A prospective,randomized controlled trial of sanguisorba oil in the treatment of tamoxifen-related vaginitisin breast cancer patients
2016-09-14YuanHongZhaoSuHengHanZhengLiXiaoYaoLuoXiuLiLongShuaiLi
Yuan-Hong Zhao*,Su-Heng Han,Zheng Li*,Xiao-Yao Luo,Xiu-Li Long,Shuai Li
1Department of Oncology,First Teaching Hospital of Tianjin University of Traditional Chinese Medicine,Tianjin,China.2Pharmaceutical Preparation Department,First Teaching Hospital of Tianjin University of Traditional Chinese Medicine,Tianjin,China.
Introduction
Tamoxifen(TAM)is one of the most important drugs of endocrine therapy for patients with breast cancer.It is reported that the recurrence rate and mortality could be respectively decreased by 14.9%and 15.2%when the patients have taken TAM for five years[1].In addition,TAM could also reduce the risk of contralateral breast cancer[2].TAM has estrogen-like and anti-estrogenic dual roles[3].Vaginal dryness,itching,burning,and irritation of the urethra are common adverse reactions after TAM treatment,and vaginal lubricants were usually used to treat above adversereactions[2-4].
Sanguisorba oil is mainly composed of 40g Sanguisorba power,10g Rhubarb powder and 50ml Sesame oil[5](Figure 1).With the advantage of hygroscopic itching and detoxification,sanguisorba oil hasbeen used to cure burnsand radiation dermatitisfor many years[6].However,whether sanguisorba oil is also effective against TAM-related vaginitis remains largely unknown.In present study,we performed a prospective,randomized controlled trial to evaluate the efficacy of sanguisorba oil in the treatment of TAM-related vaginitisin breast cancer patients.

Figure 1 Sanguisorba oil.
Materialsand methods
Patients
We collected 112 patients with breast cancer in the First Affiliated Hospital of Tianjin University of Chinese Medicine from April 2012 to April 2015.This study was approved by the Ethics Committees of First Affiliated Hospital of Tianjin University of Chinese Medicine.All the cases were included according to the following criteria:(1)by histopathology and/or cytology in line with NCCN Guidelines-Breast Cancer diagnostic criteria(Version 2,2015);(2)estrogen and/or progesterone receptor positive(+~+++),and 20mg oral tamoxifen Qd treatment;(3)KPSscore≥60,expected survival time>3 months;(4)receiving TAM therapy for 12-36 months,and patients were diagnosed with vaginal inflammation(clinical features including vaginal dryness,less vaginal secretions or vaginal itching or burning,or accompanied with urinary frequency,urgency and dysuria);(5)each patient signed the informed consent and cooperated with the treatment scheme and follow-up.The patients would be exclude if:(1)patients were diagnosed with vaginitis or urinary tract infection before the TAM treatment; (2) patients who used tinidazole effervescent tablets; (3) patients with disease progression or death;(4)patients who withdrew from the study.
Randomization
These patients were randomly assigned to receive the intervention or usual care using a random number table.(1)the sanguisorba oil group consists of 56 patients,with aged 28-67 years,mean age 52.4±6.2 years,11 cases of premenopausal and 45 cases of postmenopausal;(2)the control group consists of 56 patients,aged 29-68 years,mean age 53.5±6.6 years,10 cases of premenopausal and 46 cases of postmenopausal.There was no significant difference in the age,height,weight,duration and time of taking TAM between two groups(P>0.05)(Table1).
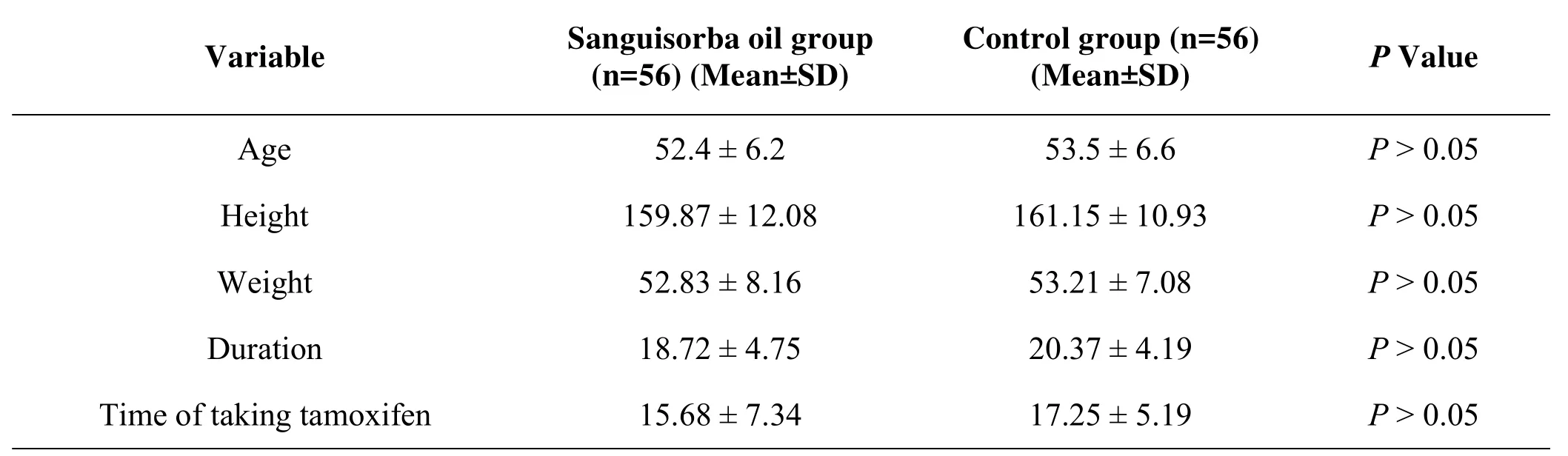
Table 1.Clinical characteristics of enrolled patients.
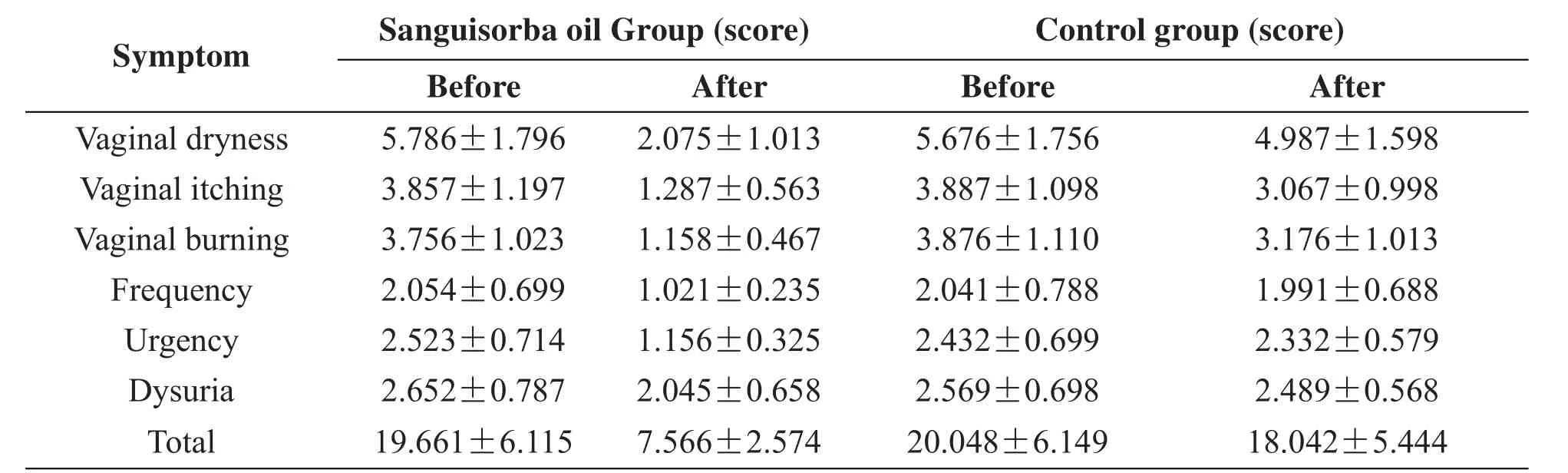
Table 2 Comparison of clinical symptom scorebetween the Sanguisorba oil group and the control group.
Procedures
All the breast cancer patients in this study were treated with routine surgery,radiotherapy or chemotherapy,followed by 20mg oral TAM Qd treatment.In the sanguisorba oil group,patients were treated with external application of sanguisorba oil(2g,Qd)after cleaning the vulva in the morning and at night(Sanguisorba oil was made by the Pharmaceutical preparation department,First Affiliated Hospital of Tianjin University of Traditional Chinese Medicine,50 g per bottle);while patients in the control group only cleaned the vulva without any topical medications.All patients were evaluated after 7 days at the end of treatment.
Outcomemeasures
Clinical performance was recorded at two time points(before the treatment and 7 days at the end of treatment),including vaginal dryness,vaginal itching,vaginal burning,frequent urination,urgency,dysuria and other symptoms.The evaluation criteria were in accordance with the female reproductive systemic inflammation extracted from “Chinese medicine clinical research guidelines”[7].In detail,vaginal dryness was recorded in 3 points,vaginal itching or burning in 2 points,and frequent,urgency and dysuria in 1 point.In addition,each symptom was calculated by multiplying the severity of each symptom.When the symptoms disappeared after treatment,we didn’t record it,which indicating that it was healed.Lastly,adverse reactions were also recorded during the treatment.
Statistical analysis
All statistical tests were performed with SPSS 17.0 software package,measurement data as mean ±standard deviation(x±s).Self-controlled data were in accordance with normal distribution using paired t test,otherwise using rank sum test.Multiple groups’means were compared with single factor analysis of variance,and the comparison between groups was performed with LSD method or Dunnett method.Qualitative variables were compared by using Chi-squaretest.
Results
Clinical symptom score before and after treatment in two groups
Before the treatment,there was no significant difference between the two groups in clinical symptom score(P>0.05).After the treatment,the sanguisorba oil group had a better clinical symptom score compared to control group(P<0.05).In addition,vaginal dryness or itching or burning,or frequent,urgency and other symptom scores were largely alleviated after 10 days treatment in sanguisorba oil group,with the difference was statistically significant(P<0.05).However,no significant difference of clinical symptom scores was found in control group after the treatment(P>0.05)(Table2).
Evaluation of curativeeffect
In the sanguisorba oil group,4 cases were healed,which indicating the cure rate was 7.1%.Whereas no patients was healed in the control group.Significant difference was found between the two groups and it was suggested that the sanguisorba oil group had a better curative effect compared to control group(P<0.05).
Adversereactions
There was no significant difference of liver and kidney function,and electrocardiogram between before and after treatment in each group.In addition,we did not found any adverse reactions related with sanguisorba oil application.
Discussion
TAM is the first generation selective estrogen receptor modulators,with an anti-estrogen and/or estrogen-like dual role in different target organs of the same patient[1-4].In breast tissue,TAM showed estrogen resistance,and then inhibited the breast tissue proliferation and tumor cell growth to suppress disease recurrence and metastasis[3-4].However,TAM function on the vaginal epithelium was slightly different in women before and after menopause.Vagina is covered by a multi-squamous non-keratinized epithelial cells,which contain estrogen receptors both in premenopausal and postmenopausal women.Upon estrogen stimulation,the surface and middle of epithelium cells will produce excessive glycogen,which makes the vaginal epithelial cell proliferation and variation [4]. Generally,postmenopausal women were not easily infected with vagina or labia Candida albicans unless excessive glycogen and estrogen stimulation.In premenopausal women,TAM exhibited anti-estrogenic effect in rich estrogen vaginal epithelium cells,which manifested as vaginal drynessand vaginal itching.In postmenopausal women,patients were easily infected with non-specific inflammatory,which attributed to ovarian dysfunction,deficiency or insufficient estrogen, and estrogen-dependent organs atrophic changes.For those women,vaginal epithelium cells exhibited mature and/or estrogen-like effect after TAM treatment,but vaginal symptoms such as vaginal dryness,decreased libido were also noted[8].In general,the vaginal symptoms in postmenopausal women can be treated with estradiol(E2)suppository or ointments.However,considering estrogen effect may aggregated cancer progression,it was not recommended in breast cancer patientswith vaginitis.In addition,several authors also advocated that silica cantraceplion ring or hormone-free vaginal lubricants to treat above symptoms. Nevertheless, the severe withdraw symptomsshould not beignored or minimized[9].
Sanguisorba oil,made by the Pharmaceutical preparation department,First Affiliated Hospital of Tianjin University of Traditional Chinese Medicine,was mainly contained sanguisorbaand and sesame oil(sesame oil was heated to 150°Cand slowly added into sanguisorba fried to dry,and then filtered).It was previously treated for burns and scalds.“Dictionary of Chinese Medicine”indicated that sanguisorba treats Yin-yang(vaginal itching)[10],“Materia Medica Will Read”reported that Shen cold blood scattered swollen and can Shufeng. Moreover, “The Classic”demonstrated that housewives who gynecological disease,pain,and evil forces meat,antiperspirant,treatment gold sores,while “Tang Materia Medica”mentioned that Ying-yang namely are Chinese“leukorrhagia”category.Besides,the sesame oil in our study wasused to relievedried vaginal mucosa.
Taken together,this study showed that sanguisorba oil for external application could reduce the clinical symptoms including vaginal dryness,itching,burning,urgency and dysuria.Sanguisorba oil was effective in the treatment TAM-related vaginitis and/or urinary irritation in breast cancer patients.In addition,sanguisorba oil improves the compliance of breast cancer patients with vaginitis,and should be promoted in clinical practicef or oncologists.
Innovation
In this study,we performed a prospective study to evaluate the sanguisorba oil with cooling blood and detoxification efficacy in the treatment of TAM-related vaginitis.This study takes full advantages of Chinese medicine to enrich the treatment of the disease,thus providing further insights into the endocrine therapy of TAM-induced vaginitisin breast cancer patients.
Conflict of Interests
The authors declare that there is no conflict of interests regarding thepublication of thispaper.
Acknowledgements
Special thanks to Shan-Qi Guo for help in translating thisarticle from theoriginal Chinese.
Reference
1.Visvanathan K,Hurley P,Bantug E,et al.Use of pharmacologic interventions for breast cancer risk reduction:American Society of Clinical Oncology clinical practice guideline.J Clin Oncol 2013,31(23):2942-2962.
2.Xu L,Zhao Y,Chen Z,et al.Tamoxifen and risk of contralateral breast cancer among women with inherited mutations in BRCA1 and BRCA2:a meta-analysis. Breast Cancer 2015, 22(4):327-334.
3.Goodsell DS. The molecular perspective:tamoxifen and the estrogen receptor.Stem Cells 2002,20(3):267-268.
4.Karn A,Jha AK,Shrestha S,et al.Tamoxifen for breast cancer.JNMA J Nepal Med Assoc 2010,49(177):62-67
5.Zhou GZ,Zhu L,Liu WY,et al.Research and discussion on Sanguisorba.J North Pharm 2012,9(4):30-32.
6.Ye ZJ,Yan L,Li H,et al.Research progression and clinical application of traditional Chinese medicine Sanguisorba officinalis L.Pharm Care Res2015,15(1):47-50.
7.People’s Republic of China Ministry of Health.Chinese medicine clinical research treatment principles(first series)1993:250-254.
8.Mortimer JE,Boucher L,Baty J,et al.Effect of tamoxifen on sexual functioning in patients with breast cancer.J Clin Oncol 1999, 17(5):1488-1492.
9.Zhang PY, Zhang DZ. Evaluation of tamoxifen-related side effects in breast cancer patients.Chin J Clin Oncol Rehabil 2004,11(3):271-273.
10.Chinese Medicine Dictionary (Volume II).Shanghai:Shanghai Scienceand Technology Press,1986,2483-2486.
杂志排行
Traditional Medicine Research的其它文章
- Dynamic changesof circulating Th1 and Th17 cellsin psoriasispatients:a report of 3 cases treated by Chineseherbal medicine
- Pharmacokinetic study of dl-tetrahydropalmatinepatchesby UPLC–MS/MSin rabbits
- A Literature Review of the Acupoint Plaster Therapy for Asthma in Summer
- Effectsof acupuncture treatment for irritablebowel syndrome:a systematic review and meta-analysis
- A new idea of electro-acupuncturetreatment for peripheral facial paralysis and thenerve-endocrinehypothesis
- Challengesand opportunitiesof applying P4 medicineand traditional Chinese medicinefor cancer treatment and prevention in the21st century:A medical oncologist’sperspectives