制备条件对PEI功能化石墨烯量子点荧光性能的影响
2016-09-06王晓敏
王 菡 王晓敏
(太原理工大学材料科学与工程学院,太原030024)
制备条件对PEI功能化石墨烯量子点荧光性能的影响
王菡王晓敏*
(太原理工大学材料科学与工程学院,太原030024)
石墨烯量子点凭借其良好的水溶性、低生物毒性等特点,被不断尝试应用于生物成像领域,但其有限的荧光性能限制了其进一步应用。为改善石墨烯量子点的荧光性能以及进一步揭示石墨烯量子点的制备机理,本文对聚乙烯亚胺(PEI)功能化石墨烯量子点的制备条件进行了探索,讨论了不同反应时间、制备温度以及混悬液pH值对其荧光性能的影响。测试结果显示,当混悬液pH值为12时,在反应釜中经过200°C高温反应20 h,所制备的功能化石墨烯量子点能取得良好的紫外吸收峰和荧光性能,同时达到较高的量子产量。
石墨烯量子点;聚乙烯亚胺;制备条件;荧光性能;量子产量
1 Introduction
Optical imaging with quantum dots has been widely used in the research of disease diagnosing,effective therapeutics and response monitoring,with the advantage of high sensitivity,multicolor imaging and easy-activation1,2.However,for most of the semiconductor quantum dots,low biocompatibility is the major handicap in further biological application3,4.
Graphene quantum dots(GQDs)inherit the excellent properties of both graphene and quantum dots,such as large surface area, high specific strength,ultra-small size,excellent fluorescent property and low toxicity,which hold promising applications in cellular imaging,in vivo labeling and long-term cellular studies thus inspiring intensive research efforts in its own right5,6.In 2008, Dai et al.7first reported the intrinsic luminescence of nano-graphene oxide(NGO),single-layer graphene oxide sheets down to a few nanometers in lateral width and also explored the biologicalapplications.Since then,many synthesis methods have been proposed sequentially,the“top-down”methods such as hydrothermal approach8,solvothermal method with N,N-dimethyl formamide(DMF)or ethylene glycol as solvent etc.9,some physical cutting methods10,11and bottom-up methods12,13which focusing on the initial synthesis of GQDs were developed as well.As a core parameter of quantum dots,the fluorescent property of GQDs has been investigated by researchers,and both excitation-dependent and pH-dependent property has been explored like most luminescent carbon nanoparticles14,15.The high quantum yield(QY)for both GQDs and functionalized GQDs has been reported as well16,17. However,there still is a long way to go before a satisfying investigation of the preparation mechanism and fluorescent property of GQDs to alter photoluminescence(PL),biocompatibility and enhance optical properties and quantum yield18,19.

Fig.1 (a)TEM image of GO sheets;(b)TEM image of the GQDs-BPEI after 1 h solvothermal treatment(inset:HRTEM image of an individual GQD-BPEI);(c)TEM image of the GQDs-BPEI after 10 h solvothermal treatment(inset:HRTEM image of the individual GQD-BPEI);(d)TEM image of the GQDs-BPEI after 20 h solvothermal treatment
Since their initial use as gene delivery system,polyethylenimine (PEI)polymers have been extensively tested in vitro and in vivo and have been found to be one of the most efficacious non-viral agents20,21.Herein,we proposed a facile solvothermal method with ethanol as solvent,to produce GQDs functionalized with branched polyethylenimine(BPEI)in our previous work,which successfully improved the PL property of GQDs and also achieved a low cell toxicity and excellent nucleus labeling ability in vitro experiments22.To further investigate the preparation mechanism and improve the optical property of GQDs-BPEI,a wide time range from 1 to 24 h,reaction temperature from 100 to 200°C and pH value of the solution from 7 to 12 were explored respectively,and most of the fluorescent parameters such as maximum excitation wavelength,maximum emission wavelength,UV-Vis absorption and PL emission spectra have been compared and discussed,the quantum yield is calculated and discussed as well.
2 Experimental
Branched polyethylenimine(BPEI,average molecular weight: 10 kDa)and quinine sulfate fluorescence standard substance were purchased from Aladdin Industrial Inc.,Shanghai(China).Ultrapure water(18 MΩ∙cm)was used in entire experimental procedures.
GO sheets was prepared from graphite powder by a modified Hummers method23.The sample GO sheets(50 mg)were dispersed in 40 mL ethanol solution.BPEI 10000(200 mg)was added into the solution with sufficient stirring and the pH value was turned to 7,9,and 12 with NH3∙H2O(or acetic acid).The mixture solution was then transferred into a 100 mL Teflon-lined stainless-steel autoclave and heated at 200°C(other temperature 100,140,and 180°C were also discussed)for 1,10,20,and 24 h, respectively.After cooling to room temperature,the resulting solution was further filtered through a 0.22 μm microporous membrane and the GQDs-BPEI ethanol solution with strong blue fluorescence was obtained.The GQDs-BPEI was then purified and separated via column chromatography24on neutral aluminum oxide,with ethanol as the eluent.
JEOL JEM 2010 microscope(JEOL,Japan)with acceleration voltage of 200 kVwas used for the high-resolutionTEM(HRTEM) images.The UV-Vis spectra of the samples dispersed in ethanol were collected with a Hitachi U-3900 UV-Vis spectrophotometer. Photoluminescence(PL)spectra were obtained with Horiba Jobin Yvon Fluromax-4 spectrofluorometer(Japan).All spectra were recorded with quartz cells of 10 mm path length.
3 Results and discussion
GQDs-BPEI was prepared by a facile one-step solvothermal reduction of GO sheets and branched PEI(MW=10000)in ethanol solution.Fig.1a shows the TEM image of the pristine GO sheets.Fig.1b shows the TEM image of the as-prepared GQDs-BPEI after 1 h solvothermal treatment(sample 1).It clearly reveals that the layer structure of GO have been destroyed,from which original GQDs-BPEI of different sizes could be obtained. The HRTEM image of individual GQDs-BPEI(Fig.1b inset)indicates the high crystallinity of the structure.Moreover,clearly Zigzag edges have been seen from the individual GQDs-BPEI by which may reveal the preparation mechanism proposed by Pan et al.8.Fig.1c shows the TEM and HRTEM image of GQDs-BPEI after 10 h solvothermal treatment,individually(sample 2).From which the GQDs-BPEI with discernible lattice structures are clearly demonstrated with a lattice parameter of 0.21 nm,the(100) lattice fringes of graphene.Fig.1d shows theTEMimage of GQDs-BPEI after 20 h solvothermal treatment(sample 3).Compared with the former groups,the collected GQDs-BPEI after 20 h solvothermal treatment is nearly monodispersed and with a rel-atively narrow size distribution between 1 and 3 nm.
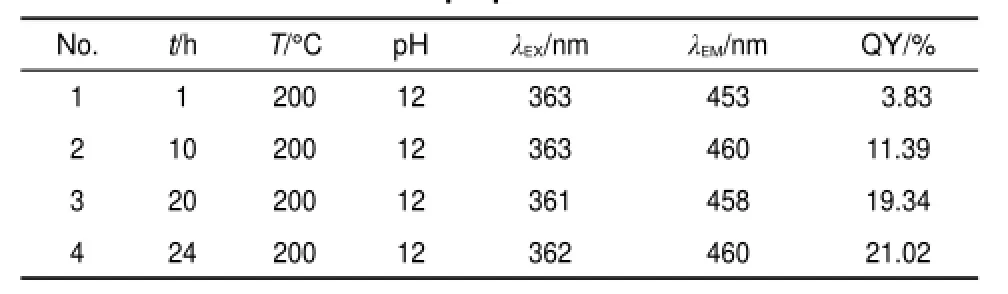
Table 1 Fluorescent parameters of GQDs-BPEI under different preparation time
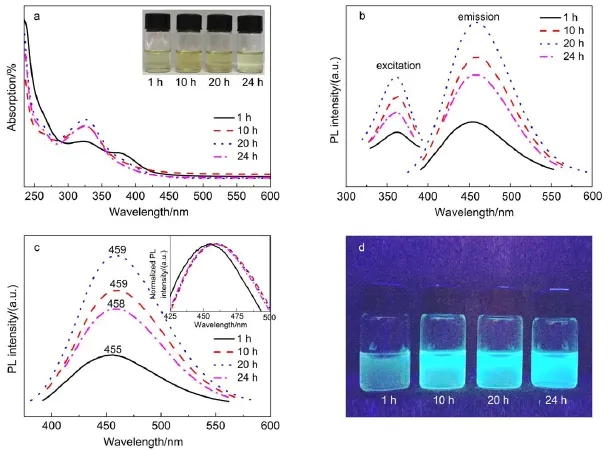
Fig.2 (a)UV-Vis absorption spectra of the GQDs-BPEI under different reaction time(inset:photographs of the GQDs-BPEI); (b)photoluminescent excitation and emission spectrum of GQDs-BPEI under different reaction time;(c)PLE spectra(at 365 nm excitation)for the GQDs-BPEI under different reaction time(inset:the corresponding normalized PLspectra); (d)photographs of the GQDs-BPEI under 365 nm UV light(color online)
To further explore the synthesis mechanism and optical properties of as-synthesized GQDs-BPEI,experiments with different reaction time ranging from 1 to 24 h,preparation temperature from 100 to 200°C and pH value of the mixture solution from 7 to 12 were developed,respectively.Fluorescent parameters such as maximum excitation wavelength,maximum emission wavelength, UV-Vis absorption and PL excitation and emission spectra have been compared and discussed,and the quantum yields is calculated and discussed as well.
3.1Reaction time
The influence of the reaction time was studied by applying reaction time from 1 to 24 h,whereas all the other reaction parameters were held constant(as shown in Table 1).Fluorescence parameters like maximum excitation wavelength,maximum emission wavelength,and quantum yield(QY)were measured and calculated respectively.
Fig.2a shows the UV-Vis absorption spectra of the GQDs-BPEI, from which the absorption peak of GQDs-BPEI can be clearly seen between 330 and 365 nm with long absorption edge,and the absorption peak at ca.330 nm keeps enhancing gradually as the reaction time ascending from 1 to 20 h,then turns into little decrease starting from 20 till 24 h.The photoluminescent excitation and emission spectra of GQDs-BPEI under different reaction time are shown in Fig.2b.It is clearly demonstrated that GQDs-BPEI can be successfully obtained within a wide reaction time ranging from 1 to 24 h.When reaction time increased to 20 h,the PL spectrum indicates that the strongest peak is at 458 nm with a Stoke shift of 97 nm(Fig.1b).To further investigate the optical properties of the as-prepared GQDs-BPEI,the PLE spectra at 365nm excitation were recorded,as shown in Fig.2c.When the preparation time increases from 1 to 10 h,the PL peak of the GQDs-BPEI red shifts to longer wavelengths with a large Stoke shift,enables fluorescent signals to easily be distinguished from scattered excited light4.From 10 to 24 h,the location of the PLE spectra peak roughly remains unchanged.
According to the classical optical reference method25,the PL quantum yield(QY)of GQDs-BPEI under different reaction time were measured and calculated using quinine sulfate as a reference, by the following formula:

where φ stands for the quantum yield,A is the integral area of fluorescence emission peak intensity of PL spectrum,Abs is the corresponding absorbance of the sample,η is the refractive index of the solvent,subscripts st and x refer to the standard(which is quinine sulfate in this paper)and the unknown,respectively.
As shown in Table 1(the detailed calculation steps are attached in Table S1(Supporting Information)),along with the preparation time ascends from 1 to 24 h,the PLquantum yield(QY)of GQDs-BPEI increases correspondingly from 3.83%to 21.02%.Considering the further reasonable optimization of the experimental conditions,the change of QY value verse reaction time was intuitively conducted in Fig.S1(Supporting Information).It is clearly shown that from 1 to 20 h the QY value of GQDs-BPEI is rapidly added,whereas the growth rate is reduced from 20 to 24 h.In the solvothermal process,the presence of ethanol may deoxidized the abundant oxygen-containing functional groups that exist in and on the surface of graphene oxide sheets where the epoxy groups are linearly arranged,ethanol can play an unzipping role26.Similar to the traditional hydrothermal method in synthesizing GQDs,the process of our solvothermal method is also a “top-down”mechanism,and with the reaction time increases the GO sheets were further cut to small pieces,which also confirmed by the TEM results(see Fig.1).The result shows that the quantum yield will be higher when the reaction time is getting longer and the particles becomes smaller,however,based on an overall consideration of factors such as time and cost,the optimal reaction time is finally set to 20 h.
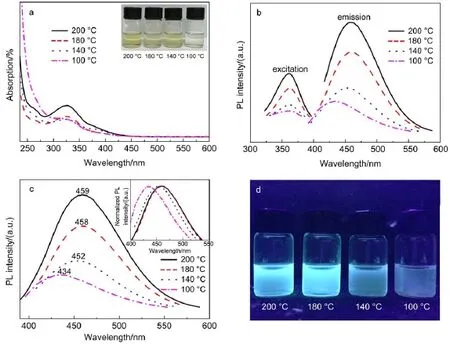
Fig.3 (a)UV-Vis absorption spectra of the GQDs-BPEI under different preparation temperatures(inset:photographs of the GQDs-BPEI); (b)photoluminescent excitation and emission spectrum of GQDs-BPEI under different preparation temperatures;(c)PLE spectra(at 365 nm excitation)for the GQDs-BPEI under different preparation temperatures(inset:the corresponding normalized PLspectra); (d)photographs of the GQDs-BPEI with different preparation temperatures under 365 nm UV light(color online)
3.2Preparation temperature
The influence of preparation temperature was investigated by setting preparation temperature from 100 to 200°C,whereas all the other reaction parameters were held constant(as shown in Table 2).When the preparation temperature is below 100°C, fluorescence from GQDs-BPEI can be hardly observed,meanwhile,limited by the autoclave the highest safe temperature is set to 200°C.
Fig.3a shows the UV-Vis absorption spectra of the GQDs-BPEI, from which the absorption peak of GQDs-BPEI can be clearly located around 330 nm with long absorption edge,and the peak keeps enhancing gradually as the preparation temperature risen from100 to 200°C.The photoluminescent excitationandemission spectra of GQDs-BPEI under different preparation temperature are shown in Fig.3b.The bright GQDs-BPEI quantum dots can be easily obtained under 100 to 200°Csolvothermal preparation,and the PLintensity of which was rapidly enhanced via the preparation temperature risen.Fig.3c shows the PLEspectra of GQDs-BPEI at 365 nmexcitation,fromwhich it is clearly observed that when the preparation temperature was changed from 100 to 200°C,the PL peaks red shift from 434 to 459 nm with larger Stokes shift.It is commonly known that,higher absorbency,PLintensity and largerStokes shift make quantumdots easy to visualize without imposing stringentrequirementsontheopticalsystem4.
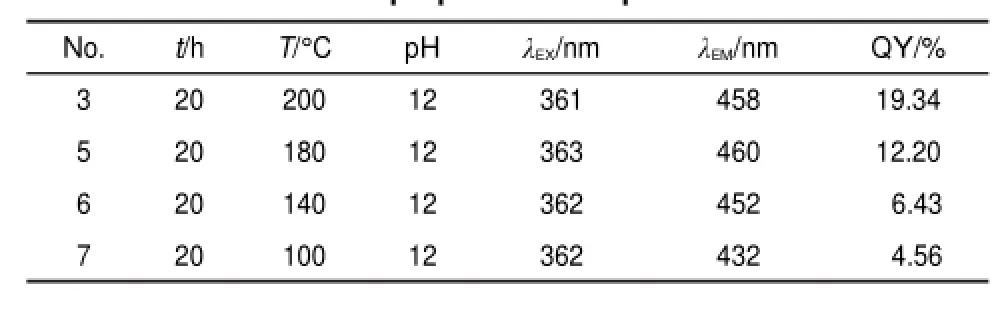
Table 2 Fluorescent parameters of GQDs-BPEI under different preparation temperatures
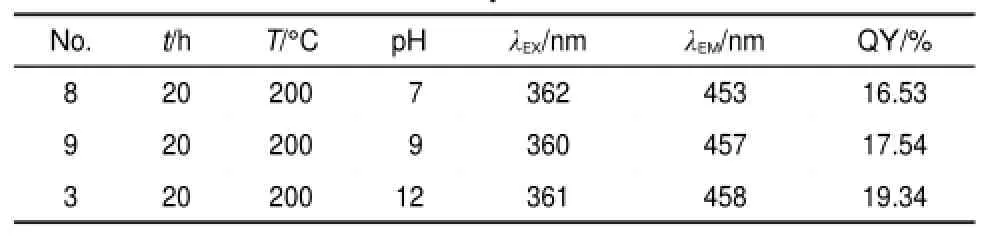
Table 3 Fluorescent parameters of GQDs-BPEI under different pH values
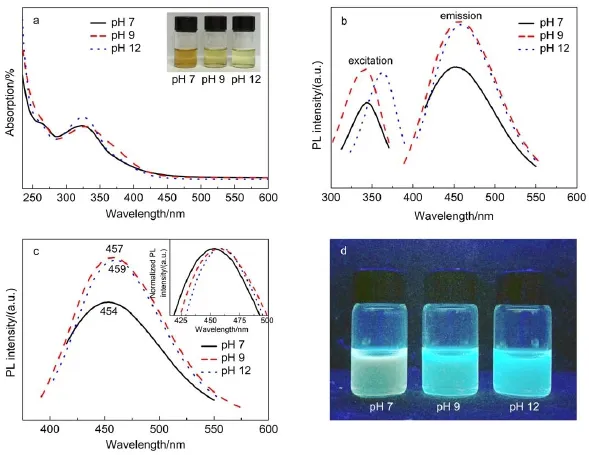
Fig.4 (a)UV-Vis absorption spectra of the GQDs-BPEI under different pH values(inset:photographs of the GQDs-BPEI); (b)photoluminescent excitation and emission spectrum of GQDs-BPEI under different pH values; (c)PLE spectra(at 365 nm excitation)for the GQDs-BPEI under different pH values(inset:the corresponding normalized PLspectra); (d)photographs of the GQDs-BPEI with different pH values under 365 nm UV light(color online)
The QY values of GQDs-BPEI under different preparation temperatures are shown in Table 2.As the preparation temperature increased from 100 to 200°C,the QY value rises correspondingly from 4.56%to 19.34%,and the growth rate raises rapidly as the temperature increases from 150 to 200°C(as shown in Fig.S2 (Supporting Information)).The results suggested that under high temperature especially above 180°C the reducibility of ethanol was further enhanced,more oxygen-containing functional groups of GO sheets were deoxidized,and the GO sheets were cut into smaller pieces with better fluorescent property and higher QY value.However,considering the temperature limit of the Teflon material(inner container of the stainless-steel autoclave),we choose 200°C as the highest safe temperature.
3.3pH value
Since the branched PEI will agglomerate under acidic conditions,the influence of the pH value of the solution was investigated within the range from 7 to 12,whereas all the other reaction parameters were held constant(as shown in Table 3).
Fig.4a shows the UV-Vis absorption spectra of the GQDs-BPEI, from which the absorption peaks of GQDs-BPEI can be clearly observed around 330 nm with long absorption edge.The photoluminescent excitation and emission spectra of GQDs-BPEI under different pH value are shown in Fig.4b.It clearly demonstrates that,compare to reaction time and preparation temperature,the pH value of the solvent has limited effect on the UV absorbency and PL intensity of the as-prepared GQDs-BPEI.It seems that under alkaline environment the GQDs-BPEI always have higher absorbency and better PL properties.Fig.4c further confirmed that under the same excitation light(365 nm)as the pH value of the solvent added,the PL peaks red shift with larger Stokes shift and smaller half peak width(PWH).Usually,for quantum dots smaller PWH means the size distribution of the particle is more uniform and concentrated.
The QY values of GQDs-BPEI under different pH values are calculated and demonstrated in Table 3.When the pH value increases from 7 to 12,the QY value increases slowly from 16.53% to 19.34%(as shown in Fig.S3(Supporting Information)).This may because GO sheets can be better dispersed in ethanol solvent under alkaline conditions and further deoxidized.Usually,by adding branched PEI the pH value of the mixture solution is ca 10.2.Therefore,a small amount of ammonia was added to ensure the consistency and repeatability of the experiment,at the same time to obtain better fluorescence properties of the outcome.
4 Conclusions
In conclusion,to obtain a better fluorescent property of the GQDs for further application in biological labeling,small graphene quantum dots functionalized with branched PEI(GQDs-BPEI)were synthesized,and the preparation conditions,reaction time,temperature,and pH value were explored,the optical properties have been further compared and discussed.Compared with the normal hydrothermal method,the solvothermal method with ethanol as solvent successfully contributes the broken-up process of GO sheets,meanwhile,the existence of PEI also passivated the surface of the as-prepared GQDs which further improved the PL property.In consideration of factors such as time and cost,when the pH value of the mixture solution set to 12,the preparation temperature ascend to 200°C,and after 20 h reaction in autoclave the as-prepared GQDs-BPEI can achieved a higher UVabsorbency and better PLproperties with a high quantum yield of 19.34%.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
References
(1)Resch-Genger,U.;Grabolle,M.;Cavaliere-Jaricot,S.; Nitschke,R.;Nann,T.Nature Methods 2008,5,763.doi: 10.1038/nmeth.1248
(2)Lu,L.P.;Li,J.;Wu,J.;Kang,T.F.;Cheng,S.Y.Acta Phys.-Chim.Sin.2015,31(3),483.[鲁理平,李娇,武静,康天放,程水源.物理化学学报,2015,31(3),483.] doi:10.3866/PKU.WHXB201501151
(3)Lee,D.E.;Koo,H.;Sun,I.C.;Ryu,J.H.;Kim,K.;Kwon,I. C.Chemical Society Reviews 2012,41,2656.doi:10.1039/ C2CS15261D
(4)Nida,D.L.;Rahman,M.S.;Carlson,K.D.;Richards-Kortum, R.;Follen,M.Gynecologic Oncology 2005,99,S89. doi:10.1016/j.ygyno.2005.07.050
(5)Shen,J.;Zhu,Y.;Yang,X.;Li,C.Chemical Communications 2012,48,3686.doi:10.1039/c2cc00110a
(6)Nurunnabi,M.;Khatun,Z.;Nafiujjaman,M.;Lee,D.G.;Lee, Y.K.ACS Applied Materials&Interfaces 2013,5,8246. doi:10.1021/am4023863
(7)Sun,X.;Liu,Z.;Welsher,K.;Robinson,J.T.;Goodwin,A.; Zaric,S.;Dai,H.Nano Research 2008,1,203.doi:10.1007/ s12274-008-8021-8
(8)Pan,D.;Zhang,J.;Li,Z.;Wu,M.Advanced Materials 2010, 22,734.doi:10.1002/adma.v22:6
(9)Zhu,S.;Zhang,J.;Qiao,C.;Tang,S.;Li,Y.;Yuan,W.;Li,B.; Tian,L.;Liu,F.;Hu,R.;Gao,H.;Wei,H.;Zhang H.Chemical Communications 2011,47,6858.doi:10.1039/c1cc11122a
(10)Ponomarenko,L.;Schedin,F.;Katsnelson,M.;Yang,R.;Hill, E.;Novoselov,K.;Geim,A.Science 2008,320,356.doi: 10.1126/science.1154663
(11)Tang,L.;Ji,R.;Cao,X.;Lin,J.;Jiang,H.;Li,X.;Teng,K.S.; Luk,C.M.;Zeng,S.;Hao,J.ACS Nano 2012,6,5102.doi: 10.1021/nn300760g
(12)Lu,J.;Yeo,P.S.E.;Gan,C.K.;Wu,P.;Loh,K.P.Nature Nanotechnology 2011,6,247.doi:10.1038/nnano.2011.30
(13)Yan,X.;Cui,X.;Li,L.S.Journal of the American Chemical Society 2010,132,5944.doi:10.1021/ja1009376
(14)Baker,S.N.;Baker,G.A.Angewandte Chemie International Edition 2010,49,6726.doi:10.1002/anie.200906623
(15)Feng,C.;Deng,X.Y.;Ni,X.X.;Li,W.B.Acta Phys.-Chim. Sin.2015,31(12),2349.[冯昌,邓晓燕,倪晓晓,李卫兵.物理化学学报,2015,31(12),2349.]doi:10.3866/PKU. WHXB201510281
(16)Shen,J.;Zhu,Y.;Yang,X.;Zong,J.;Zhang,J.;Li,C.New Journal of Chemistry 2012,36,97.doi:10.1039/C1NJ20658C (17)Hu,C.;Liu,Y.;Yang,Y.;Cui,J.;Huang,Z.;Wang,Y.;Yang, L.;Wang,H.;Xiao,Y.;Rong,J.Journal of Materials Chemistry B 2013,1,39.doi:10.1039/C2TB00189F
(18)Wang,Z.;Qu,Y.;Gao,X.;Mu,C.;Bai,J.;Pu,Q.Materials Letters 2014,129,122.doi:10.1016/j.matlet.2014.05.016
(19)Fan,Z.;Li,Y.;Li,X.;Fan,L.;Zhou,S.;Fang,D.;Yang,S. Carbon 2014,70,149.doi:10.1016/j.carbon.2013.12.085
(20)Boussif,O.;Lezouale′H,F.;Zanta,M.A.;Mergny,M.D.;Scherman,D.;Demeneix,B.;Behr,J.P.Proceedings of the National Academy of Sciences of the United States of Amercia 1995,92,7297.doi:10.1073/pnas.92.16.7297
(21)Brownlie,A.;Uchegbu,I.;Schätzlein,A.International Journal of Pharmaceutics 2004,274,41.doi:10.1016/j. ijpharm.2003.12.029
(22)Wang,H.;Wang,X.RSC Adv.2015,5,75380.doi:10.1039/ C5RA13509E
(23)Li,D.;Muller,M.B.;Gilje,S.;Kaner,R.B.;Wallace,G.G. Nat.Nanotechnol 2008,3,101.doi:10.1038/nnano.2007.451
(24)Li,H.;He,X.;Kang,Z.;Huang,H.;Liu,Y.;Liu,J.;Lian,S.; Tsang,C.H.A.;Yang,X.;Lee,S.T.Angewandte Chemie International Edition 2010,49,4430.doi:10.1002/ anie.200906154
(25)Crosby,G.A.;Demas,J.N.The Journal of Physical Chemistry 1971,75,991.doi:10.1021/j100678a001
(26)Kosynkin,D.V.;Higginbotham,A.L.;Sinitskii,A.;Lomeda, J.R.;Dimiev,A.;Price,B.K.;Tour,J.M.Nature 2009,458, 872.doi:10.1038/nature07872
Effect of Preparation Conditions on the Optical Properties of PEI-Functionalized Graphene Quantum Dots
WANG HanWANG Xiao-Min*
(College of Materials Science and Engineering,Taiyuan University of Technology,Taiyuan 030024,P.R.China)
Because of their low toxicity and excellent water-solubility,graphene quantum dots have been highly anticipated for use in cellular imaging.However,their limited optical properties are hampering this use.To address this issue,graphene quantum dots surface passivated by branched polyethylenimine(GQDs-BPEI) were proposed in this paper.We discussed optical properties when prepared under different conditions including reaction time,temperature,and pH value.The results indicate that GQDs-BPEI prepared at pH 12,at a temperature of 200°C,and with a 20 h reaction in an autoclave can achieve a higher UV absorbency and better PL properties with a high quantum yield.
Graphene quantum dot;Polyethylenimine;Preparation condition;Optical property; Quantum yield
December 7,2015;Revised:February 29,2016;Published on Web:March 1,2016.
O644
10.3866/PKU.WHXB201603014
*Corresponding author.Email:wangxiaomin@tyut.edu.cn;Tel:+86-351-6018639.
The project was supported by the National Natural Science Foundation of China(51372160,51572184).国家自然科学基金(51372160,51572184)资助项目
