性高血压的作用,推测CST可能与肾性高血压的发生发展有关。
高血压,肾性;儿茶酚抑素;一氧化氮 ;去甲肾上腺素
【DOI】10.13459/j.cnki.cjap.2016.03.006
儿茶酚抑素(catestatin,CST)是嗜铬颗粒蛋白A (chromograninA,Chga)的酶切产物,Chga主要分布于肾上腺嗜铬细胞和肾上腺能神经元胞质颗粒中[1]。已有研究表明CST通过自分泌/旁分泌的方式发挥多种生物学效应:具有扩张血管、降低血压、降低心肌收缩力等生物效应[2];O'Connor[3]等的研究发现高血压患者的CST水平下降;具有高血压遗传倾向者在高血压早期甚至是在未出现血压升高时,其血浆中CST含量也低于正常水平,提示CST与高血压的发病机制之间存在密切关系。肾性高血压是最常见的继发性高血压疾病,药物疗效较差,心血管并发症多,易发展成为恶性高血压[4]。肾交感神经系统兴奋性增强导致肾血管强烈收缩,是其发病的主要病理生理机制之一。CST与其发病是否存在关系,及CST在肾性高血压中的作用目前仍未明了。本文在两肾一夹法复制的大鼠肾性高血压模型上对其进行初步探讨,以期对CST介导的血压调节机制有更深入的了解,为寻找肾性高血压的有效治疗药物提供理论依据。
1 材料与方法
1.1 研究对象及分组
清洁级雄性SD大鼠36只,由温州医科大学实验动物中心提供,动物许可证号为SYXK(浙)2010-0044。体重(BW)180~220 g,适应性喂养1周后,随机分为假手术组(Sham组)(n=15)和肾性高血压模型组(Model组)(n=21)。手术6周后,Model组再随机分为两肾一夹高血压组(2K1C组)(n=15)与2K1C+CST组(n=6)。2K1C+CST组经右侧颈外静脉一次性给予CST(80μg/100 g体重),Sham组与2K1C组给予等容积的生理盐水。饲养温度保持在20℃~25℃,湿度在40%~60%,日光灯模拟昼夜,食物及饮水充足,定期消毒。
1.2 两肾一夹肾性高血压大鼠模型的制备
模型制备参照戴勇等[5]的方法,即将Model组大鼠做腹正中切口打开腹腔,沿肾静脉下方钝性分离出左肾动脉,将直径0.25mm的针灸针与肾动脉血管长轴平行放置,用无菌丝线结扎肾动脉和针灸针,然后抽出针灸针,造成单侧肾动脉狭窄。Sham组的手术操作同Model组,但不放置针灸针和不用丝线结扎左肾动脉。术后3 d内腹腔注射青霉素钠(3×104U/d)(山西联邦制药,国药准字H14022390)预防感染,正常进食、饮水。
1.3 大鼠动脉压测定
术后采用尾动脉测定仪检测动脉血压,每周测1次,每次测量重复3次;造模后第42天,各组大鼠称重、异氟烷麻醉后行左颈总动脉插管测量血压,待血压记录稳定15 min后,经右颈外静脉缓慢推注CST或NS,并实时记录动脉血压的变化。
1.4 标本的采集
各组大鼠给药后30 min,抽取颈总动脉血3 ml分别置于含EDTA-Na2和肝素的抗凝管中,轻摇混匀后,3 000 r/min离心15min,取上清,-80℃保存用于测定血浆CST和NO的含量。动物处死后取出心脏分离左心室和室间隔(left ventricle+septum,LV+ S),滤纸吸干水分,称重,计算(LV+S)/BW作为左室肥大指数。取右肾、肾上腺髓质及延髓组织置于-80℃保存待测。
1.5 检测指标及方法
1.5.1 血浆去甲肾上腺素(NE)及CST含量测定取血浆200μl加入0.1mol/L高氯酸800μl,8 000 r/ min离心10 min去蛋白,取上清至EP管中8 000 r/ min再次离心10 min,采用高效液相色谱-电化学法(high performance liquid chromatography,HPLC),Agilent(安捷伦)1100系列色谱仪测定血浆NE含量。用酶联免疫吸附法(ELISA)测定血浆CST的含量,严格按照试剂盒说明操作。
1.5.2 血浆及左心室中NO含量的测定 采用硝酸还原酶法测定左心室及血浆NO3-及NO2-浓度之和来代表其NO水平。取左心室50 mg,加入1 ml PBS,用高速离散器制成心室肌匀浆液,4℃,3 000 r/min离心20min,取其上清液;将血浆、心室肌匀浆液加入试剂盒反应体系(南京建成)进行显色,测定吸光度值。
1.5.3 相关组织中Chga、eNOS及iNOS蛋白的测定每组随机选取6只大鼠,称取适量组织(50~100 mg),加入含有蛋白酶抑制剂的RIPA裂解液(普利莱,北京)提取组织总蛋白,BCA法测定蛋白浓度。100℃煮沸5min使蛋白变性,行SDS-PAGE电泳、电转,将PVDF膜置于5%脱脂奶粉中室温封闭2 h,加入稀释一抗后(Chga为1∶100,内皮型一氧化氮合酶(endothelial nitric oxide synthase,eNOS)及一氧化氮合成酶(inducible nitric oxide synthasei,iNOS)为1∶400,内参为1∶500)4℃孵育过夜,TBST洗膜4次,加入相应的二抗后(1∶10 000),室温孵育2 h。TBST洗膜,5min×4次。ECL显色剂,显影。Bio-rad凝胶成像分析系统曝光、拍片。采用ImageJ软件计算条带的灰度值,计算待测蛋白与内参蛋白灰度的比值,对得到的待测蛋白的相对表达量进行比较。
1.6 统计学处理
实验数据采用GraphPad Prism 5.0软件分析。实验数据以均数±标准差(±s)表示,两组间比较采用独立样本 t检验,多组间比较采用单因素方差分析(One-Way ANOVA)。
2 结果
2.1 手术前后不同时期大鼠尾动脉血压的变化
术后所有大鼠均存活。造模前两组大鼠尾动脉血压均在正常范围,血压、体重均无显著性差异。与同期Sham组比较,术后21 d,Model组尾动脉血压较Sham组增高19%(P<0.05),术后28 d增高31%,术后35 d增高52%,术后42 d增高68%,差异均有统计学意义(P<0.01,表1)。
Tab.1 Changes in tail-artery blood pressure in each group rats(mmHg,±s)

Tab.1 Changes in tail-artery blood pressure in each group rats(mmHg,±s)
*P<0.05,**P<0.01 vs Sham
Group n 0 d 14 d 21 d 28 d 35 d 42 d ** Sham 15 109.2±11.7 101.5±10.9 98.8±13.4 106.7±16.7 104.1±10.8 110.5±16.0 Model 21 107.8±13.5 106.6±14.7 117.9±15.8* 140.3±18.8** 158.3±16.2** 185.6±21.3
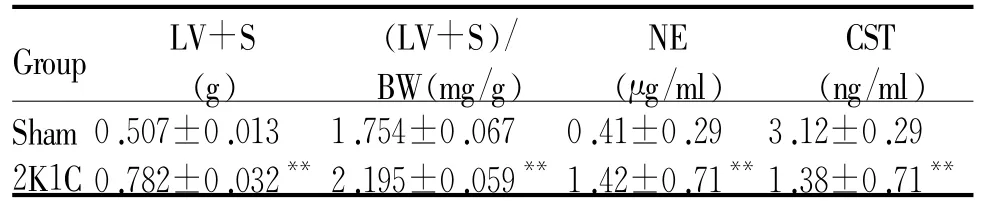
2.2 大鼠左心肥大指数、血浆NE和CST含量的变化
左室肥大指数显示,2K1C组较Sham组出现明显的心室肥厚(P<0.01)。高效液相电化学及ELISA结果显示,2K1C组血浆NE含量比Sham组增高246%(P<0.01),而CST含量比Sham组降低56%(P<0.01,表2)。
Tab.2 Changes in left ventricle/body weight ratio,NE and CST in plasma of each group rats(±s,n =15)
Tab.2 Changes in left ventricle/body weight ratio,NE and CST in plasma of each group rats(±s,n =15)
LV+S:Left ventricular plus interventricular septum;BW: Bodyweight;NE:Norepinephrine;CST:Catestatin**P<0.01 vs Sham group
Group LV+S (g)(LV+S)/ BW(mg/g)NE (μg/ml)CST (ng/ml)Sham 0.507±0.013 1.754±0.067 0.41±0.29 3.12±0.29 2K1C 0.782±0.032**2.195±0.059**1.42±0.71**1.38±0.71**
2.3 大鼠延髓、肾上腺髓质、左心室及肾脏组织中Chga蛋白表达的变化
与Sham组相比,2K1C组延髓中Chga蛋白表达增高108%;肾上腺髓质中Chga蛋白表达无显著差异;左心室心肌Chga蛋白表达降低60%;右肾Chga蛋白表达降低30%(P<0.05,P<0.01,图1,表3)。
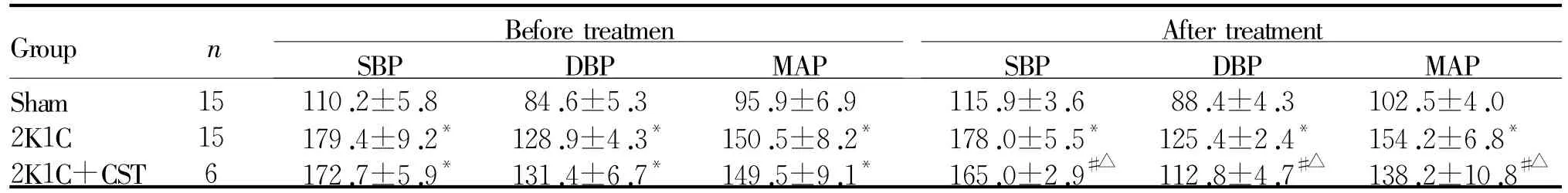
2.4 外源性CST对大鼠血压的影响
颈总动脉血压测量结果显示:2K1C组比Sham组的收缩压(systolic blood pressure,SBP)高63%(P<0.05),舒张压(diastolic blood pressure,DBP)高50% (P<0.05),平均压(mean arterial pressure,MAP)高56%(P<0.05);2K1C+CST组给药前SBP、DBP、MAP均显著高于Sham组(P<0.05),与2K1C组无显著差异。2K1C+CST组给药后SBP、DBP、MAP均显著低于2K1C组和2K1C+CST组给药前(P<0.05,表4)。而Sham组、2K1C组处理前后的血压无差别。

Fig.1 RepresentativeWestern blot showed the 2K1C surgery altered expression of ChgaA:Medullaoblongata;B:Adrenal gland;C:Myocardium;D:Kidney;Chga:ChromograninA
Tab.3 Densitometric analyses are presented as the relative ratio of Chga inmedulla oblongata,adrenal gland,myocardium,and kidney to thatofβ-actin in differentgroups(± s,n =6)

Tab.3 Densitometric analyses are presented as the relative ratio of Chga inmedulla oblongata,adrenal gland,myocardium,and kidney to thatofβ-actin in differentgroups(± s,n =6)
Chga:ChromograninA*P<0.05,**P<0.01 vs Sham
Kidney Sham 0.419±0.013 0.543±0.09 1.111±0.292 0.763±0.09 2K1C 0.871±0.08* 0.592±0.06 0.444±0.01**0.534±0.04 Group Medullaoblongata Adrenalgland Myocardium **
Tab.4 Changes incarotid arterial pressure before and after treatmentof each group rats(mmHg,±s)
Tab.4 Changes incarotid arterial pressure before and after treatmentof each group rats(mmHg,±s)
SBP:Systolic blood pressure;DBP:Diastolic blood pressure;MAP:Mean arterial pressure*P<0.05 vs Sham;#P<0.05 vs2K1C;△P<0.05 vs before treatment
Group MAP Sham 15 110.2±5.8 84.6±5.3 95.9±6.9 115.9±3.6 88.4±4.3 102.5±4.0 2K1C 15 179.4±9.2* 128.9±4.3* 150.5±8.2* 178.0±5.5* 125.4±2.4* 154.2±6.8*2K1C+CST 6 172.7±5.9* 131.4±6.7* 149.5±9.1* 165.0±2.9#△ 112.8±4.7#△ 138.2±10.8 n Before treatmen SBP DBP MAP After treatment SBP DBP#△
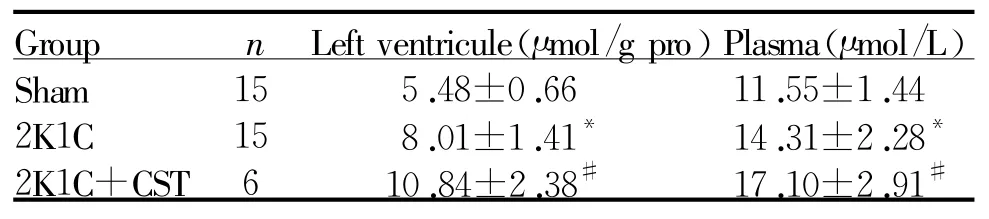
2.5 CST对大鼠血浆及左心室NO表达的影响
与Sham组相比,2K1C组的左心室NO含量增高46%(P<0.05),血浆NO含量增高24%(P<0.05);与2K1C组相比,2K1C+CST组左心室NO含量增高35%(P<0.05);血浆NO含量增高19%(P <0.05,表5)。
Tab.5 NO content in leftventriculeand plasmaof each group rats(±s)
Tab.5 NO content in leftventriculeand plasmaof each group rats(±s)
*P<0.05 vs Sham;#P<0.05 vs2K1C
?
2.6 CST对大鼠左心室eNOS、iNOS蛋白表达的影响
与Sham组相比,2K1C组左心室中eNOS、iNO蛋白分别高66%和高40%;与2K1C组相比,2K1C+ CST组eNOS蛋白表达高50%,iNOS蛋白表达无显著统计学差异(图2,表6)。
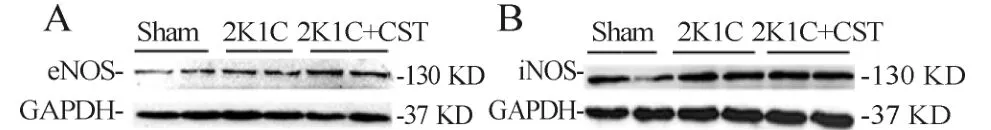
Fig.2 CST treatment altered eNOS(A)and iNOS(B)expression levels inmyocardium
Tab.6 Densitometricanalysesarepresentedastherelativeratio oftheproductofeNOSandiNOSinmyocardiumindifferentgroups(±s,n=6)
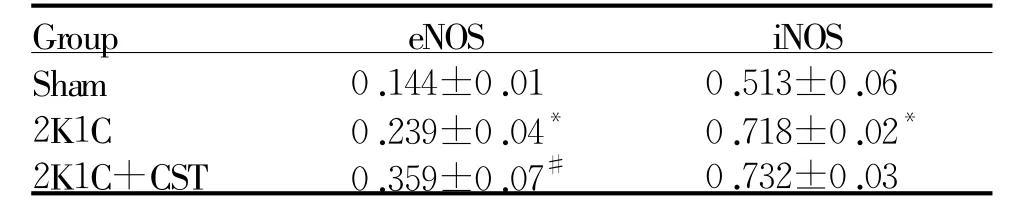
Tab.6 Densitometricanalysesarepresentedastherelativeratio oftheproductofeNOSandiNOSinmyocardiumindifferentgroups(±s,n=6)
eNOS:Endothelial nitric oxide synthase;iNOS:Inducible nitric oxide synthase*P<0.05 vs Sham;#P<0.05 vs2K1C
?
3 讨论
肾性高血压在成人高血压中的发病率较高,约为5%~10% ,为临床上较为常见的肾病并发症[6]。目前各种肾性高血压模型多采用大鼠,使其肾动脉缩窄以形成肾性高血压。本文采用改良的两肾一夹法成功造成左肾动脉狭窄 ,结果与刘洁[7]等类似。
肾性高血压的发病机制与多因素相关,其中肾交感神经的兴奋作用不容忽视,肾缺血代谢产物可刺激肾的化学感受器,导致交感神经兴奋活性增强,调节肾素的释放引起水钠潴留,使动脉收缩增加血管阻力致高血压的发生[7]。儿茶酚抑素CST是源于Chga、能够抑制肾上腺嗜铬细胞分泌儿茶酚胺的多肽,Gaede等[8]已证实CST可以通过降低外周交感神经兴奋,负反馈抑制儿茶酚胺的释放而降低血压。延髓在血压和交感神经活动调控方面起十分关键的作用。本实验结果显示2K1C大鼠血浆中去甲肾上腺素含量明显升高,交感神经活性增高;延髓Chga蛋白表达明显上调,中枢代偿性的Chga表达增加以增强负反馈抑制儿茶酚胺释放,抑制交感紧张性;而2K1C大鼠左心室肌及肾脏的Chga蛋白的表达下调,血浆中CST水平明显降低,推测外周器官所产生并储存在囊泡内的CST在高血压早期即对交感神经兴奋产生拮抗作用,中枢Chga的高表达依旧不能满足全身交感神经兴奋的抑制效应,提示肾性高血压的发生可能与体内CST负反馈抑制儿茶酚胺释放效应不足有关,这一作用可能参与肾性高血压的发病过程。外源给予CST后,短时间内即观察到2K1C大鼠的血压明显降低,进一步说明CST的分泌不足可能与肾性高血压的发生有关并对其具有降压作用。
本实验结果显示2K1C大鼠左心室eNOS表达增高,血浆及左心室NO含量增高,左心室代偿性增加NO的表达负反馈调节血压的现象与Zhou的实验结果一致[10]。已有报道CST能通过活化磷酸肌醇-3激酶/蛋白激酶B(PI3K/Akt)通路,促进心内膜内皮细胞中eNOS磷酸化,合成释放NO,拮抗缺血再灌注过程中代偿性增多的儿茶酚胺类物质对心肌的损伤[9]。本实验结果推测 CST可能通过促进eNOS的表达使NO的释放增加,从而起到舒张血管发挥其降低肾性高血压大鼠动脉血压的作用。
综上所述,肾性高血压大鼠CST表达异常,这种异常可能参与了肾性高血压的发生发展,外源给予CST可以降低肾性高血压大鼠血压,可能为肾性高血压的治疗提供新的思路。
[1]Mahata SK,MahataM,TaylorCV L,et al.TheNovelCatecholamine Release-Inhibitory Peptide Catestatin(Chromogranin A344-364)[M].Springer,2002,263-277.
[2]AngeloneT,QuintieriAM,BrarBK,et al.The antihypertensive chromogranin a peptide catestatin acts as a novel endocrine/paracrinemodulator of cardiacinotropismand lusitropism[J].Endocrinol,2008,149(10):4780-4793.
[3]O'Connor DT,KailasamMT,Kennedy BP,et al.Early decline in thecatecholamine release-inhibitory peptidecatestatin in humans at geneticrisk of hypertension[J].JHypertens,2002,20(7):1335-1345.
[4]James PA,Oparil S,Carter BL,et al.2014 evidence-based guideline for the management of high blood pressure in adults:report from the panelmembersappointed to the Eighth JointNational Committee(JNC 8)[J].JAMA,2014,311 (5):507-520.
[5]戴 勇,彭武建,徐卓佳,等.“两肾一夹”肾性高血压大鼠模型的改进[J].实验动物科学与管理,2006,23 (2):60-62.
[6]刘 洁,白 桦,李 丽 ,等.肾性和自发性高血压大鼠心肌肥大前后心肌Gαq/11含量的变化[J].中国应用生理学杂志,2003,19(2):200-201.
[7]CamposRR,Oliveira-Sales E,NishiEE,etal.Mechanisms of renal sympathetic activation in renovascular hypertension Experimental[J].Exp Physiol,2015,100(5):496-501.
[8]Gaede AH,LungMS,Pilowsky PM.Catestatin attenuates the effects of intrathecalnicotine and isoproterenol[J].Brain Res,2009,1305:86-95.
[9]Bassino E,Fornero S,Gallo MP,et al.A novel catestatininduced antiadrenergicmechanism triggered by the endothelial PI3K-eNOS pathway in the myocardium [J].Cardiovasc Res,2011,91(4):617-624.
[10]Zhou MS,Jaimes EA,Raij L.Atorvastatin prevents end-organ injury in salt-sensitive hypertension:role of eNOS and oxidant stress[J].Hypertension,2004,44(2):186-190.
Role of catestatin in 2K1C-induced renal hypertension in rats and the underlying mechanism
DING Lu,ZHENGQing-qing,LIYang,CHEN Xuan-ying,CHEN Ran,WANG Xue-rui,GONG Yong-sheng,FAN Xiao-fang△
(Institute of Hypoxia Medicine,Wenzhou Medical University,Wenzhou 325035,China)
【ABSTRACT】Objective:To investigate the rolesof catestatin(CST)in 2-kidney-1 clip(2K1C)-induced renalhypertension in rats,and to explore theunderlyingmechanism.Methods:Thirty sixmale SD ratswere randomly divided into Sham group(n=15)andModelgroup(n= 21).Themodelgroup was performed by 2K1C operation.For 2K1C operation,the left renal arterieswere narrowed by cotton thread.The Sham groupwas treated with the same condition as the 2K1C group except the renal arterywas narrowed.Tail-cuff systemic blood pressure of ratswasmeasured before and everyweeksafter 2K1Coperation.Sixweeksafter2K1Coperation,a carotid artery catheterwas inserted tomeasure blood pressure of rats under anesthesia.Then,themodel group was randomly subdivided into 2K1C group(n=15)and 2K1C+CST group(n=6).The ratsof2K1C+CSTgroupwere intravenousgiven CST(80μg/100 g)and the ratsof Sham or 2K1C groupweregiven normal saline.All ratswere sacrificed after blood pressurewasmeasured and bloodwas collected.Then,the leftventricular plus interventricular septum weight(LV+S)wasweighted and the ratio of(LV+S)/bodyweight(BW)was calculated as the index of leftventricularhypertrophy. Norepinephrine(NE)contents in plasmawere determined by high performance liquid chromatography(HPLC)and CST contents in plasma by ELISA.The nitrite/nitrate contents in the leftventricular tissue and plasmaweremeasured by nitrate reductionmethod to representnitric oxide (NO)contents.Expression levelsof CST in the leftventricle,kidney,medulla oblongata and adrenalgland,aswell as eNOS and iNOS,were tested byWestern blot.Results:①The2K1C group had higher tail-artery blood pressure(P<0.01)andweremoremarked presenceof right ventricular hypertrophy than those of sham group(P<0.01).Comparedwith Sham group,plasma CST content in 2K1C groupwas decreased by 226%(P<0.01),while plasma NE content in 2K1C group was increased by 246%(P<0.01),expression levels of chromograminA (Chga)inmedulla oblongata of 2K1C groupwere increased by 108%,in leftventricle and kidneywere decreased by 60%and 30%,respectively(P<0.05).the contentof NO in leftventricular and plasmawere increased by 46%and 24%respectively.②The carotid arterialblood
pressure of 2K1C groupmarkedly reduced after administration of CST.③Compared with 2K1C group,the contentof NO in left ventricul and plasma of 2K1C+CSTgroupwere increased by 35%and 19%respectively(P<0.05).Theexpression of eNOSin leftventricularof 2K1C+ CST group were also obviously increased.Conclusion:The CST expression of 2K1C-induced renalhypertension rats is reduced and the effects of exogenous CST lowering their blood pressuremay be related to NO/NOSsystem.Therefore,we speculate CST could contribute to the pathogenesis and progression of renal hypertension.
hypertension; renal; catestatin; nitric oxide;norepinephrine
R331.3
A
1000-6834(2016)03-214-05
国家自然科学基金青年项目(81200039);浙江省自然基金项目 (LY12H01003);浙江省教育厅科研项目(Y201326833)
2015-05-28
2016-02-17
Tel:0577-86699521;E-mail:fxbwzmc@126.com
