咪唑立宾对单侧输尿管梗阻小鼠肾小管上皮间质转化的影响
2016-08-05于方邓海月姜红
于方,邓海月,姜红
(中国医科大学附属第一医院儿科,沈阳 110001)
咪唑立宾对单侧输尿管梗阻小鼠肾小管上皮间质转化的影响
于方,邓海月,姜红
(中国医科大学附属第一医院儿科,沈阳 110001)
目的观察咪唑立宾(MZR)对单侧输尿管梗阻(UUO)小鼠肾小管上皮间质转化(EMT)的影响,探讨其抗肾间质纤维化机制。方法将24只CD1小鼠随机分为假手术组、UUO模型组和MZR治疗组,每组8只。造模前1 d,MZR治疗组给予MZR [10 mg/(kg·d)]灌胃,假手术组、UUO模型组给予等量生理盐水灌胃。术后14 d收集小鼠血液,记录血肌酐(Scr)和尿素氮(BUN);收集梗阻侧肾脏,HE染色、Masson染色观察肾脏病理改变;免疫组化、Western blot检测α⁃平滑肌肌动蛋白(α⁃SMA)和E⁃钙黏蛋白(E⁃Cad)在肾脏的表达。结果与假手术组相比,UUO模型组血肌酐、尿素氮显著升高,肾组织病理改变明显,肾组织中α⁃SMA表达增加而E⁃Cad表达减少,差异有统计学意义(P<0.05);与UUO模型组相比,MZR治疗组Scr、BUN及肾组织病理改变均不同程度改善,肾组织中α⁃SMA表达受抑而E⁃Cad表达上调,差异有统计学意义(P<0.05)。结论MZR可抑制UUO小鼠肾小管EMT的发展,减轻肾小管间质纤维化程度,改善肾功能。
单侧输尿管梗阻;上皮间质转化;咪唑立宾
网络出版地址
肾小管间质纤维化是所有慢性肾脏病发展为终末期肾衰竭的共同通路,其发生机制复杂,临床缺乏有效的治疗措施[1]。研究[2]发现肾小管上皮间质转化(epithelial⁃mesenchymal transition,EMT)在肾小管间质纤维化的发生中起重要作用,抑制EMT的发展可以起到抗肾纤维化的治疗作用。如何抑制或逆转EMT已成为国内外研究的热点。咪唑立宾(mizoribine,MZR)是一种新型免疫抑制剂,主要用于器官移植及自身免疫性疾病的治疗。研究[3]发现,MZR可以改善肾间质纤维化程度,保护肾功能。但MZR对肾小管EMT是否有影响尚无明确定论。本研究通过构建小鼠单侧输尿管梗阻(unilater⁃al ureteral obstruction,UUO)模型,观察梗阻侧肾组织中α⁃平滑肌肌动蛋白(α⁃smooth muscle actin,α⁃SMA)、E⁃钙黏蛋白(E⁃cadherin,E⁃Cad)的表达,分析MZR对肾小管EMT的影响,为临床治疗慢性肾脏病提供新靶点。
1 材料与方法
1.1材料
动物:SPF级健康雄性CD1小鼠,3~4周龄,体质量18~22 g,由北京维通利华生物技术有限公司提供[许可证号:SCXK(京)2012⁃0001]。主要试剂:MZR(大连美仑生物技术有限公司,批号:A1202A);兔抗鼠α⁃SMA抗体(美国Epitomics公司,批号:1181⁃4)、兔抗鼠E⁃Cad抗体(北京博奥森生物公司,批号:ZS⁃7870);Masson三色染色试剂盒(南京森贝伽生物科技有限公司);SP免疫组化试剂盒及DAB显色试剂盒(北京中杉金桥生物技术有限公司)。
1.2方法
1.2.1动物分组及处理:24只CD1小鼠饲养于中国医科大学实验动物中心,自由摄食及饮水。适应性喂养1周后随机分为假手术组、UUO模型组及MZR治疗组,每组8只小鼠。模型制备过程为10%水合氯醛3 mL/kg腹腔注射麻醉小鼠,俯卧位固定于小鼠手术台上,备皮消毒后于小鼠左侧背部肋脊角处纵向切开约0.5 cm切口,游离左肾及左侧输尿管,于肾底处及其下方0.5 cm处双向结扎输尿管并于两结扎点之间离断,观察无活动性出血后逐层缝合腹腔。假手术组仅游离左侧输尿管不结扎。给药过程为术前1 d,MZR治疗组给予MZR 10 mg/(kg·d)溶于0.1 mL生理盐水中灌胃,假手术组及UUO模型组给予等量生理盐水灌胃。术后14 d处死全部小鼠。处死方法为眼球摘除法留取1.5~2 mL血液,断颈处死小鼠后迅速取出梗阻侧肾脏,去除肾包膜,生理盐水冲洗后沿冠状面切开,部分迅速固定于4%多聚甲醛中,部分迅速放于-80℃冰箱中冷冻。
1.2.2指标检测:将小鼠全血放入离心机中,以3 500 r/min,4℃离心10 min,留取血清。用全自动生化分析仪测血肌酐(serum creatinine,Scr)及尿素氮(blood urea nitrogen,BUN)含量。
1.2.3肾脏病理组织学:肾组织固定48 h后常规石蜡包埋,切片厚4 μm,行HE、Masson染色。其中,Masson染色切片置于400倍光学显微镜下观察,每张切片随机选取10张不含肾小球及小血管的不重复视野,以肾间质蓝色胶原沉积为阳性信号,使用Image⁃Pro Plus 6.0图像分析软件计算肾间质阳性蓝染面积占整个视野面积的百分比,取均值作为该标本的肾间质纤维化相对面积。
1.2.4免疫组织化学染色:采用SP法检测肾组织中α⁃SMA、E⁃Cad的表达。常规石蜡包埋、切片、烤片、脱蜡、水化;3%过氧化氢消除内源性过氧化物酶;微波修复抗原;正常山羊血清工作液封闭20 min;滴加一抗α⁃SMA、E⁃Cad(1∶100),4℃孵育过夜;加入生物素标记二抗15 min;显微镜下控制DAB显色时间;苏木素复染5 min,脱水、透明,封片。以图片中出现棕黄色颗粒为阳性结果,PBS作一抗阴性对照。400倍光学显微镜下,每张切片随机选取5个不含肾小球的小管间质区域,用Image⁃Pro Plus 6.0图像分析软件计算平均光密度值(累积光密度值/视野面积),取均值作比较。
1.2.5Western blot检测α⁃SMA、E⁃Cad的表达:用匀浆器充分研磨、匀浆冻存的肾组织,加入细胞裂解液裂解细胞,冰浴30 min,分装于离心管中,于4℃16 000 r/min离心30 min,取上清;BCA法定量蛋白质;50 μg蛋白上样,行12%聚丙烯酰胺凝胶电泳,半干法转移至PVDF膜上,5%脱脂牛奶封闭;加入一抗α⁃SMA、E⁃Cad(1∶500),4℃孵育过夜;加入辣根过氧化物酶标记的二抗(1∶5 000),室温孵育2 h,ECL显影。以GAPDH为内参,采用Image J图像分析软件进行分析。
1.3统计学分析
2 结果
2.1Scr和BUN结果
与假手术组相比,UUO模型组及MZR治疗组小鼠Scr、BUN明显升高,差异有统计学意义(P<0.01);与UUO模型组相比,MZR治疗组小鼠Scr、BUN降低,差异有统计学意义(P<0.01)。见表1。
2.2肾组织病理改变
2.2.1HE染色:假手术组无明显病理改变;UUO模型组肾小管萎缩,管腔明显扩张,可见蛋白管型,间质内大量炎性细胞浸润;MZR治疗组上述改变较UUO模型组不同程度减轻。见图1。
2.2.2Masson染色:假手术组肾间质未见异常;UUO模型组肾间质内可见较多蓝色胶原纤维沉积,肾间质纤维化相对面积较假手术组明显增加,差异有统计学意义(P<0.01);MZR治疗组肾纤维化相对面积较UUO模型组减小,差异有统计学意义(P<0.01)。见图2,表2。
表1 各组小鼠血清Scr和BUN的变化(±s)Tab.1 Changes of serum creatinine and blood urea nitrogen in mice of each group(x±s)
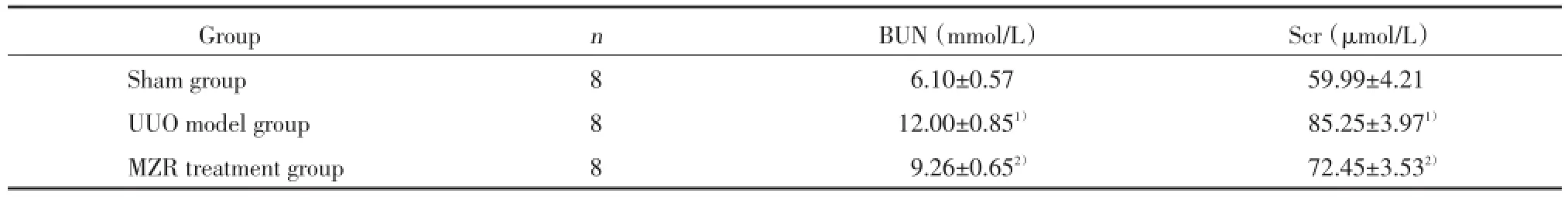
表1 各组小鼠血清Scr和BUN的变化(±s)Tab.1 Changes of serum creatinine and blood urea nitrogen in mice of each group(x±s)
1)P<0.01 compared with sham group;2)P<0.01 compared with UUO model group.
Group n BUN(mmol/L) Scr(μmol/L)Sham group 8 6.10±0.57 59.99±4.21 UUO model group 8 12.00±0.851) 85.25±3.971)MZR treatment group 8 9.26±0.652) 72.45±3.532)
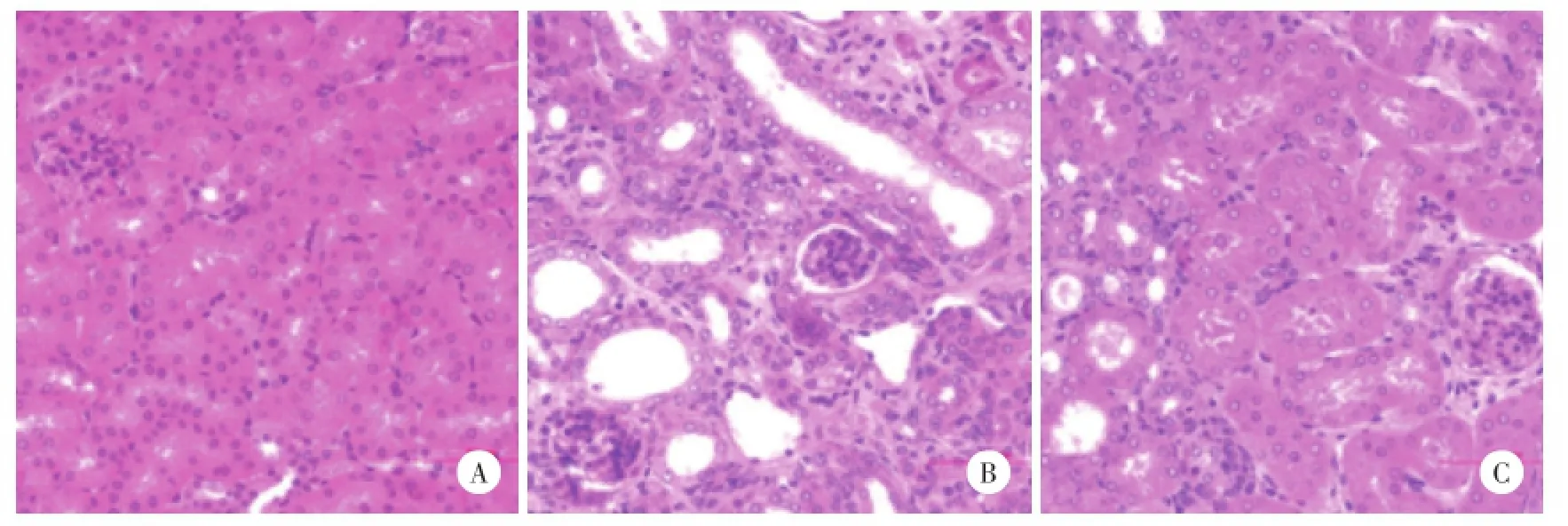
图1 各组小鼠肾组织病理改变 HE×400Fig.1 Histological changes of kidney tissue in each group HE×400
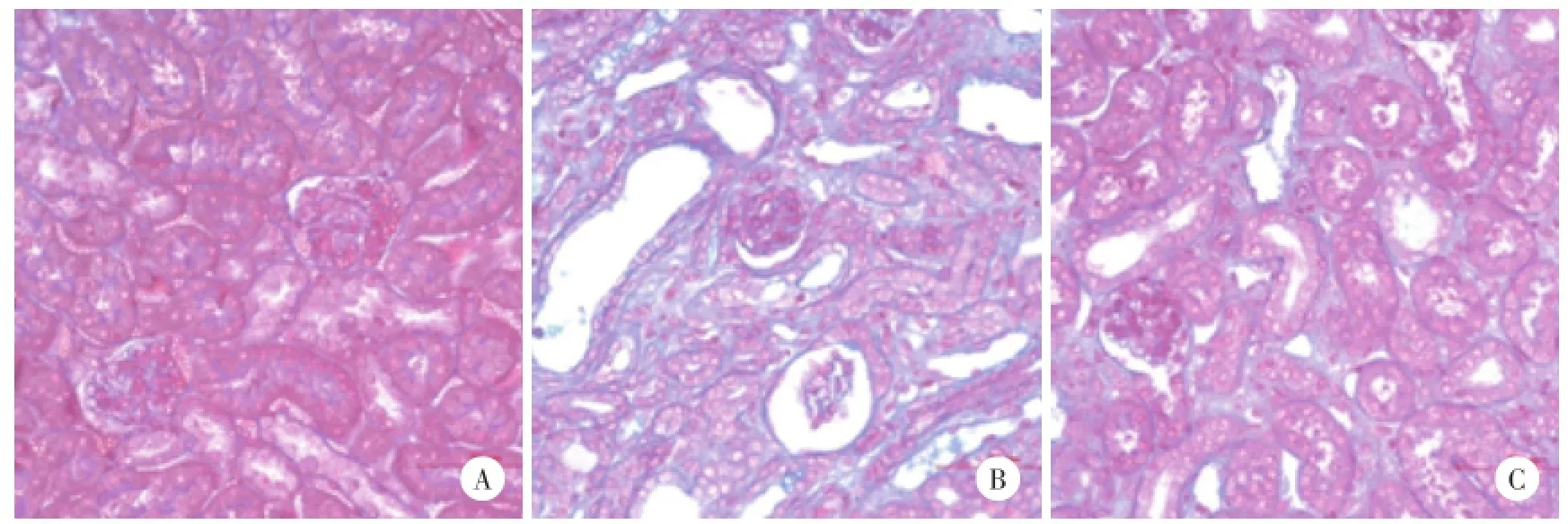
图2 各组小鼠肾组织病理改变 Masson×400Fig.2 Histological changes of kidney tissue in each group Masson×400
表2 各组小鼠肾间质纤维化相对面积(±s)Tab.2 The relative area of renal interstitial fibrosis in mice of each group(±s)
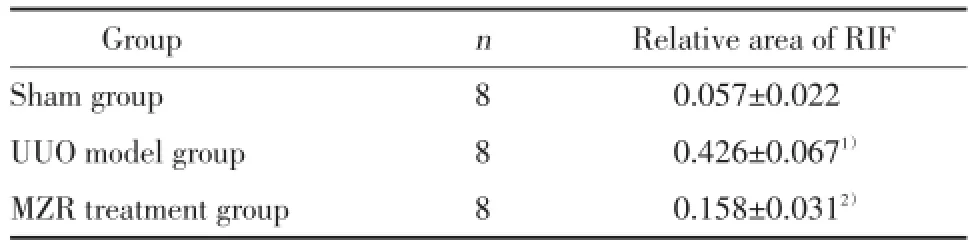
表2 各组小鼠肾间质纤维化相对面积(±s)Tab.2 The relative area of renal interstitial fibrosis in mice of each group(±s)
RIF,renal interstitial fibrosis.1)P<0.01 compared with sham group;2)P<0.01 compared with UUO model group.
Group n Relative area of RIF Sham group 8 0.057±0.022 UUO model group 8 0.426±0.0671)MZR treatment group 8 0.158±0.0312)
2.3免疫组化检测α⁃SMA、E⁃Cad在肾组织中的表达
2.3.1α⁃SMA在肾脏的表达:假手术组小鼠肾小管上皮细胞几乎不表达α⁃SMA;UUO模型组肾小管上皮细胞大量表达α⁃SMA,差异有统计学意义(P<0.01);MZR治疗组小鼠α⁃SMA表达较UUO模型组减少,差异有统计学意义(P<0.01)。见图3,表3。
2.3.2E⁃Cad在肾脏的表达:假手术组肾小管上皮细胞大量表达E⁃Cad;UUO模型组E⁃Cad表达较假手术组明显减少,差异有统计学意义(P<0.01);MZR治疗组E⁃Cad表达较UUO模型组增加,差异有统计学意义(P<0.01)。见图4,表3。

图3 α⁃SMA在各组小鼠肾脏的表达 SP法×400Fig.3 Expression of α⁃SMA in the kidney of mice in each group SP×400
表3 各组小鼠肾组织α⁃SMA、E⁃Cad表达的平均光密度值(±s)Tab.3 The mean optical density value of α⁃SMA and E⁃Cad expression in the kidney tissue of each group(±s)
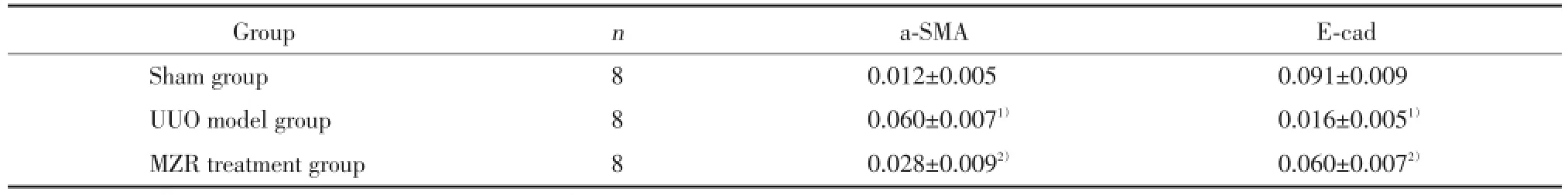
表3 各组小鼠肾组织α⁃SMA、E⁃Cad表达的平均光密度值(±s)Tab.3 The mean optical density value of α⁃SMA and E⁃Cad expression in the kidney tissue of each group(±s)
1)P<0.01 compared with sham group;2)P<0.01 compared with UUO model group.
Group n a⁃SMA E⁃cad Sham group 8 0.012±0.005 0.091±0.009 UUO model group 8 0.060±0.0071) 0.016±0.0051)MZR treatment group 8 0.028±0.0092) 0.060±0.0072)
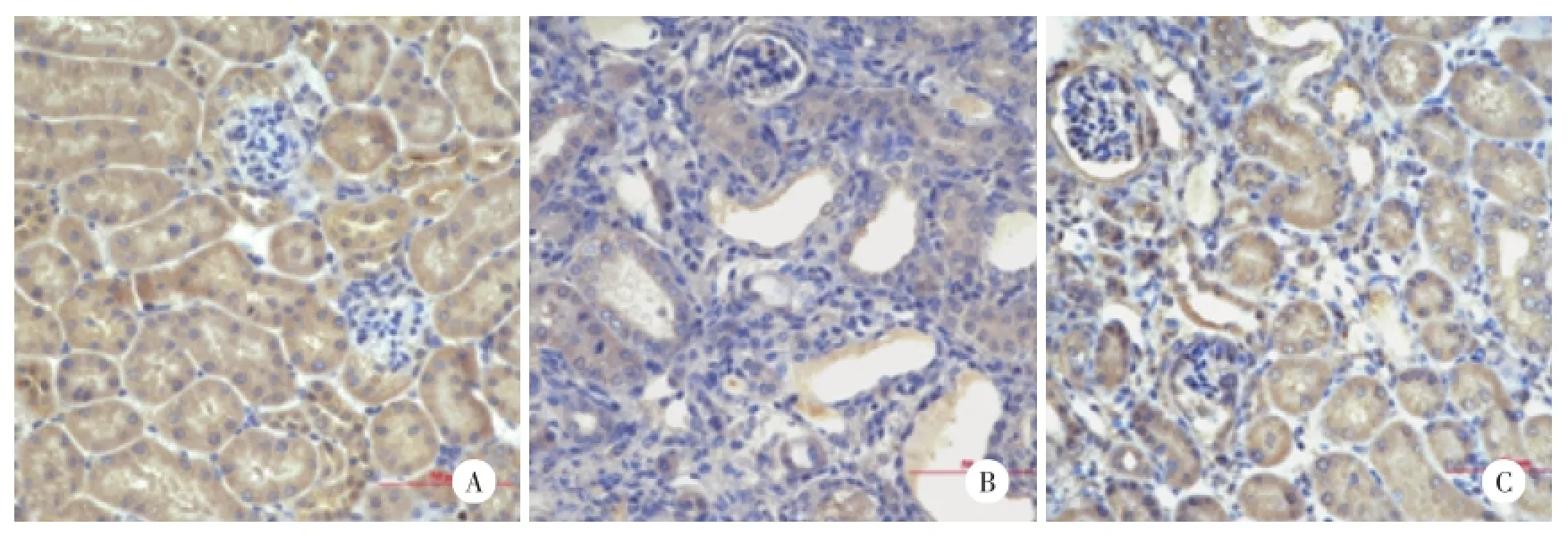
图4 E⁃Cad在各组小鼠肾脏的表达 SP法×400Fig.4 Expression of E⁃Cad in the kidney of mice in each group SP×400
2.4肾组织α⁃SMA、E⁃Cad的表达
Western blot结果显示,与假手术组相比,UUO模型组小鼠肾组织中α⁃SMA表达增加而E⁃Cad表达减少,差异有统计学意义(P<0.05);与UUO模型组相比,MZR治疗组小鼠肾组织α⁃SMA表达减少而E⁃Cad表达增加,差异有统计学意义(P<0.05)。见图5。
3 讨论
小鼠UUO模型可以反映人体肾间质纤维化过程,是研究肾小管EMT及评价慢性肾脏病治疗方法的理想模型,目前已广泛应用[4⁃5]。肾小管EMT即肾小管上皮细胞转化为成纤维细胞或肌成纤维细胞的过程,主要表现为细胞上皮标志物表达减少而间质标志物表达增多,细胞获得迁移能力向间质内迁移,细胞外基质合成增多,从而导致肾间质纤维化的发展[6⁃7]。
MZR是日本学者于1971年从土壤的真菌培养液中分离获得的咪唑核苷类抗代谢药,能特异性地抑制快速增长的淋巴细胞的分裂和增殖。口服吸收迅速,药物浓度在2 h达到峰值[8]。其作用机制主要是抑制核酸代谢中的嘌呤合成,竞争性的抑制次黄嘌呤核苷酸脱氢酶和鸟苷酸合成酶,使鸟苷酸合成减少,阻止细胞由G1期进入S期,从而抑制细胞的增殖[8]。临床上,MZR最早用于预防肾移植术后的排斥反应,与其他免疫抑制剂相比,其不良反应较少,安全性较高,能有效的降低免疫抑制剂治疗后的病毒感染[9⁃11]。目前逐渐用于狼疮性肾炎、肾病综合征、紫癜性肾炎、IgA肾病[12⁃15]等治疗,并取得了一定的疗效,但具体机制尚不完全清楚,可能与其抗感染及免疫抑制作用相关。早期研究[3]发现MZR可以通过抑制UUO大鼠肾组织中巨噬细胞浸润及α⁃SMA表达改善肾间质纤维化。最新研究[16]发现MZR联合应用血管紧张素Ⅱ受体拮抗剂类药物对大鼠慢性肾毒性具有协同保护作用。MZR联合应用直接肾素抑制剂阿利吉仑可更明显地抑制UUO大鼠肾间质纤维化程度[17]。此外,YAMABE等[18]发现MZR可通过抑制单核细胞趋化蛋白1及巨噬细胞炎性蛋白2,从而抑制大鼠肾小球上皮细胞的增殖。
本研究中MZR治疗组小鼠Scr、BUN及肾组织病理改变较UUO模型组改善,提示MZR可以保护肾功能、减轻肾脏的病理损害。此外,免疫组化及Western blot结果显示UUO模型组小鼠肾组织中α⁃SMA表达明显增加而E⁃Cad表达明显减少,说明模型组肾间质纤维化过程中存在EMT;MZR治疗组小鼠肾组织中α⁃SMA表达较UUO模型组受抑而E⁃Cad表达较UUO模型组上调,提示MZR可在一定程度上抑制肾小管EMT发展。
综上所述,MZR作为一种新型免疫抑制剂,可有效保护肾功能,改善肾脏病理损害,对延缓肾间质纤维化起积极作用,其机制可能与其抑制肾小管EMT有关,为临床治疗慢性肾脏病提供了新思路。调控肾小管EMT的系统复杂[6],MZR通过何种途径抑制EMT有待于进一步研究。
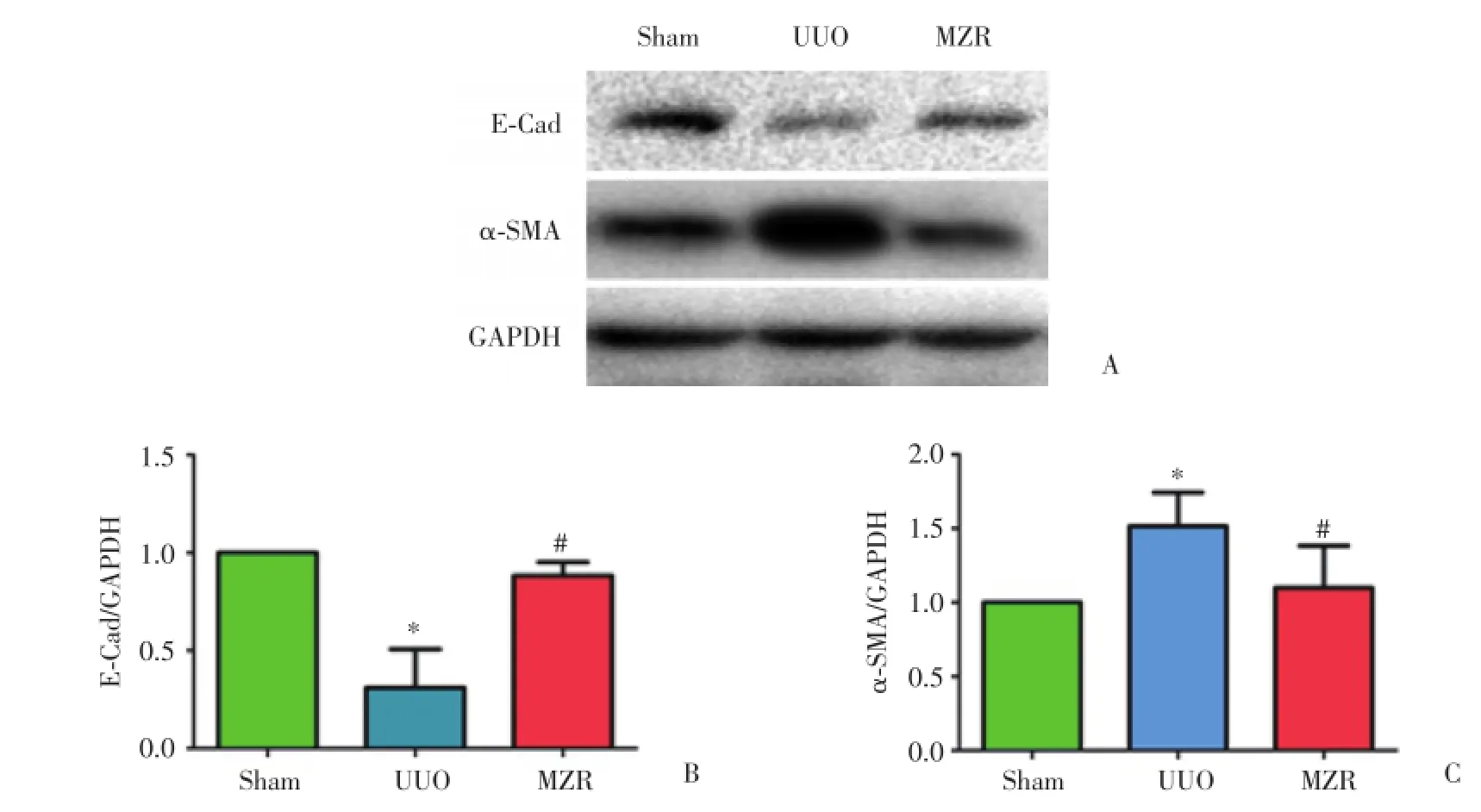
图5 Western blot检测各组小鼠肾组织α⁃SMA和E⁃Cad的表达Fig.5 α⁃SMA and E⁃Cad expression in kidney tissue in each group by means of Western blot
[1]FARRIS AB,COLVIN RB.Renal interstitial fibrosis:mechanisms and evaluation[J].Curr Opin Nephrol Hypertens,2012,21(3):289-300.DOI:10.1097/MNH.0b013e3283521cfa.
[2]GUARINO M,TOSONI A,NEBULONI M,et al.Direct contribution of epithelium to organ fibrosis:epithelial⁃mesenchymal transition [J].Hum Pathol,2009,40(10):1365-1376.DOI:10.1016/j.hump⁃ath.2009.02.020.
[3]SAKAI T,KAWASAKI T,SHIRASAWA T,et al.Mizoribine im⁃proves renal tubulointerstitial fibrosis in unilateral ureteral obstruc⁃tion(UUO)⁃treated rat by inhibiting the infiltration of macrophages and the expression of alpha⁃smooth muscle actin[J].J Urol,1997,158(6):2316-2322.
[4]UCERO AC,BENITO⁃MARTIN A,IZQUIERDO MC,et al.Unilater⁃al ureteral obstruction:beyond obstruction[J].Int Urol Nephrol,2014,46(4):765-776.
[5]赵成广,张慧,吴玉斌.EGCG对梗阻性肾病大鼠肾小管上皮细胞转分化的干预作用及机制[J].中国医科大学学报,2011,40 (6):516-519.
[6]CAREW RM,WANG B,KANTHARIDIS P,et al.The role of EMT in renal fibrosis[J].Cell Tissue Res,2012,347(1):103-116. DOI:10.1007/s00441⁃011⁃1227⁃1.
[7]LIU Y.New insights into epithelial⁃mesenchymal transition in kid⁃ney fibrosis[J].J Am Soc Nephrol,2010,21(2):212-222.DOI:10.1681/ASN.2008121226.
[8]谢院生.咪唑立宾在肾脏中的应用[J].中国中西医结合肾病杂志,2008,9(7):565-568.
[9]ZHANG X,FU S,HAN S,et al.The argument for the use of mizorib⁃ine in renal transplantation:a meta⁃analysis and systemic review [J].Transpl Immunol,2013,28(2/3):106-111.DOI:10.1016/j. trim.2012.12.003.
[10]XING S,YANG J,ZHANG X,et al.Comparative efficacy and safe⁃ty of mizoribine with mycophenolate mofetil for Asian renal trans⁃plantation—a meta⁃analysis[J].Clin Biochem,2014,47(7/8):663-669.
[11]NISHIMURA K,UCHIDA K,YUZAWA K,et al.Excellent results with high⁃dose mizoribine combined with cyclosporine,corticoste⁃roid,and basiliximab in renal transplant recipients:multicenter study in Japan[J].Transplant Proc,2012,44(1):147-149.
[12]TANAKA H,AIZAWA T,WATANABE S,et al.Efficacy of mizoribine⁃tacrolimus⁃based induction therapy for pediatric lupus nephritis[J].Lupus,2014,23(8):813-818.
[13]MATSUMOTO Y,SHIMADA Y,NOJIMA Y,et al.Efficacy of mizoribine followed by low⁃dose prednisone in patients with idio⁃pathic membranous nephropathy and nephrotic⁃range proteinuria [J].Ren Fail,2013,35(7):936-941.DOI:10.1177/0961203314 528553.
[14]KAWASAKI Y,SUYAMA K,HASHIMOTO K,et a1.Methylpred⁃nisolone pulse plus mizoribine in children with Henoch⁃Schoen⁃lein purpura nephritis[J].Clin Rheumatol,2011,30(4):529-535.DOI:10.3109/0886022X.2013.808133.
[15]XIE YJ,HUANG SM,WANG L,et al.Efficacy and safety of mizoribine combined with losartan in the treatment of IgA ne⁃phropathy:a multicenter,randomized,controlled study[J].Am J Med Sci,2011,341(5):367-372.DOI:10.1097/MAJ.0b013e318 207e02d.
[16]ENDO A,SOMEYA T,NAKAGAWA M,et al.Synergistic protec⁃tive effects of mizoribine and angiotensinⅡreceptor blockade on cyclosporine a nephropathy in rats[J].Pediatr Res,2014,75(1):38-44.DOI:10.1038/pr.2013.169.
[17]KOJI S,AMANE E,TOMONOSUKE S,et al.The synergistic effect of mizoribine and a direct renin inhibitor,aliskiren,on unilateral ureteral obstruction induced renal fibrosis in rats[J].J Urol,2014,191(4):1139-1146.DOI:10.1016/j.juro.2013.10.053.
[18]YAMABE H,SHIMADA M,MURAKAMI R,et al.Mizoribine sup⁃presses proliferation of rat glomerular epithelial cells in culture and inhibits increase of monocyte chemoattractant protein⁃1 and macrophage inflammatory protein⁃2 stimulated by thrombin[J]. Biol Pharm Bull,2012,35(5):705-708.
(编辑于溪)
Effects of Mizoribine on Renal Tubular Epithelial⁃mesenchymal Transition in Unilateral Ureteral Obstruction Mice
YU Fang,DENG Haiyue,JIANG Hong
(Department of Pediatrics,The First Hospital,China Medical University,Shenyang 110001,China)
ObjectiveTo observe the effects of mizoribine(MZR)on renal tubular epithelial⁃mesenchymal transition(EMT)of mice which have been performed unilateral ureteral obstruction(UUO),and study the mechanism of its anti⁃fibrosis of renal interstitial.MethodsA total of 24 CD1 mice were randomly divided into sham group,UUO model group and MZR treatment group,with 8 mice in each group.The day before op⁃eration,mice of MZR treatment group had been given MZR 10 mg/kg/d lavage,those of sham group and UUO model group had been given equal saline lavage.Fourteen days after the operation,blood was collected and serum creatinine and blood urea nitrogen were measured;the obstruction kidneys were harvested for section,HE staining and Masson staining were employed to observe the changes of kidney pathological;the expression of α⁃SMA and E⁃Cad in kidney with detected by immunohistochemical and Western blot method.ResultsCompared with sham group,serum creatinine and blood urea nitrogen of mice in UUO model group and MZR treatment group were significantly elevated,kidney pathological chang⁃es and the expression of α⁃SMA in renal tissue were increased and that of E⁃Cad was reduced,the differences were all statistically significant(P<0.05);compared with UUO model group,mice in MZR treatment group had different degree of improvements in serum creatinine,blood urea ni⁃trogen and kidney pathological changes,the expression of α⁃SMA in renal tissue was inhibited and that of E⁃Cad was increases,and the differences were statistically significant(P<0.05).ConclusionMZR may inhibit the development of renal tubular EMT in UUO mice,thereby reduce the level of renal tubule interstitial fibrosis and improve renal function.
unilateral ureteral obstruction;epithelial⁃mesenchymal transition;mizoribine
R692.6
A
0258-4646(2016)08-0728-05
10.12007/j.issn.0258⁃4646.2016.08.014
高等学校博士学科点专项科研基金(20122104110001)
于方(1992-),女,硕士研究生.
姜红,E-mail:jianghong724@163.com
2015-11-12
网络出版时间:
