高压氧对神经病理性疼痛大鼠p38 MAPK信号通路的影响
2016-08-05韩光李璐赵平
韩光,李璐,赵平
(中国医科大学附属盛京医院麻醉科,沈阳 110004)
高压氧对神经病理性疼痛大鼠p38 MAPK信号通路的影响
韩光,李璐,赵平
(中国医科大学附属盛京医院麻醉科,沈阳 110004)
目的探讨高压氧对神经病理性疼痛大鼠p38 MAPK信号通路的影响,从而明确其作用机制。方法实验分为2部分,每部分选用30只SD大鼠,又随机分为3组:假手术组(S组)、坐骨神经慢性缩窄组(CCI组)和高压氧组(HBO组),每组10只。第一部分实验测定术后3,7,14,28 d疼痛行为学指标,并在术后28 d取脊髓标本Western blot方法检测磷酸化p38表达,免疫组化法检测P2X4受体;第二部分实验先对各组大鼠行腰段鞘内置管,并将p38 MAPK信号通路阻断剂(SB203580)连续注入蛛网膜下腔中,测定阻断p38 MAPK信号通路后3,7,14,28 d疼痛行为学指标及术后28 d免疫组化法检测P2X4受体。结果第一部分实验中CCI组、HBO组大鼠疼痛行为学评分较S组明显降低(P<0.05),且CCI组比HBO组降低显著(P<0.05)。CCI组、HBO组的磷酸化p38及P2X4受体含量较S组增加(P<0.05),且CCI组磷酸化p38及P2X4受体的表达比HBO组显著增加(P<0.05)。第二部分实验中大鼠p38 MAPK信号通路抑制后,CCI组、HBO组疼痛行为学评分无统计学差异(P>0.05),但与未给抑制剂第一部分实验的CCI组、HBO组比较明显增高(P<0.05);CCI组及HBO组的P2X4受体表达比S组显著增高(P<0.05),但2组间比较无统计学差异(P>0.05)。结论高压氧可能通过P2X4受体介导的p38 MAPK信号通路影响神经病理性疼痛大鼠。
高压氧;坐骨神经结扎;p38;P2X4受体
网络出版地址
神经病理性疼痛发病机制十分复杂,治疗效果欠佳。近年来很多报道[1]显示,外周神经损伤后,丝裂原激活的蛋白激酶(p38 MAPK)通路的某些重要信号蛋白、受体发生明显变化。高压氧是一种新型、无创、有效的治疗手段,已广泛应用在神经病理性疼痛领域,但高压氧的作用机制还不是很清楚。本研究通过研究p38 MAPK信号通路中P2X4受体在高压氧抗神经病理性疼痛中的作用,深入了解其在高压氧抗神经病理性疼痛的信号传导作用机制,从而为发现更具有针对性的疼痛治疗靶点提供依据。
1 材料与方法
1.1一般资料
雄性SD大鼠60只,体质量280~320 g,由中国医科大学盛京医院实验动物中心提供。实验分为2部分,每部分30只SD大鼠。各部分又随机均分为3组:假手术组(S组),暴露却不结扎坐骨神经;坐骨神经慢性缩窄(chronic constrictive injury,CCI)组,右侧坐骨神经结扎;高压氧(hyperbaric oxygen,HBO)组,术后第1天实施HBO,连续进行5 d。
1.2方法
1.2.1CCI模型的制备:腹腔注射1%戊巴比妥钠(40 mg/kg)麻醉,在右后肢外侧找寻坐骨神经主干,分离出坐骨神经,4⁃0丝线围绕并轻轻结扎坐骨神经,使神经外膜稍稍受压,同时肌肉出现快速收缩抽搐。最后分层缝合,归笼饲养。假手术组的大鼠仅暴露右侧坐骨神经。
1.2.2实验鼠的HBO处理:HBO组大鼠被放入舱内,舱内先进行纯氧洗舱,匀速加压至0.25 MPa,高压状态下停留1 h,又匀速减到常压。1次/d,连续5 d。其他组大鼠放置在舱内,模拟高压氧组的实验环境与条件。
1.2.3鞘内p38抑制剂大鼠模型的制备:第2部分大鼠腹腔注射1%戊巴比妥钠(40 mg/kg)麻醉,将大鼠俯卧位固定,剃毛、消毒、铺巾,在两侧骼棘联线上方L3⁃4间隙行纵行切口,暴露L3、L4棘突。分离组织后,再用细针穿透黄韧带,置入一根导管,使脑脊液从导管慢慢流出,固定导管后观察2 d,选取无感觉及运动障碍的大鼠。通过导管,定时定量给予p38抑制剂—SB203580(2.5 μg),连续5 d。
将大鼠腹腔麻醉后,在左心室插管,灌注生理盐水冲洗,待流出液无血性液体后,取腰段L4~6脊髓,-80℃冰箱保存一部分,其余的浸泡在4%多聚甲醛中待用。
1.3观察指标
1.3.1大鼠疼痛行为学评分的测定:在术后3、7、14、28 d上午9:00⁃12:00进行大鼠疼痛行为学检测。(1)机械性缩足反射阈值(mechanical withdrawal threthold,MWT),将大鼠置金属网格上,罩上透明玻璃罩。使用von Frey纤维刺激大鼠结扎侧足底的中部,使纤维丝在6~8 s内弯成S形,观察大鼠是否缩足。(2)热刺激爪退缩阈值(thermal withdrawal laten⁃cy,TWL),使用热测痛仪将聚光灯产生的光辐射焦点对准大鼠结扎侧足底中部,当大鼠抬腿时为爪退缩阈值。连续测量5次,取平均值。
1.3.2Western blot方法测定组织中磷酸化p38含量:用ECL发光法试剂盒(SW2040,北京索莱宝科技有限公司)显色,结果采用美国BIO⁃RAD凝胶成像系统(Gel DocTM XR,美国BIO⁃RAD公司)拍照,用Quantity One 1⁃D软件(美国BIO⁃RAD公司)分析,记录每条蛋白电泳带的灰度值。
1.3.3免疫组化方法观察P2X4受体含量:4%多聚甲醛固定并包埋大鼠脊髓组织,加入P2X4抗体及二抗,光镜观察,棕色染色的为阳性细胞。显微照相及图像分析应用U⁃MCB Olympus图像采集系统、METAMORPH/COOL SNAP+X/AX70显微(荧光)图像分析系统(VIC/ROPER OLYMPUS)US/JP对切片进行分析处理,每只大鼠取4张切片,测定阳性细胞数值,放大400倍照相。
1.4统计学分析
2 结果
2.1各组大鼠疼痛行为学评分的测定结果比较
第一部分大鼠CCI术后CCI组、HBO组疼痛行为学评分较S组明显降低,且CCI组比HBO组降低更明显(均P<0.05)。第二部分大鼠在给予SB203580后,S组MWT及TWL值无明显变化,但CCI组与HBO组大鼠MWT及TWL值显著增加(P<0.05),但CCI组与HBO组之间比较无统计学差异(P>0.05)。见表1。
2.2各组大鼠磷酸化p38表达比较
第一部分大鼠Western blot结果显示:与S组(0.61±0.08)比较,CCI组(0.98±0.08)与HBO组(0.89±0.11)磷酸化p38表达上升(P<0.05)。但HBO组大鼠磷酸化p38血清含量显著低于CCI组(P<0.05),见图1。
2.3各组大鼠P2X4受体表达比较
两部分试验中CCI组与HBO组大鼠P2X4受体与S组比较在脊髓表达增加。第一部分实验CCI组增加均较HBO组显著(P<0.05),第二部分实验这2组无统计学差异(P>0.05),见表2,图2、3。
表1 各组大鼠疼痛行为学评分的测定结果比较(±s)Tab.1 Pain⁃related behavior of rats in each group(±s)
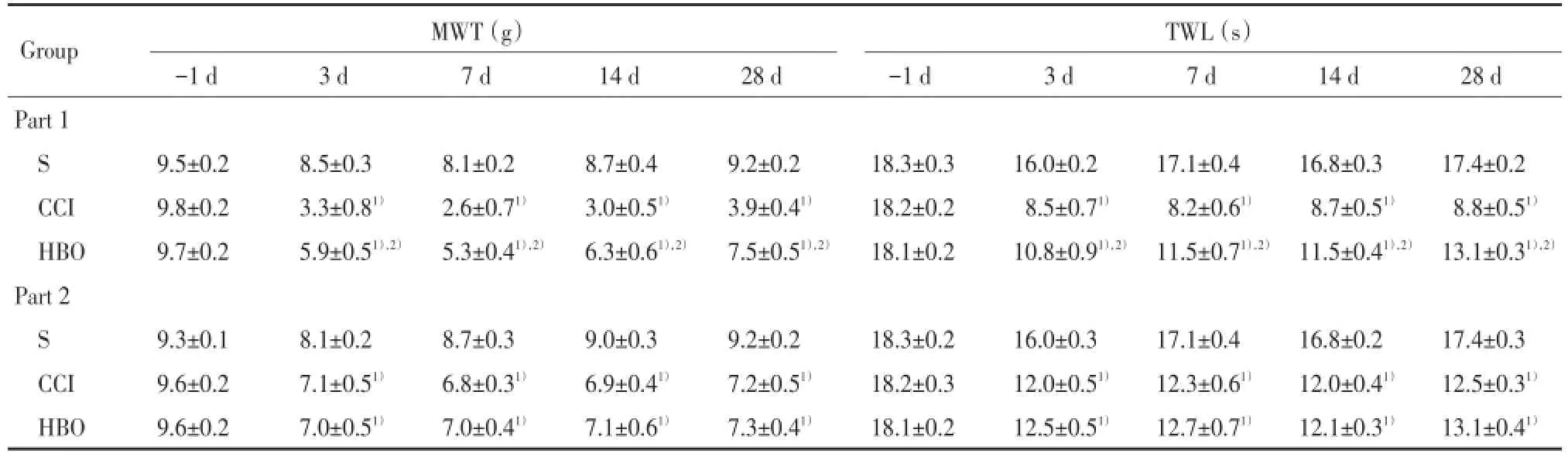
表1 各组大鼠疼痛行为学评分的测定结果比较(±s)Tab.1 Pain⁃related behavior of rats in each group(±s)
1)P<0.05 vs group S in the same part;2)P<0.05 vs group CCI in the same part.
Group MWT(g)TWL(s)-1 d 3 d 7 d 14 d 28 d -1 d 3 d 7 d 14 d 28 d Part 1 S 9.5±0.2 8.5±0.3 8.1±0.2 8.7±0.4 9.2±0.2 18.3±0.3 16.0±0.2 17.1±0.4 16.8±0.3 17.4±0.2 CCI 9.8±0.2 3.3±0.81) 2.6±0.71) 3.0±0.51) 3.9±0.41) 18.2±0.2 8.5±0.71) 8.2±0.61) 8.7±0.51) 8.8±0.51)HBO 9.7±0.2 5.9±0.51),2) 5.3±0.41),2) 6.3±0.61),2) 7.5±0.51),2) 18.1±0.2 10.8±0.91),2) 11.5±0.71),2) 11.5±0.41),2) 13.1±0.31),2)Part 2 S 9.3±0.1 8.1±0.2 8.7±0.3 9.0±0.3 9.2±0.2 18.3±0.2 16.0±0.3 17.1±0.4 16.8±0.2 17.4±0.3 CCI 9.6±0.2 7.1±0.51) 6.8±0.31) 6.9±0.41) 7.2±0.51) 18.2±0.3 12.0±0.51) 12.3±0.61) 12.0±0.41) 12.5±0.31)HBO 9.6±0.2 7.0±0.51) 7.0±0.41) 7.1±0.61) 7.3±0.41) 18.1±0.2 12.5±0.51) 12.7±0.71) 12.1±0.31) 13.1±0.41)

图1 第一部分各组大鼠磷酸化p38的表达结果Fig.1 The phosphorylative p38 expression of rats in three groups of part one

表2 各组大鼠P2X4受体表达比较Tab.2 P2X4 receptor expression of rats in groups
1)P<0.05 vs group S in the same part;2)P<0.05 vs group HBO in the same part.
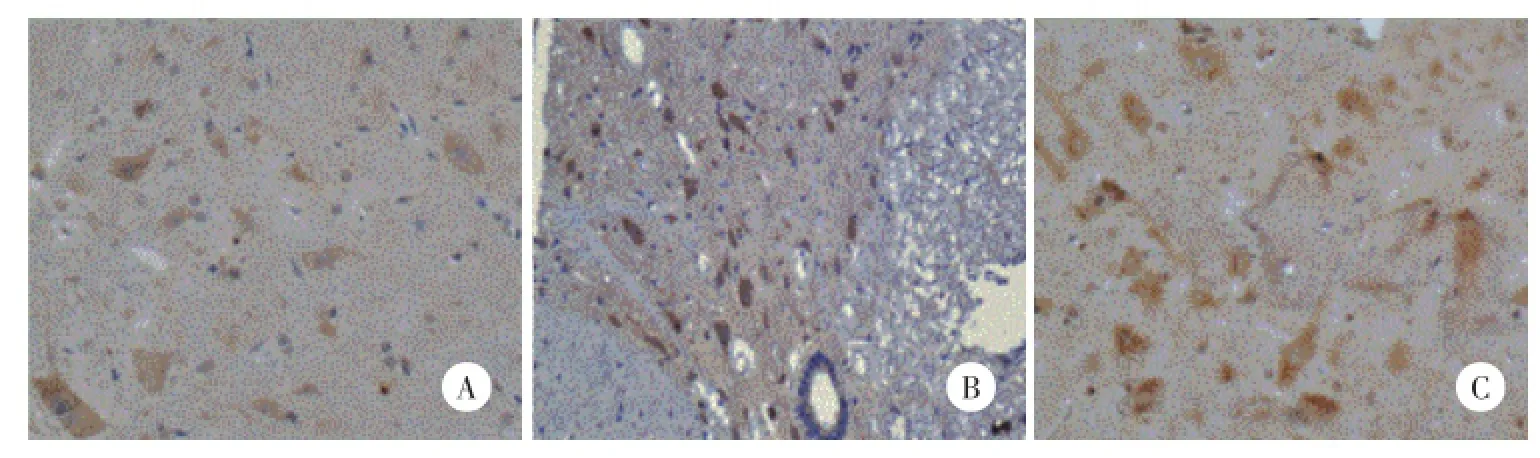
图2 第一部分各组大鼠P2X4受体的免疫组化结果×400Fig.2 P2X4 receptor immunohistochemistry expression in spinal cord in each group of part one×400
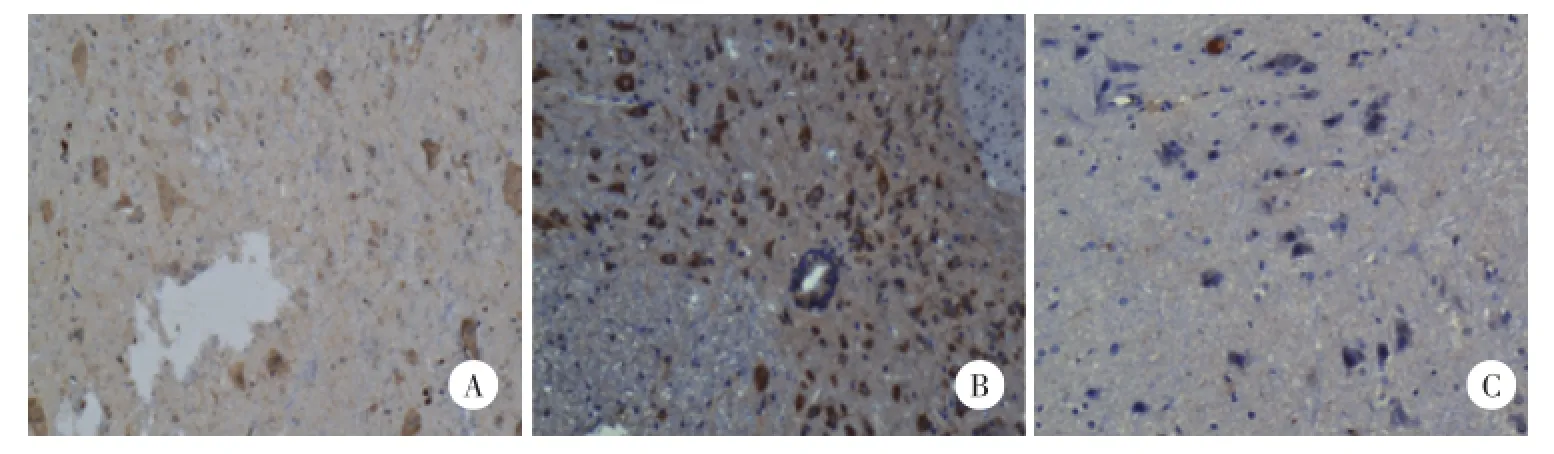
图3 第二部分各组大鼠在给予p38抑制剂后P2X4受体的免疫组化结果×400Fig.3 P2X4 receptor immunohistochemistry expression after SB203580 administration in spinal cord in each group of part two×400
3 讨论
神经损伤后,脊髓中磷酸化p38 MAPK免疫组化阳性细胞显著增加,尤其以p38 MAPK磷酸化水平明显增加[2]。在多种炎性疼痛的动物模型中,p38 MAPK参与炎性痛觉过敏的形成,并可以通过多种机制和信号系统发挥作用[3⁃5]。文献[6]报道皮下注射辣椒素可引起疼痛,同时伴随周围皮肤的p38受体MAPK增加,使用p38 MAPK抑制剂SB203580就能达到镇痛的作用。同时可以抑制炎性组织中P2X4的增加并减轻动物的机械性痛敏及热痛敏[7⁃8]。因此我们推测p38 MAPK信号通路可能通过调节P2X4受体蛋白的释放,参与疼痛的形成。
HBO能增加减轻神经水肿,使组织抗氧化,加速自由基的清除,提高携氧能力、产生ATP,促进毛细血管再生,缓解疼痛[9⁃10]。有学者[11⁃12]认为HBO的神经保护作用机制可能是抑制氧化应激反应。因为HBO可降低白细胞的大量聚集,降低循环中炎性因子的释放[13⁃14]。它在早期抑制炎性因子的产生与释放方面有着重要的作用。最近有研究[15]表明,HBO通过增高脑源性神经营养因子的水平,抑制p38 MAPK信号通路,达到脑保护。说明 p38 MAPK信号通路调节的细胞功能在脑保护中可能扮演了重要的角色,它为HBO的神经保护作用机制的阐明开辟了新的道路。本研究结果表明:与S组大鼠相比CCI组及HBO组大鼠磷酸化p38水平显著增加,说明外周神经损伤后p38 MAPK被激活。磷酸化p38 MAPK伴随痛觉过敏,在术后3 d开始升高。P38 MAPK被阻断后,疼痛明显缓解。但是,p38 MAPK只是MAPK信号通路众多亚族中的一种,MAPK信号通路的上下游分子也需要进一步寻找。另外,高压氧是否通过减少P2X4受体表达影响p38 MAPK信号通路的作用还不清楚,仍需要进行深入探讨。
曾有报道[16]在甲醛造成的大鼠致炎性痛模型中,脊髓小胶质细胞的P2X4受体上调。P2X4受体蛋白随时间逐渐增加,这种变化与神经损伤后引起的痛觉超敏变化相一致。目前外周神经损伤后,脊髓小胶质细胞P2X4受体调控的机制还不是很清楚,可能与小胶质细胞活化后P2X4受体mRNA及蛋白的转录有关[17⁃18]。已有研究[19⁃20]表明,神经损伤后通过激活p38 MAPK、ERK等细胞内信号分子进一步活化P2X4受体转录,反之应用这些分子的抑制剂可有效减轻疼痛,预防脊髓神经痛觉超敏。本研究证明高压氧能有效减少P2X4受体的表达,而且这种抗神经病理性疼痛作用在给予P38 MARK抑制剂后变得不明显,提示它很可能通过P38 MARK信号通路起作用。
[1]CAO J,WANG JS,REN XH,et al.Spinal sample showing p⁃JNK and P38 associated with the pain signaling transduction of glial cell in neuropathic pain[J].Spinal Cord,2015,53(2):92-97.DOI:10.1038/sc.2014.188.
[2]NI HD,YAO M,HUANG B,et al.Glial activation in the periaque⁃ductal gray promotes descending facilitation of neuropathic pain through the p38 MAPK signaling pathway[J].J Neurosci Res,2016,94(1):50-61.DOI:10.1002/jnr.23672.
[3]LIN ML,LIN WT,HUANG RY,et al.Pulsed radiofrequency inhibit⁃ed activation of spinal mitogen⁃activated protein kinases and amelio⁃rated early neuropathic pain in rats[J].Eur J Pain,2014,18(5):659-⁃670.
[4]LIN X,WANG M,ZHANG J,et al.p38 MAPK:a potential target of chronic pain[J].Curr Med Chem,2014,21(38):4405-4418.
[5]TATSUMI E,YAMANAKA H,KOBAYASHI K,et al.RhoA/ROCK pathway mediates p38 MAPK activation and morphological changes downstream of P2Y12/13 receptors in spinal microglia in neuropath⁃ic pain[J].Glia,2015,63(2):216-228.DOI:10.1002/glia.22745.
[6]HU JH,YANG JP,LIU L,et al.Involvement of CX3CR1 in bone cancer pain through the activation of microglia p38 MAPK pathway in the spinal cord[J].Brain Res,2012,1465:1-9.DOI:10.1016/j. brainres.2012.05.020.
[7]CHIANG RP,HUANG CT,TSAI YJ.Melatonin reduces median nerve injury⁃induced mechanical hypersensitivity via inhibition of microglial p38 mitogen⁃activated protein kinase activation in rat cu⁃neate nucleus[J].J Pineal Res,2013,54(2):232-244.DOI:10.1111/jpi.12029.
[8]ZHOU TT,WU JR,CHEN ZY,et al.Effects of dexmedetomidine on P2X4Rs,p38⁃MAPK and BDNF in spinal microglia in rats with spared nerve injury[J].Brain Res,2014,1568:21-30.DOI:10.1016/j.brainres.
[9]ANAND P,SHENOY R,PALMER JE,et al.Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury[J].Eur J Pain,2011,15(10):1040-1048.DOI:10.1016/j.ejpain.2011.04.005.
[10]LIU X,YANG J,LI Z,et al.Hyperbaric oxygen preconditioning promotes neovascularization of transplanted skin flaps in rats[J]. Int J Clin Exp Pathol,2014,7(8):4734-4744.
[11]HAN G,LI L,MENG LX.(2013)Effects of hyperbaric oxygen on pain⁃related behaviors and nitric oxide synthase in a rat model of neuropathic pain[J].Pain Res Management,2013,18(3):137-141.
[12]ZHANG Y,LV Y,LIU YJ,et al.Hyperbaric oxygen therapy in rats attenuates ischemia⁃reperfusion testicular injury through blockade of oxidative stress,suppression of inflammation,and reduction ofnitric oxide formation[J].Urology,2013,82(2):489.e9-489.e15. DOI:10.1016/j.urology.
[13]QI Z,GAO CJ,WANG YB,et al.Effects of hyperbaric oxygen pre⁃conditioning on ischemia⁃reperfusion inflammation and skin flap survival[J].Chin Med J(Engl),2013,126(20):3904-3909.
[14]ZHAO BS,MENG LX,DING YY,et al.Hyperbaric oxygen treat⁃ment produces an antinociceptive response phase and inhibits as⁃trocyte activation and inflammatory response in a rat model of neu⁃ropathic pain[J].J Mol Neurosci,2014,53(2):251-261.DOI:10.1007/s12031⁃013⁃0213⁃3.
[15]ZHANG Y,STOLZ PA,SHIRACHI DY,et al.Reduced antinoci⁃ceptive responsiveness to hyperbaric oxygen in opioid⁃tolerant mice [J].Eur J Pain,2014,18(7):1032-1039.DOI:10.1002/j.1532⁃2149.2013.00448.x.
[16]WILSON HD,WILSON JR,FUCHS PN.Hyperbaric oxygen treat⁃ment decreases inflammation and mechanical hypersensitivity in an animal model of inflammatory pain[J].Brain Res,2006,1098 (1):126-128.
[17]ASE AR,HONSON NS,ZAGHDANE H,et al.Identification and characterization of a selective allosteric antagonist of human P2X4 receptor channels[J].Mol Pharmacol,2015,87(4):606-616. DOI:10.1124/mol.114.096222.
[18]IGAWA T,HIGASHI S,ABE Y,et al.Preparation and character⁃ization of a monoclonal antibody against the refolded and function⁃al extracellular domain of rat P2X4 receptor[J].J Biochem,2013,153(3):275-282.DOI:10.1093/jb/mvs143.
[19]HERNANDEZ⁃OLMOS V,ABDELRAHMAN A,EL⁃TAYEB A,et al.N⁃substituted phenoxazine and acridone derivatives:structure⁃activity relationships of potent P2X4 receptor antagonists[J].J Med Chem,2012,55(22):9576-9788.DOI:10.1021/jm300845v.
[20]BERNIER LP,ASE AR,BOUÉ⁃GRABOT E,et al.P2X4 receptor channels form large noncytolytic pores in resting and activated mi⁃croglia[J].Glia,2012,60(5):728-737.DOI:10.1002/glia. 22301.
[21]BIBER K,TSUDA M,TOZAKI⁃SAITOH H,et al.Neuronal CCL21 up⁃regulates microglia P2X4 expression and initiates n europathic pain development[J].EMBO J,2011,30(9):1864-1873.DOI:10.1038/emboj.2011.89.
(编辑武玉欣)
Effect of Hyperbaric Oxygen on p38 MAPK Signal Transduction Pathway in Neuropathic Rats
HAN Guang,LI Lu,ZHAO Ping
(Department of Anesthesiology,Shengjing Hospital,China Medical University,Shenyang 110004,China)
ObjectiveTo investigate the effect of hyperbaric oxygen(HBO)on p38 MAPK signal pathway in neuropathic pain,and explore its mechanism.MethodsThe experiment was divided into two parts with thirty SD rats in each part.Each part was then randomly divided into 3 groups:sham operation group(S group),chronic constriction group(CCI group)and hyperbaric oxygen group(HBO group),with 10 rats in each group.Part one:Pain⁃related behavior were detected on 3 day,7 day,14 day,28 day after operation.Phosphorylative p38 was detected by Western blot method and the P2X4 receptor was detected by immunohistochemistry method on the 28th day.Part two:All the rats were treated with SB203580.Pain⁃related behavior were detected on 3 day,7 day,14 day,28 day after operation.P2X4 receptor was detected by immunohistochem⁃istry method on the 28th day.ResultsCCI and HBO groups were significantly lower than S group in pain⁃related behaviors(P<0.05).CCI group was significantly lower than that in HBO group(P<0.05).The content of phosphorylative p38 and P2X4 receptor in CCI and HBO group were increased(P<0.05),and the expression of phosphorylative p38 and P2X4 in CCI group was significantly increased than that in HBO group (P<0.05).When p38 MAPK was inhibited,pain⁃related behavior in HBO and CCI group increased significantly than the pain⁃related behavior of HBO and CCI group in Part 1(P<0.05).But the difference between group CCI and HBO was not significant(P>0.05).Though the expression of P2X4 receptor in CCI group and HBO group was significantly higher than that in S group(P<0.05),the difference between group CCI and HBO was not significant(P>0.05).ConclusionHyperbaric oxygen therapy can affect the expression of p38 MAPK through P2X4 receptor in rats of neuropathic pain.
hyperbaric oxygen;chronic constrictive injury;p38;P2X4 receptor
R614
A
0258-4646(2016)08-0723-05
10.12007/j.issn.0258⁃4646.2016.08.013
沈阳市科学技术计划(F15⁃199⁃1⁃35)
韩光(1978-),女,副教授,硕士.
赵平,E-mail:zhaop@sj⁃hospital.org
2016-01-11
网络出版时间:
