芳酰腙铜(Ⅱ)和锌(Ⅱ)配合物的合成、晶体结构及抗菌活性
2016-07-22海士坤娄淑芳仇晓阳商丘医学高等专科学校公共学科部商丘47600商丘师范学院功能材料制备工程研究中心商丘476000
海士坤 娄淑芳 仇晓阳(商丘医学高等专科学校公共学科部,商丘 47600)(商丘师范学院功能材料制备工程研究中心,商丘 476000)
芳酰腙铜(Ⅱ)和锌(Ⅱ)配合物的合成、晶体结构及抗菌活性
海士坤1娄淑芳1仇晓阳*,2
(1商丘医学高等专科学校公共学科部,商丘476100)
(2商丘师范学院功能材料制备工程研究中心,商丘476000)
摘要:合成了一对结构类似的双核铜和锌配合物,Cu2(L1)2(1)和[Zn2(L2)2(CH3OH)2](2),其中L1和L2分别是2-溴-N′-(2-羟基-5-甲基苯亚甲基)苯甲酰肼(H2L1)和2-氯-N′-(2-羟基-5-甲基苯亚甲基)苯甲酰肼(H2L2)的二价阴离子,通过元素分析、红外光谱以及单晶X射线衍射表征了它们的结构。配合物1以三斜晶系P1空间群结晶,其晶体学参数:a=0.914 11(6)nm,b=1.180 04(7)nm,c= 1.359 36(9)nm,α=101.928(2)°,β=91.399(2)°,γ=107.873(2)°,V=1.359 3(2)nm3,Z=2,R1=0.054 0,wR2=0.118 9,GOF=0.970。配合物2以单斜晶系P21/c空间群结晶,其晶体学参数:a=1.216 97(9)nm,b=1.214 96(9)nm,c=1.212 83(9)nm,β=110.939(1)°,V= 1.674 8(2)nm3,Z=2,R1=0.034 1,wR2=0.068 9,GOF=1.024。X射线分析表明2个化合物都是中心对称的双核配合物,其中配合物1中的Cu原子是平面正方形配位构型,配合物2中的Zn原子是四方锥配位构型。还通过MTT法研究了这两个配合物的抗细菌(大肠杆菌,金黄色葡萄球菌,枯草芽孢杆菌和铜绿色假单胞菌)和抗真菌(白假丝酵母菌和黑曲霉菌)活性。
关键词:席夫碱;铜配合物;锌配合物;双核配合物;晶体结构;抗菌活性
河南省教育厅基金(No.14B150036)资助项目。
*通信联系人。E-mail:xiaoyang_qiu@126.com
Schiff bases are a kind of biological active compounds bearing the-N=CH-functional groups,which can be prepared by the condensation reactions of carbonyl-containing compounds with primary amines. The compounds have been attracted considerable attention for their wide range of biological activities,such as antibacterial[1-2],antifungal[3],antitumor[4-5],antiinflammatory[6-7],and cytotoxic[8-9].Aroylhydrazones bearing the-C(O)-NH-N=CH-functional groups are a kind of special Schiff bases,which possess interesting biological activities[10-13].It was reported that Schiff bases bearing electron-withdrawing groups can improve their antimicrobial activities[14-15].Rai and co-workers reported a series of fluoro,chloro,bromo,and iodosubstituted compounds,and found that they have significant antimicrobial activities[16-18].As a continuation of work on the exploration of novel antimicrobial agents,in this paper,two new dinuclear copper(Ⅱ)and zinc(Ⅱ) complexes,Cu2(L1)2(1)and[Zn2(L2)2(CH3OH)2](2),where L1and L2are the dianionic form of 2-bromo-N′-(2-hydroxy-5-methylbenzylidene)benzohydrazide(H2L1)and 2-chloro-N′-(2-hydroxy-5-methylbenzylidene)benzohydrazide(H2L2),respectively(Scheme 1),were prepared and their antimicrobial activities were investigated.

Scheme 1 Aroylhydrazones
1 Experimental
1.1General methods and materials
5-Methylsalicylaldehyde,2-bromobenzohydrazide,and2-chlorobenzohydrazidewerepurchasedfrom Sigma-Aldrichandusedasreceived.Allother reagents were of analytical reagent grade.Elemental analyses of C,H and N were carried out in a Perkin-Elmer automated model 2400 SeriesⅡCHNS/O analyzer.FT-IR spectra were obtained on a Perkin-Elmer 377 FT-IR spectrometer with samples prepared as KBr pellets.Molar conductance was measured with a Shanghai DDS-11A conductometer.X-ray diffraction was carried out on a Bruker SMART 1000 CCD diffractometer.
1.2Synthesis of the aroylhydrazones
The aroylhydrazones were synthesized as follows To the methanolic solution(30 mL)of 5-methylsalicyl aldehyde(0.02 mol,2.72 g)was added a methanolic solution(20 mL)of 2-bromobenzohydrazide(0.02 mol 0.043 g)or 2-chlorobenzohydrazide(0.02 mol,0.034 g)with stirring.The mixtures were stirred for 30 min at room temperature to give colorless precipitation Thesolventwasevaporatedtogivecolorless crystalline product,which was recrystallized from methanol and dried in vacuum containing anhydrous CaCl2.
For H2L1:Yield 87%.Characteristic IR data (cm-1):3 433(m),3 217(w),1 638(s).Anal.Calcd for C15H13BrN2O2(%):C,54.1;H,3.9;N,8.4.Found (%):C,53.9;H,4.0;N,8.5.For H2L2:Yield 92% Characteristic IR data(cm-1):3 441(m),3 223(w)1 637(s).Anal.Calcd.for C15H13ClN2O2(%):C,62.4 H,4.5;N,12.3.Found(%):C,62.3;H,4.7;N,9.6.
1.3Synthesis of complex 1
H2L1(0.1 mmol,33.3 mg)and copper nitrate trihydrate(24.2 mg,0.1 mmol)were mixed in methanol(10 mL).The mixture was refluxed for 1 h and then cooled to room temperature.Single crystals of the complex,suitable for X-ray diffraction,were grown from the solution upon slow evaporation within a few days.The crystals were isolated by filtration washed with methanol and dried in vacuum containing anhydrous CaCl2.Yield:41%.Characteristic IR data (cm-1):1 623(s).Anal.Calcd.for C30H22Br2Cu2N4O (%):C,45.6;H,2.8;N,7.1.Found(%):C,45.8;H 2.7;N,7.0%.
1.4Synthesis of complex 2
Complex 2 was prepared and crystallized by the similar method as described for complex 1,with H2L replaced by H2L2(0.1 mmol,28.9 mg),and coppe nitrate trihydrate replaced by zinc nitrate hexahydrate (29.7 mg,0.1 mmol).Yield 52%.Characteristic IR data(cm-1):3 453(m),1 621(m).Anal.Calcd.fo C32H30Cl2N4O6Zn2(%):C,50.0;H,3.9;N,7.3.Found (%):C,49.8;H,4.1;N,7.3.
1.5X-ray crystallography
X-ray diffraction analysis was carried out at a Bruker SMART 1000 CCD area diffractometer equipped with Mo Kα radiation(λ=0.071 073 nm).The diffraction data were collected with SMART and reduced with SAINT[19],and multi-scan absorption correction was performed using SADABS[20].The structures of the complexes were solved by direct method,and refined against F2by full-matrix least-squares method using SHELXTL[21].All of the non-hydrogen atoms were refined anisotropically.The methanol hydrogen of complex 2 was located from a difference Fourier map,and refined isotropically with O-H distance restrained to 0.085(1)nm.The remaining hydrogen atoms were placed in calculated positions and constrained to ride ontheirparentatoms.Themethanolligandin complex 2 disordered over two sites,with occupancies of 0.638(2)and 0.362(2).The crystallographic data and refinement parameters for the compounds are listed in Table 1.Selected bond lengths and angles are listed in Table 2.
CCDC:1447054,1;1447056,2.

Table 1 Crystallographic information for the complexes
1.6Antimicrobial assay
The antibacterial activities of the synthesized compounds was tested against B.subtilis,S.aureus,E.coli,and P.aeruginosa using LB medium.The antifungal activities of the compounds were tested against C.albicans and A.niger using RPMI-1640 medium.The IC50(half inhibitory concentration)of the test compounds were determined by a colorimetricmethod using the dye MTT(3-(4,5-di-methylth-iazol-2-yl)-2,5-diphenyltetrazoliumbromide).Astock solution of the synthesized compound(1 mg·mL-1)in DMSO was prepared and graded quantities of the test compounds were incorporated in specified quantity of sterilizedliquidLBmedium.Suspensionofthe microorganism was prepared and applied to 96-well assay plate with serially diluted compounds to be tested.10 μL of tested samples at pre-set concentrations were added to wells with Penicillin G as a positive reference and the solvent control(5%DMSO)in medium and incubated at 37℃ for 24 h.After 24 h exposure,10 μL of PBS(phosphate buffered saline 0.01 mol·L-1,pH=7.4)containing 4 mg·mL-1of MTT was added to each well.After 4 h,the medium was replaced by 150 μL DMSO to dissolve the purple formazan crystals produced.The absorbance at 492 nm of each well was measured with an ELISA plate reader.
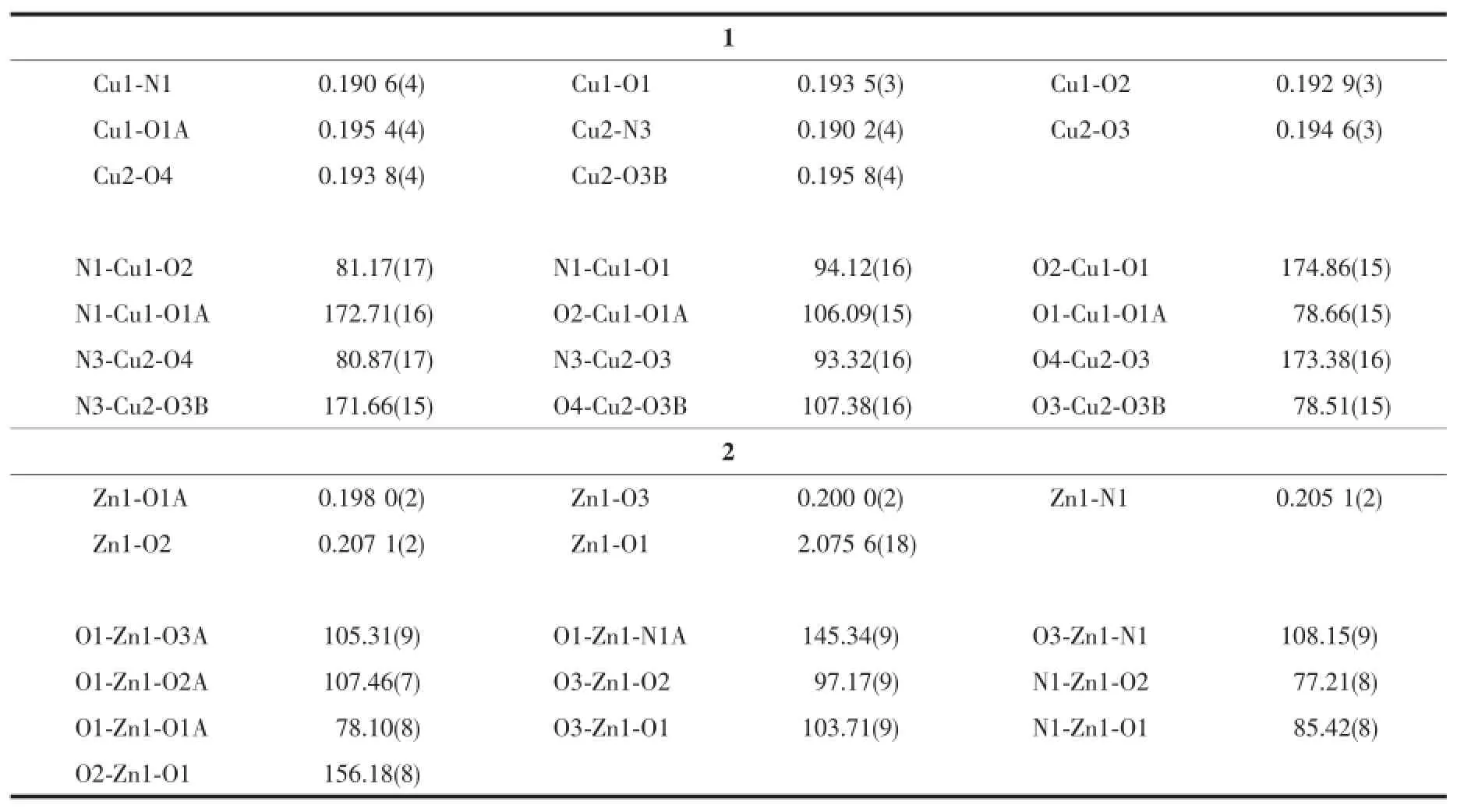
Table 2 Selected bond lengths(nm)and angles(°)for the complexes
2 Results and discussion
2.1Synthesis and characterization
The aroylhydrazones H2L1and H2L2were readily prepared by the condensation reaction of 1∶1 molar ratio of 5-methylsalicylaldehyde with 2-bromobenzohydrazide and 2-chlorobenzohydrazide,respectively,in methanol.Complexes 1 and 2 were prepared by the reaction of the aroylhydrazones with copper nitrate and zinc nitrate,respectively,in methanol,followed by recrystallization.Elemental analyses of the compl exes are in accordance with the molecular structures proposed by the X-ray analysis.FT-IR spectra of both complexes are of similar type.The complexes show typical C=N absorptions at 1 623 cm-1for 1 and 1 621 cm-1for 2.Both complexes are stable in air at room temperature.The molar conductivity of the complexes measured in absolute methanol at concentration of 1 mmol·L-1are 18.0 Ω-1·cm2·mol-1for 1 and 22 Ω-1· cm2·mol-1for 2,indicating the non-electrolytic nature of the complexes in solution[22].
2.2Structure description of complex 1
The molecular structure of complex 1 is shown in Fig.1.The asymmetric unit of the complex contains two independent molecules.The complex is a centro symmetric dinuclear copper(Ⅱ)species,withthe inversion center located at the midpoint of the two Cu atoms.The Cu…Cu distances are 0.300 9(2)and 0.302 3(2)nm.Each Cu atom of the complex is coor dinated by the phenolate oxygen,imino nitrogen and enolate oxygen of the aroylhydrazone ligand,and thephenolate oxygen of the symmetry related aroylhydrazone ligand,forming square planar geometry.The distortion of the square planar coordination can be observed from the bond distances and bond angles. The cis bond angles are from 81.2(2)°to 106.1(2)°for Cu1,and from 78.5(2)°to 107.4(2)°for Cu2,and the trans bond angles are 172.7(2)°and 174.9(2)°for Cu1,and 171.7(2)°and 173.4(2)°for Cu2.The Cu-O and Cu-N bonds in the complex are comparable to those observed in copper(Ⅱ) complexes with aroylhydrazone ligands[23-24].The two benzene rings of the aroylhydrazone ligand form dihedral angle of 25.7(3)°.

Fig.1 A perspective view of complex 1 with the atom labeling scheme
2.3Structure description of complex 2
The molecular structure of complex 2 is shown in Fig.2.The complex is a centrosymmetric dinuclear zinc(Ⅱ) species,with the inversion center located at the midpoint of the two Zn atoms.The Zn…Zn distance is 0.315 0(2)nm.Each Zn atom of the complex is coordinated in a square pyramidal geometry,with the phenolate oxygen,imino nitrogen and enolate oxygen of the aroylhydrazone ligand,and the phenolate oxygen of the symmetry related aroylhydrazone ligand,defining the basal plane,and with the methanol oxygen occupying the apical position.The Zn atom deviates from the least-squares plane defined by the four basal donor atoms by 0.046 8(2)nm.The distortion of the square pyramidal coordination can be observed from the bond distances and bond angles.The cis bond angles in the basal plane are from 77.21(8)°to 108.15(9)°,and the trans bond angles are 145.34(9)° and 156.18(8)°.The angles among the apical and basal donor atoms are in the range of 97.17(9)°~105.31(9)°. The Zn-O and Zn-N bonds in the basal plane of the complex are comparable to those observed in zinc(Ⅱ)complexes with aroylhydrazone ligands[25-26].The two benzene rings of the aroylhydrazone ligand form dihedral angle of 28.3(5)°.

Fig.2 A perspective view of complex 2 with the atom labeling scheme

Fig.3 Emission spectrum of complex 2
2.4Fluorescence character of complex 2
The fluorescence property of complex 2 was studied at room temperature(Fig.3).The emission spectrum of the complex is from 420 to 500 nm,with λmax=465 nm(λex=266 nm).For zinc(Ⅱ)complexes,noemission originating from metal-centered MLCT/LMCT excited states are expected,since zinc(Ⅱ)ion is difficult to oxidize or reduce due to its stable d10configuration.Thus,the emission observed in the complex is tentatively assigned to the π-π*intraligand fluorescence.
2.5Antimicrobial activity
The complexes and the free aroylhydrazones were screened for antibacterial activity against two Gram(+)bacterial strains(B.subtilis and S.aureus)and two Gram(-)bacterial strains(E.coli and P.aeruginosa)by MTT method.The IC50values of the compounds against four bacteria are listed in Table 3.Penicillin G was used as the standard drug.The aroylhydrazone H2L1showed medium activity against the bacteria B.subtilis,while no activity against the other bacteria. The aroylhydrazone H2L2showed medium activities against the bacteria B.subtilis,S.aureus,and E.coli,while no activity against P.aeruginosa.Thus,the Clsubstitute group is better than the Br-substitute group for the antibacterial activity of S.aureus and E.coli. The copper complex has strong activity against B. subtilis,medium activity against S.aureus,and no activity against the other bacteria.The zinc complex has medium activity againstB.subtilis,and n activity against the other bacteria.From the results,i is difficult to give a definite conclusion about which one is good for the antibacterial activities of the aroylhydrazoneandthecomplexes.Forexample complexes 1 and 2 have stronger activities against B subtilis than the aroylhydrazones.However,as for S aureus,complex 1 has stronger activity than H2L1,yet H2L2has stronger activity than complex 2.As for E coli,both H2L1and complexes 1 and 2 have n activity,while H2L2has effective activity.The particula interest is that complex 1 showed the most effective activity againstB.subtilis,which is even more effective than Penicillin G.
The antifungal activities of the complexes and the aroylhydrazones were also evaluated against two fungal strains(C.albicans and A.niger)by MTT method Ketoconazole was used as a reference drug.It is interesting that complex 1 has effective activity,with IC50value of 7.27 μg·mL-1.However,both the aroylhydrazone and the zinc complex have no activity against the fungal strains.

Table 3 IC50values of the tested material
References:
[1]You Z L,Zhu H L.Z.Anorg.Allg.Chem.,2006,632:140-146
[2]Samanta B,Chakraborty J,Choudhury C R,et al.Struct. Chem.,2007,18:33-41
[3]Chohan Z H,Sumrra S H,Youssoufi M H,et al.Eur.J.Med. Chem.,2010,45:2739-2747
[4]TAI Xi-Shi(台夕市),ZHAO Wen-Hua(赵文华),LI Fa-Hui(李法辉).Chinese J.Inorg.Chem.(无机化学学报),2013,29 (6):1328-1332
[5]Kamel M M,Ali H I,Anwar M M,et al.Eur.J.Med.Chem., 2010,45:572-580
[6]Sondhi S M,Arya S,Rani R,et al.Med.Chem.Res.,2012 21:3620-3628
[7]Alam M S,Choi J H,Lee D U.Bioorg.Med.Chem.,2012 20:4103-4108
[8]Sunil D,Isloor A M,Shetty P,et al.Med.Chem.Res.,2011 20:1024-1032
[9]Iqbal A,Siddiqui H L,Ashraf C M,et al.Chem.Pharm Bull.,2007,55:1070-1072
[10]Ferrari M B,Capacchi S,Reffo G,et al.J.Inorg.Biochem. 2000,81:89-97
[11]Hearn M,Cynamon M H,Chen M F,et al.Eur.J.Med. Chem.,2009,44:4169-4178
[12]Kucukguzel S G,Mazi A,Sahin F,et al.Eur.J.Med.Chem.,2003,38:1005-1013
[13]Raparti V,Chitre T,Bothara K,et al.Eur.J.Med.Chem.,2009,44:3954-3960
[14]Shi L,Ge H M,Tan S H,et al.Eur.J.Med.Chem.,2007,42:558-564
[15]Zhang M,Xian D M,Li H H,et al.Aust.J.Chem.,2012,65:343-350
[16]Rai N P,Narayanaswamy V K,Govender T,et al.Eur.J. Med.Chem.,2010,45:2677-2682
[17]Rai N P,Narayanaswamy V K,Shashikanth S,et al.Eur.J. Med.Chem.,2009,44:4522-4527
[18]Meletiadis J,Meis J F G M,Mouton J W,et al.J.Clin. Microbiol.,2000,38:2949-2954
[19]SMART and SAINT,Bruker AXS Inc,Madison,2002.
[20]Sheldrick G M.SADABS,University of Göttingen,Germany,1996.
[21]Sheldrick G M.SHELXTL V5.1,Software Reference Manual,Bruker AXS Inc,Madison,1997.
[22]Geary W J.Coord.Chem.Rev.,1971,7:81-122
[23]Bai Y,Dang D B,Cao X,et al.Inorg.Chem.Commun.,2006,9:86-89
[24]Gordon R J,Campbell J,Henderson D K,et al.Chem. Commun.,2008:4801-4803
[25]CHEN Xiao-Hua(陈小华),WU Qiong-Jie(吴琼洁),LIANG Zhi-Yu(梁志瑜),et al.Chinese J.Inorg.Chem.(无机化学学报),2009,25(5):910-914
[26]HU Zhong-Qiu(胡宗球),SHI Shao-Min(施少敏),HE Hong-Wu(贺红武),et al.Chinese J.Inorg.Chem.(无机化学学报),2007,23(2):323-328
中图分类号:O614.121;O614.24+1
文献标识码:A
文章编号:1001-4861(2016)05-0906-07
DOI:10.11862/CJIC.2016.099
收稿日期:2016-01-13。收修改稿日期:2016-03-11。
Syntheses,Crystal Structures and Antimicrobial Activity of Copper(Ⅱ)and Zinc(Ⅱ)Complexes with Aroylhydrazones
HAI Shi-Kun1LOU Shu-Fang1QIU Xiao-Yang*,2
(1Department of public Subject,Shangqiu Medical College,Shangqiu,Henan 476100,China)
(2Engineering Research Center of Functional Material Preparation,Shangqiu Normal University,Shangqiu,Henan 476000,China)
Abstract:A pair of structurally similar dinuclear copper(Ⅱ) and zinc(Ⅱ) complexes,Cu2(L1)2(1)and[Zn2(L2)2(CH3OH)2](2),where L1and L2are the dianionic form of 2-bromo-N′-(2-hydroxy-5-methylbenzylidene)benzohydrazide(H2L1)and 2-chloro-N′-(2-hydroxy-5-methylbenzylidene)benzohydrazide(H2L2),respectively,have been synthesized and characterized by elemental analysis,infrared spectra and single-crystal X-ray diffraction. Complex 1 crystallizes in the triclinic space group P1,with unit cell parameters:a=0.914 11(6)nm,b=1.180 04(7)nm,c=1.359 36(9)nm,α=101.928(2)°,β=91.399(2)°,γ=107.873(2)°,V=1.359 3(2)nm3,Z=2,R1=0.054 0,wR2= 0.118 9,GOF=0.970.Complex 2 crystallizes as the monoclinic space group P21/c,with unit cell parameters:a= 1.216 97(9)nm,b=1.214 96(9)nm,c=1.212 83(9)nm,β=110.939(1)°,V=1.674 8(2)nm3,Z=2,R1=0.034 1,wR2= 0.068 9,GOF=1.024.X-ray analysis indicates that the complexes are centrosymmetric dinuclear species,with the Cu atoms in 1 in square planar coordination,and with the Zn atoms in 2 in square pyramidal coordination.The complexes were evaluated for their antibacterial(B.subtilis,S.aureus,E.coli and P.aeruginosa)and antifungal (C.albicans and A.niger)activities by MTT(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide)method.CCDC:1447054,1;1447056,2.
Keywords:Schiff base;copper complex;zinc complex;dinuclear complex;crystal structure;antimicrobial activity
