配体结构对铀配位性能影响的理论研究
2016-09-09匙芳廷魏贵林易发成
匙芳廷,文 君,熊 洁,胡 胜,魏贵林,易发成
1.西南科技大学,核废物与环境安全国防重点学科实验室,四川 绵阳 621010;2.中国工程物理研究院 核物理与化学研究所,四川 绵阳 621900
配体结构对铀配位性能影响的理论研究
匙芳廷1,2,文君2,*,熊洁2,胡胜2,魏贵林1,易发成1
1.西南科技大学,核废物与环境安全国防重点学科实验室,四川 绵阳621010;2.中国工程物理研究院 核物理与化学研究所,四川 绵阳621900
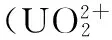
海水提铀;密度泛函;配体结构设计;分子轨道

1 计算方法
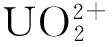
2 结果与讨论
2.1几何结构优化
(1)

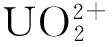
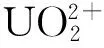

图2 配体的构型Fig.2 Geometric configuration of ligands
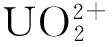
2.2热力学参数、配位稳定常数
配离子(配原子)的配位稳定常数用K来表示,配位稳定常数越大,形成配离子(配原子)越稳定。配合物的形成过程可以简单的理想化为反应式(2)。
(2)
通过计算配位过程中焓变(ΔH)、熵变(ΔS)、热力学能变(ΔU)、吉布斯自由能(ΔG)和配位稳定常数(lgK),进一步比较了配合物的稳定性,上述参数列入表3。ΔH、ΔG和lgK可以用公式(3)—(5)求得。
(3)

(4)

(5)
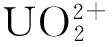
表3配合物[UO2(Ax)(H2O)3]+的热力学参数与配位稳定常数
Table 3Thermodynamic parameters and coordination stability constants of uranyl ligand complexes([UO2(Ax)(H2O)3]+)

配体类型气态ΔS/(cal·mol-1·K-1)ΔU/(kcal·mol-1)ΔH/(kcal·mol-1)ΔG/(kcal·mol-1)lgKk2-A1-135.8927.969-245.219-204.72335.897k2-A2-126.6658.553-458.861-421.11573.840k2-A3-127.4028.952-469.399-431.43375.649k2-A4-140.0587.839-448.537-406.80071.330η2-A4-125.2969.240-466.554-429.21675.260配体类型水溶液ΔS/(cal·mol-1·K-1)ΔU/(kcal·mol-1)ΔH/(kcal·mol-1)ΔG/(kcal·mol-1)lgKk2-A1-130.117.485-82.063-43.2907.590k2-A2-129.6976.779-126.612-87.96215.424k2-A3-127.7456.754-137.049-98.98117.356k2-A4-123.8668.323-119.726-82.81414.521η2-A4-120.9558.395-136.974-100.92917.697
2.3分子轨道


(a)——[UO2(η2-A4)(H2O)3]+,(b)——[UO2(k2-A1)(H2O)3]+,(c)——[UO2(k2-A2)(H2O)3]+,(d)——[UO2(k2-A3)(H2O)3]+,(e)——[UO2(k2-A4)(H2O)3]+ OLP:氧原子的孤对电子;NLP:氮原子的孤对电子;π(N-O):N-O形成的π键 图3 配合物[UO2(Ax)(H2O)3]+典型的分子轨道(MO)Fig.3 Representative diagram of molecular orbital of [UO2(Ax)(H2O)3]+ complexes

[UO2(Ax)(H2O)3]+轨道能量/eV分子轨道(MO)分子轨道主要组成[UO2(η2-A4)(H2O)3]+-8.558OLP,π(N-O)1δuOpz,Npz,Ufz-10.140π(N-O)3σuOpz,Npz,Uf3z-10.192OLP,NLP1φuOpx,Opy,Ufx-11.165OLP3σuNpz,Opz,Cpz,Uf3z-11.319π(N-O)2πuOpx,Opy,Npx,Uf2zx,Uf2zy-11.6162πuOpx,Opy,Uf2zx,Uf2zy-13.108π(N-O)1πgOpz,Cpz,Npz,Ud3z[UO2(k2-A1)(H2O)3]+-15.460OLP2σgOpx,Npx,Ufx,Uf2zx-17.012OLP1σgOpz,Udxz[UO2(k2-A2)(H2O)3]+-10.994N-OLPσgUf3z,Opz-12.047C=OLPσgOpx,Opy,Uf2zx,Uf2zy-12.049N-OLPσgOpx,Opy,Opz,Uf2zx,Uf2zy-12.356N-OLPσgOpz,Opx,Opy,Ud2z,Uf2zx-12.629C=OLPσgOpx,Opy,Opz,Udyz,Udxz[UO2(k2-A3)(H2O)3]+-10.651NLPσgUf3z,Npz-10.798OLPσgNpz,Uf3z,Ufz,Uf2zy-11.798OLPσgOpx,Opy,Uf2zy,Uf2zx-11.853NLPσgNpx,Uf2zx[UO2(k2-A4)(H2O)3]+-10.840OLPσgUf3z,Opz-11.934NLPσgNpy,Uf2zy-13.100π(N-O)1πgUdzy,Opy,Npz

由图3(e)可知,与[UO2(η2-A4)(H2O)3]+相比,π电子云没有有效地与U轨道重叠,只有Opz轨道重叠形成σ键,同时N连有两个H因此电子云密度低于[UO2(k2-A3)(H2O)3]+中A3的N的电子云密度,综合两个因素,k2-A4与U配位能力低于η2-A4和k2-A3。
通过分析配合物典型的成键分子轨道,得到配位原子O或N主要成键轨道为p轨道,中心原子主要成键轨道为d、f轨道;当配体螯合构型配位时,主要形成σ键,当配位原子的邻位是给电子基团时有利于σ键的形成;配体π电子云重叠成键时,π键周围有给电子时有利于配位;因此容易与U配位的是k2-A3和η2-A4,分子轨道分析与水溶液条件下配位常数lgK计算结果一致。
3 结 论
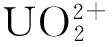
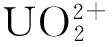
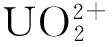
(3) 通过热力学参数、分子轨道分析,当配体螯合构型(k2)配位时,主要形成σ键,当配位原子的邻位是给电子基团时有利于σ键的形成;当配体π电子云重叠成键时,π键周围有给电子基并能形成共轭时有利于配位;因此容易与U配位的是k2-A3和η2-A4,与水溶液条件下配位常数lgK计算结果一致。
[1]Zhou L, Bosscher M, Zhang C S, et al. A protein engineered to bind uranyl selectively and with femtomolar affinity[J]. Nature Chem, 2014, 6: 236-241.
[2]Davies R V, Dr Kennedy J, Mcilroy R W, et al. Extraction of uranium from sea water[J]. Nature, 1964, 203(495): 1110-1115.

[4]Kim J S, Tsouris C, Mayes R T, et al. Recovery of uranium from seawater: a review of current status and future research needs[J]. Sep Sci Technol, 2013, 48: 367-387.
[5]Sinisa V, Watson L A, Kang S O, et al. How amidoxime binds the uranyl cation[J]. Inorg Chem, 2012, 51: 3855-3859.
[6]Astheimer L, Schenk H J, Witte E G, et al. Development of sorbers for the recovery of uranium from seawater part 2: the accumulation of uranium from seawater by resins containing amidoxime and imidoxime functional groups[J]. Sep Sci Technol, 1983, 18: 307-339.
[7]Rao L F. Recent international R&D activities in the extraction of uranium from seawater, LBNL-4034E[R]. Berkeley: Lawrence Berkeley National Laboratory, 2010.
[8]Sun X Q, Xu C, Tian G X, et al. Complexation of glutarimidedioxime with Fe(Ⅲ), Cu(Ⅱ), Pb(Ⅱ), and Ni(Ⅱ), the competing ions for the sequestration of U(Ⅵ) from seawater[J]. Dalton Trans, 2013, 42: 14621-14627.
[9]Barber P S, Kelley S P, Rogers R D. Highly selective extraction of the uranyl ion with hydrophobic amidoxime-functionalized ionic liquids via eta-2 coordination[J]. Rsc Adv, 2012, 2(22): 8526-8530.
[10]Abney C W, Liu S B, Lin W B. Tuning amidoximate to enhance uranyl binding: a density functional theory study[J]. J Phy Chem A, 2013, 117, 11558-11565.
[11]Xu C F, Su J, Xu X, et al. Theoretical studies on the complexation of uranyl with typical carboxylate and amidoximate ligands[J]. Sci China Chem, 2013, 11: 1525-1532.
[12]Bühl M, Diss R, Wipff G, et al. Coordination environment of aqueous uranyl(Ⅵ) ion[J]. J Am Chem Soc, 2005, 127: 13506-13507.
[13]Jia T T, Gao T, Zhang Y G, et al. Structural and spectroscopic properties of small Pun(n=2-5) molecules[J]. Chin Phys B, 2011, 20(11): 215-221.
[14]Maite G, Christa W, Sven K. Systematic DFT study of gas phase and solvated uranyl and neptunyl complexes[AnO2X4]n(An: U, Np; X: F, Cl, OH,n=-2; X: H2O,n=+2)[J]. Inorg Chem, 2006, 37: 1356-1366.
[15]Tian X F, Long C S, Zhu Z H, et al. Molecular dynamics simulation of collective behavior of Xe in UO2[J]. Chin Phys B, 2010, 19: 477-482.
[16]Zhang Y G, Li Y D. Relativistic density functional investigation of UX6(X=F, Cl, Br and I)[J]. Chin Phys B, 2010, 19(3): 274-280.
[17]Stipdonk M J V, Winnie C, Kellis B, et al. Gas-phase uranyl-nitrile complex ions[J]. J Phys Chem A, 2006, 110: 959-970.
[18]Hagberg D, Karlstroem G, Roos B O, et al. The coordination of uranyl in water: a combined quantum chemical and molecular simulation study[J]. J Am Chem Soc, 2005, 127: 14250-14256.
[19]Groenewold G S, Gianotto A K, Cossel K C, et al. Vibrational spectroscopy of mass-selected [UO2(ligand)n]2+complexes in the gas phase: comparison with theory[J]. J Am Chem Soc, 2006, 128: 4802-4813.
[20]Vallet V, Wahlgren U, Schimmelpfennig B, et al. The mechanism for water exchange in [UO2(H2O)5]2+and [UO2(oxalate)2H2O]2-, as studied by quantum chemical methods[J]. J Am Chem Soc, 2001, 123: 11999-12008.
[22]Abney C W, Liu S B, Lin W B. Tuning amidoximate to enhance uranyl binding: a density functional theory study[J]. J Phys Chem A, 2013, 117, 11558-11565.
[23]Vukovic S, Watson L A, Kang S O, et al. How amidoximate binds the uranyl cation[J]. Inorg Chem, 2012, 51: 3855-3859.
Density Functional Theory Study on Selective Complexation of Uranyl(Ⅵ) With Ligands Possessing Different Configuration
CHI Fang-ting1,2, WEN Jun2,*, XIONG Jie2, HU Sheng2, WEI Gui-lin1, YI Fa-cheng1
1.Fundamental Science on Nuclear Wastes and Environmental Safety Laboratory,Southwest University of Science and Technology, Mianyang 621010, China;2.Institute of Nuclear Physics and Chemistry, China Academy of Engineering Physics, Mianyang 621900, China

recovery uranium from seawater; density functional theory; designation of ligand structure; molecular orbital
2015-09-11;
2015-11-19
国家自然科学基金项目(21401152);西南科技大学博士研究基金项目(13zx7130);西南科技大学核废物与环境安全国防重点学科实验室预研项目(15yyhk08);西南科技大学核废物与环境安全国防重点学科实验室团队项目(14tdhk01)
匙芳廷(1982—),女,山东聊城人,博士,副教授,从事海水提铀吸附剂研究与放射性废物处理研究
*通信联系人:文君(1985—),男,四川乐山人,副研究员,放射化学专业,E-mail: junwen@caep.cn
TL941
A
0253-9950(2016)04-0238-09
10.7538/hhx.2016.38.04.0238
