具有π…π作用的三维Cu(Ⅱ)配位聚合物的合成结构表征和荧光性质
2016-07-22黄廷洪朱胜兰四川理工学院材料与化工学院自贡643000
黄廷洪 朱胜兰 杨 虎 赵 彬 阳 龑(四川理工学院材料与化工学院,自贡 643000)
具有π…π作用的三维Cu(Ⅱ)配位聚合物的合成结构表征和荧光性质
黄廷洪*朱胜兰杨虎赵彬阳龑
(四川理工学院材料与化工学院,自贡643000)
摘要:合成了2个铜(Ⅱ)的配位聚合物{[Cu4(4-bpo)4(CH3CN)4](BF4)4}n(1)和{[Cu3(4-bpo)2(4,4′-bipy)(CH3CN)6](BF4)3}n(2)(4,4′-bipy=4,4 -bipyridine,4-bpo=2,5-bis(4-pyridyl)-1,3,4-oxadiazole),并对其进行了红外、氢谱、氟谱、硼谱等相关表征,利用X射线单晶衍射仪测定了聚合物的结构。晶体结构研究表明,配位聚合物1通过2,5-二(4-吡啶基)-1,3,4-噁二唑桥连配位作用构筑‘8’形状的结构单元,形成了三维网络结构。引入4,4′-联吡啶配体,导致配合物的结构发生改变:在配合物2中,一维螺旋链通过π…π氢键相互作用组装成不同二维和三维网络结构,说明引入第二配体对改变配合物的超分子结构起关键作用。此外,还研究了配合物的固态发光性能,显示其存在ILCT电荷跃迁。
关键词:铜(Ⅱ);配合物;晶体结构;荧光性质
四川理工学院人才引进项目(No.2014RC29)四川省教育厅重点项目(No.15ZA0223)材料腐蚀与防护四川省重点实验室开放基金项目(No.2015CL03)和精细化工助剂及表面活性剂四川省高校重点实验室开放基金(No.2015JXZ01)资助。
*通信联系人。E-mail:hth_chem@126.com
0 Introduction
In the past two decades,the design and synthesis of supramolecular coordination complexes are of great current interest[1-3],not only because of their intriguing variety of structural features[4-5]but also because of their potential applications in gas adsorption,catalysis,luminescence,electronics and chemistry[6-10].Synthetic strategies for the building of supramolecular structures are very import[11-12]generally based on a good understanding of the connection between the building blocksandthe resulting lattice networks[13-15].In general,the most efficient approach to afford this type of building blocks are mainly based on the selfassemblyoffunctionalinorganicandorganic components[16-17],especially the chemical structure of organic ligands chosen,in which the organic units play an important structural role in the control of the final supramolecular architectures[18-20].Under this direction,awiderangeoforganiclinkerswith different types of functional groups,such as pyridyl,carboxylate and phosphine moiety,have been designed for assemblies of different kinds ofcoordination systems,from infinite 1D polymeric chain to 2D or 3D supramolecular networks[21-24].Moreover,as well known,supramolecularinteractionscontaincoordination bonds and noncovalent interactions[25],such as C-H…π or π…π stacking interactions,and hydrogen bonds,contributing significantly in constructing supramolecular networks[26-27].For this purpose,the introduction ofsecondaryligandscoordinatetometalions effectively provides more variability to build much more complicated and fantastic topologies by the weak interactions[28-29].However,the influential laws in a two-ligand system are not well understood because of the complexity and difficult prediction of the resulting supramolecular structures.Hence,an extensive study needs to be done and deliberately rational design will be undertaken for further understanding of this.
To further study on the effects of secondary ligands on supramolecular architectures of coordination polymers,one main ligand and one secondary ligand were cogitatively selected.The symmetrical pyridyl benzoate ligand,2,5-bis(4-pyridyl)-1,3,4-oxadiazole(4-bpo)was selected as the main ligand.The ligand,4,4′-bipyridine,was selected as the secondary ligands.In this research,one 3D coordination polymer,{[Cu4(4-bpo)4(CH3CN)4](BF4)4}n(1),and one 1D coordination polymers{[Cu3(4-bpo)2(4,4′-bipy)(CH3CN)6](BF4)3}n(2),were obtained,indicating diverse and interesting 1D chain,2D layer and 3D extended supramolecular structures constructed by inter-chain weak interactions(intermolecular π…π interactions and hydrogen bonds).In addition,Solid-state emission spectrum of complex 1 was also investigated.

Scheme 1 Structure of 4-bpo
1 Experimental
1.1General methods and materials
All chemicals were of AR grade and were used as received without further purification.Ligand 2,5-bis (4-pyridyl)-1,3,4-oxadiazole(4-bpo)wasprepared according to references[30].Elemental analyses were performed with a Carlo ERBA 1106 analyzer.IR spectra were recorded as KBr pellets on a Nicolet 6700 spectrometer in the range of 4 000~450 cm-1.1H NMR,11B NMR and19F NMR spectra were recorded on a Bruck 400 spectrometer at 400.15,128.3 and 376.5 MHz,respectively.The luminescent spectrum of complex 1 were measured at room temperature at a FL3-P-TCSPC fluorescence spectrophotometer.
1.2Synthesis of{[Cu4(4-bpo)4(CH3CN)4](BF4)4}n(1)
A mixture of[Cu(CH3CN)4]BF4(0.031 6 g,0.1 mmol)and 4-bpo(0.022 4 g,0.1 mmol)in 5 mL CH3CN/DMF was stirred at room temperature for 0.5 h. The vapor diffusion of diethyl ether into the solution gave yellow block crystals.The complex was obtained by filtration,washed with diethyl ether and dried in vacuo.Yield:63%.Anal.Calcd.for C56H44B4Cu4F16N20O4(%):C,40.46;H,2.67;N,16.85.Found(%):C,39.72;H,2.81;N,16.56.IR(cm-1):3 433(br),3 030 (w),2 922(w),2 253(w),1 617(w),1 565(w),1 539(w),1480(m),1424(s),1058(vs),836(m),744(m),715(m),503(s).1H NMR(CD3SOCD3,25℃,TMS):δ 2.06(12H,-CH3),8.51~11.59 (32H,bipy).19F NMR(CD3SOCD3,25℃,TMS):δ-148.4.11B NMR(CD3SOCD3,25℃,TMS):δ-1.34.
1.3Synthesis of{[Cu3(4-bpo)2(4,4′-bipy)(CH3CN)6](BF4)3}n(2)
A mixture of[Cu(CH3CN)4]BF4(0.047 4 g,0.15 mmol)and 4-bpo(0.022 4 g,0.1 mmol)in 5 mL CH3CN/DMF was stirred at room temperature for 0.5 h and then 4,4′-bipyridine(0.007 8 g,0.05 mmol)was added.The following procedure was similar to the preparation of 1.Yield:38%.Anal.Calcd.for C46H42B3Cu3F12N16O2(%):C,42.43;H,3.25;N,17.21.Found (%):C,40.78;H,3.01;N,15.84.IR(cm-1):3 453 (br),2 921(w),2 268(w),1 620(m),1 567(w),1 540(w),1 480(w),1 422(m),1 059(vs),840(w),748(w),716(w),507(w).1H NMR(CD3SOCD3,25℃,TMS):δ 2.06(18H,-CH3),8.02~9.39(24H,bipy).19F NMR(CD3SOCD3,25℃,TMS):δ-148.2.11B NMR(CD3SOCD3,25℃,TMS):δ-1.32.
1.4X-ray crystallography
Diffraction data for complexes 1 and 2 wer collected on a Bruker APEX CCD diffractomete with graphite monochromated Mo Kα radiation(λ= 0.071 073 nm)using the φ-ω scan technique at room temperature.The crystal structures were solved by direct methods and refined by full-matrix least-square on F2using SHELXTL.All hydrogens were generated geometrically,assigned fixed isotropic thermal para meters,and included in structure factor calculations During the structure refinement of 2,two F atoms o the BF4-anions(F5 and F6)are disordered,but the molecule structure can be determined.The details o the crystal data are ruled out in Table 1,and selected bond lengths and angles for complexes 1 and 2 ar summarized in Table 2.
CCDC:1043969,1;1043970,2.

Table 1 Crystal data and structure refinement details of complexes 1 and 2
2 Results and discussion
2.1Syntheses
The reaction of copper(Ⅱ)salt with 4-bpo in a 11molar ratio afforded three-dimensional coordination polymers{[Cu4(4-bpo)4(CH3CN)4](BF4)4}n(1),while complex 2 is obtained by reaction of copper(Ⅱ) salt with 4-bpo and 4,4′-bipy at the molar ratio of 3∶2∶1. At the room temperature,complexes 1 and 2 are soluble in DMF,DMSO,CH3CN,slightly soluble in CH2Cl2,hardly soluble in CHCl3and toluene.
The IR spectra for 1 and 2 mainly reflect the binding patterns of 4-bpo,4,4′-bipyridine,CH3CN and BF4-moieties.The absorption peak near 1 480 cm-1are δC=N-Nin agreement with complexes containing 4-bpo.The strong peak near 1 058 cm-1is assigned to B-F stretches of BF4-groups,while the absorption peaks near 1 540~1 567 cm-1,in the range of 840~715 cm-1and near 507 cm-1are δC-CH(in the plane),δC-C(in and out the plane)and νCu-p.The absorption peak at 1 617~1 620 cm-1is νC=Nin 1 and 2,while the absorption peak of 2 253~2 268 cm-1is νC≡Nof 1 and 2.The1H NMR spectra of 1 and 2 reveal expected resonances typical for the coordinated 4-bpo and 4,4′-bipyridine.Moreover,the binding pattern of BF4-was also reflected in19F NMR and11B NMR(two singlets near-148.4 and-1.32).
2.2Structural description
2.2.1{[Cu4(4-bpo)4(CH3CN)4](BF4)4}n(1)
Crystallization of 4-bpo with[Cu(CH3CN)4]BF4in theCH3CN/DMFmixedsolventsystematroom temperature afforded the infinite three-dimensional polymeric complex 1 in 63%yield.X-ray diffraction analysis for complex 1 confirms,as shown in Fig.1a and Fig.S1,that the asymmetric unit contains two crystallographically independent Cu(Ⅱ) centers,both of which reside on centers of symmetry.The first kind of Cu(Ⅱ)atom possesses a distorted tetrahedral geometry with the coordination of four N atoms from three 4-bpo ligand and one CH3CN molecule(Fig.1a).The bond distances of Cu-N(0.200 6(4),0.201 0(4),0.202 7(6)and 0.218 2(5)nm)are within the normal ranges[31]and corresponding N-Cu-N bond angle ranges from 79.76(12)°to 112.05(14)°(Table 2).The second kind of Cu(Ⅱ) center lies in a similar distorted tetrahedral {CuN4}environment(Fig.S1)and the average distance of Cu-N is 0.205 8 nm,slightly longer than the corresponding values in the Cu(1)center.

Fig.1 (a)Coordination environment of the Cu(Ⅱ)atoms in 1 with displacement ellipsoids drawn at the 10%probability level;(b)“8”-shaped building unit in 1
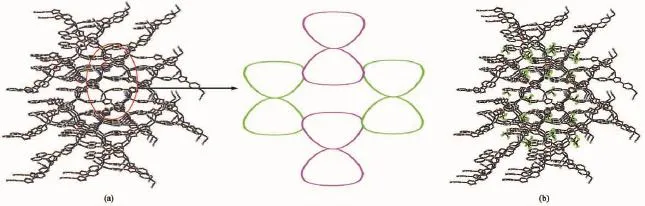
Fig.2 (a)3D network of 1 with displacement ellipsoids drawn at the 10%probability level;(b)Ordered-layer-lattice BF4-in 1 located between 3D networks with displacement ellipsoids drawn at the 10%probability level
In the solid state,4-bpo acts as a tridentate ligand,linking three Cu(Ⅱ)ions using two Npyridyl atoms and one Noxadiazoleatom,while Cu(Ⅱ) centers are linked by both pyridyl and oxadiazole N-donors of 4-bpo ligands into a“8”-shape building unit(Fig.1b).The metal ions are separated by the Cu(Ⅱ)…Cu(Ⅱ) distances o in the range from 0.696 0 to 0.904 0 nm.The“8”-shape building unit interacts with another four neighbouring ones via the 4-bpo-bridged Cu(Ⅱ)coordination polymers,forming a 3D network(Fig.2a).The ordered-layerlattice BF4-locates between these 3D networks(Fig. 2b).In the 3D network,intermolecular B-F…π interactions[32-33]are observed with B…πcentroiddistances from 0.385 to 0.401 nm,F…πcentroiddistances from 0.300 to 0.324 nm and angles from 108°to 124°.The 3D structure also involves π…π interactions,with centroid -to-centroid distances from 0.350 7 to 0.382 9 nm and dihedral angles from 4.92°to 7.11°,and C-H…F hydrogen bonds between BF4-and the 3D network(Table S1).
2.2.2{[Cu3(4-bpo)2(4,4′-bipy)(CH3CN)6](BF4)3}n(2)
Reaction of copper(Ⅱ) salt with 4,4′-bipyridin and 4-bpo in CH3CN/DMF solution gives the infinite one-dimensional polymeric complex 2 in 38%yield Single-crystal analysis shows,as shown in Fig.3,tha there are two different Cu(Ⅱ) centers present in the asymmetric unit.The first Cu(Ⅱ)center adopts distorted tetrahedral geometry constructed by two Npyridyl atoms from 4-bpo and 4,4′-bipyridine and two Nacetonitrileatoms from CH3CN,respectively.The Cu-N bond lengths are in the range of 0.192 2(7)to 0.207 3(4 nm and corresponding N-Cu-N bond angles vary from 101.24(18)°to 122.0(3)°,comparable with those in similar complexes[34-35].The second Cu(Ⅱ)center,on the other hand,lies in a similar distorted tetrahedra coordination environment which consists of four N donors from two 4-bpo ligands and two CH3CN molecules(Fig.3).The bond distances of Cu-N(1.931(6)and 0.207 4(4)nm)and the N-Cu-N bond angles in the range from 100.0(2)°to 109.78(19)°are within the normal ranges[36].
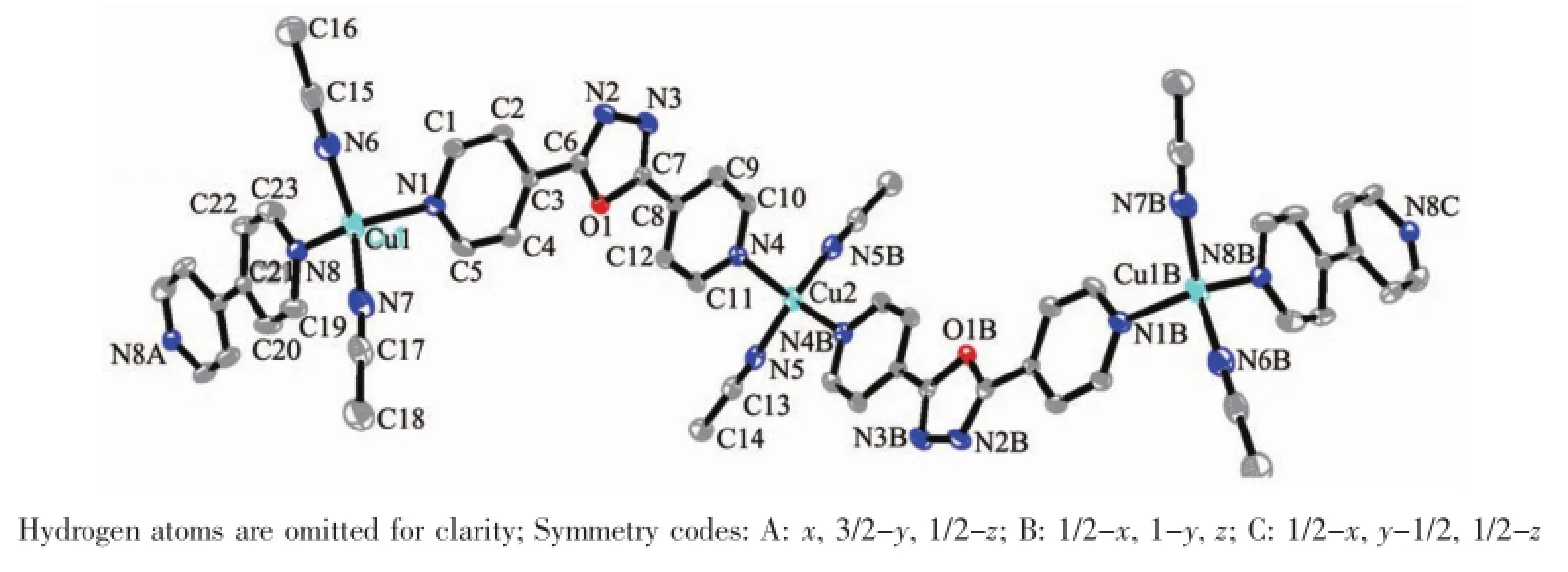
Fig.3 Coordination environments of the Cu(Ⅱ)atoms in 2 with displacement ellipsoids drawn at the 10%probability level

Fig.4 A view of the 1D[Cu3(4-bpo)2(4,4′-bipy)]nchain with displacement ellipsoids drawn at the 10%probability level

Fig.5 (a)Intermolecular π…π interaction of 2 with displacement ellipsoids drawn at the 10%probability level;(b)Infinite 2D multilayer architecture in 2 formed by π…π stacking interaction with displacement ellipsoids drawn at the 10%probability level
In the solid state,the Cu(1)and Cu(2)centers are connected to each other by 4-bpo and 4,4′-bipyridine ligands through both terminal N-donors into one-dimensional(1D)meso-helical chain along the b axis,as shown in Fig.4,which contains two different individual“links”.The metal ions are separated by the Cu…Cu distances of 1.116 6 and 1.386 5 nm,respectively,in 1D meso-helical chain.These onedimensional chains further stack together through intermolecular π… π interactions parallel to the crystallographic bc plane to produce infinite 2D multilayer architecture(Fig.5a and Fig.5b).Intermolecular π…π stacking interactions of 2 display two types of centrosymmetric pairwise embrace.The lower embrace involves two π…π interactions between theoxadiazole and pyridyl rings from two distinct 4-bpo,respectively,andtheplanesofpyridyland oxadiazoleare approximately parallel with a centroid…centroid distance of 0.373 nm and an offset dihedral angle of 3.04°.The short contacts of atom…atom between rings are in the range from 0.361 2 to 0.409 4 nm,which are close to the sums of the van der Waals radii[37],revealing typical π…π stacking interactions. The upper embrace involves two π…π interactions between pyridyl rings from two distinct 4,4′-bipyridine (centroid…centroid distance:0.374 nm,dihedral angle: 12.21°).Neighboring 2D chains of 2 are further connected by the C-H…F hydrogen bonds,with the H…F distances of 0.249 and 0.250 nm,and the C…F distances of 0.306 and 0.340 nm,respectively,leading to the construction of a hydrogen-bonded 3D supra molecular network by the stacking of 2D supra molecular network along the a axis(Fig.6).
2.3Luminescent property
Coordination compounds constructed fromd1metal centers and conjugated organic ligands are promising candidates for hybrid luminescent materials with potential applications such as dye-sensitized solar cells,light-emitting or electrochemical devices As shown in Fig.7,solid-state emission spectrum o complex 1 was recorded at room temperature,showing that excitation of the microcrystalline sample affords fluorescentemissions,withthemaximumpeaks occurring at 445 nm for 1(λex=371 nm).Moreover,the maximal emission of 4-bpo ligands is observed at 417 nm(λex=370 nm)according to references[38].For 1,th very similar profiles of their emission peaks,in comparison with that of the free 4-bpo,indicate tha their photoluminescent mechanism may be properly ascribed to intraligand(IL)π→π*transition[39].

Fig.6 3D network formed by the C-H…F hydrogen bonds in 2 with displacement ellipsoids drawn at the 10%probability level
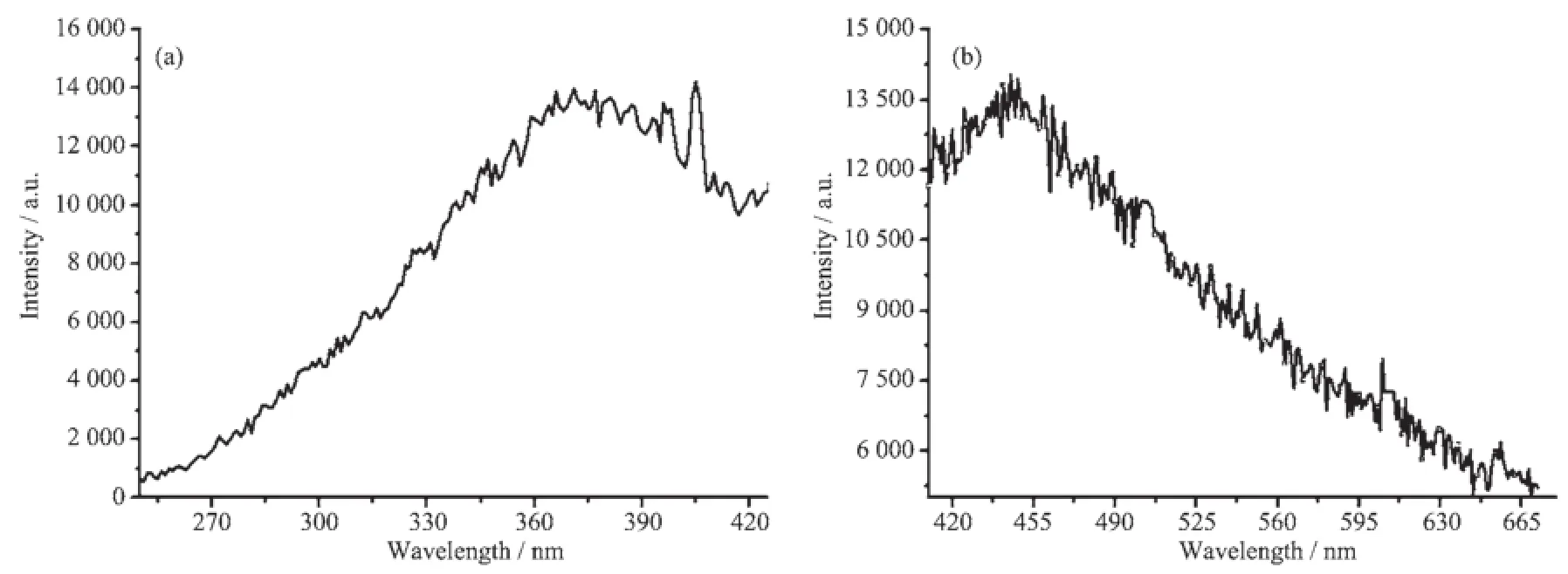
Fig.7 Maximum excitation(a)and emission(b)spectra of compound 1 in the solid state at room temperature
3 Conclusions
In this work,we have presented a new family of coordination polymers formulated as{[Cu4(4-bpo)4(CH3CN)4](BF4)4}n(1)and{[Cu3(4-bpo)2(4,4′-bipyridine)(CH3CN)6](BF4)3}n(2),respectively.These polymers show different supramolecular architectures:1 has a three dimensional(3D)network constructed by“8”-shap building unit interacting 4-bpo with another fou neighboring ones;2 contains 1D meso-helical chain 2D multilayer architecture and 3D network.All these results undoubtedly reveal that the diverse structures of coordination polymers mainly depend on the Cu(Ⅱ)ions with 4-bpo,readily interacting with flexibleangular ligand 4,4′-bipyridine to result in a variety of 1D chains,2D grid-like layers and 3D framework. Moreover,the extended supramolecular networks of the lower-dimensional coordination polymers can be well modified by the secondary interactions such as intermolecular π…πstackinginteractionsandhydrogen bonds.Solid-state emission spectrum of complex 1 was observed at room temperature,showing that its photoluminescentmechanismmaybeproperly attributed to ILCT excited states.
Supporting information is available at http://www.wjhxxb.cn
References:
[1]Chen K,Kang Y S,Zhao Y,et al.J.Am.Chem.Soc.,2014,136(48):16744-16747
[2]Evans N H,Beer P D.Angew.Chem.Int.Ed.,2014,53(44): 11716-11754
[3]Jo M,Seo J,Lindoy L F,et al.Dalton Trans.,2009(31):6096 -6098
[4]An H,Hu Y,Wang L,et al.Cryst.Growth Des.,2014,15(1): 164-175
[5]Daniliuc C,Druckenbrodt C,Hrib C G,et al.Chem.Commun.,2007(20):2060-2062
[6]Chatterjee B,Noveron J C,Resendiz M J E,et al.J.Am. Chem.Soc.,2004,126(34):10645-10656
[7]Huang T H,Yan J,Liu Y F,et al.Aust.J.Chem.,2015,68 (7):1144-1151
[8]Kanoo P,Matsuda R,Kitaura R,et al.Inorg.Chem.,2012,51(17):9141-9143
[9]Huang T H,Yan J,Du H M,et al.J.Coord.Chem.,2015,68:1514-1527
[10]Liu J,Chen L,Cui H,et al.Chem.Soc.Rev.,2014,43(16): 6011-6061
[11]Wijesinghe L P,Lankage B S,Maille G M O,et al.Chem. Commun.,2014,50(73):10637-10640
[12]Rambaran V H,Balof S,Moody L,et al.CrystEngComm,2009,11(4):580-582
[13]Li C P,Yu Q,Chen J,et al.Cryst.Growth Des.,2010,10(6): 2650-2660
[14]Housecroft C E.Dalton Trans.,2014,43(18):6594-6604
[15]Grill L,Dyer M,Lafferentz L,et al.Nat.Nano,2007,2(11): 687-691
[16]Wang X L,Qin C,Wang E B,et al.Chem.Commun.,2005(38):4789-4791
[17]Janiak C.Angew.Chem.Int.Ed,1997,36(13/14):1431-1434
[18]Zhang L,Marzec B,Clerac R,et al.Chem.Commun.,2013,49(1):66-68
[19]Meng Q G,Yan S T,Kong G Q,et al.CrystEngComm,2010,12(3):688-690
[20]Dong Y B,Ma J P,Huang R Q,et al.Dalton Trans.,2003 (8):1472-1479
[21]Shankar S,Balgley R,Lahav M,et al.J.Am.Chem.Soc.,2014,137(1):226-231
[22]Choi C L,Yen Y F,Sung H H Y,et al.J.Mater.Chem.,2011,21(24):8547-8549
[23]Rawe B W,Chun C P,Gates D P.Chem.Sci.,2014,5(12): 4928-4938
[24]Jones M W,Mantovani G,Ryan S M,et al.Chem.Commun.,2009(35):5272-5274
[25]Chen Z,Qin S,Liu D,et al.Cryst.Growth Des.,2013,13(8): 3389-3395
[26]Khavasi H R,Azizpoor Fard M.Cryst.Growth Des.,2010,10 (4):1892-1896
[27]Nishio M,Umezawa Y,Honda K,et al.CrystEngComm,2009,11(9):1757-1788
[28]Bunting P,Chisholm M H,Gallucci J C,et al.J.Am.Chem. Soc.,2011,133(15):5873-5881
[29]Mukhopadhyay U,Choquesillo Lazarte D,Niclos Gutierrez J,et al.CrystEngComm,2004,6(102):627-632
[30]Bentiss F,Lagrenée M.J.Heterocycl.Chem.,1999,36(4): 1029-1032
[31]Tan X,Li L,Zhang J,et al.Chem.Mater.,2011,24(3):480-485
[32]Banerjee S,Dastidar P.CrystEngComm,2013,15(45):9415-9428
[33]Berger R,Resnati G,Metrangolo P,et al.Chem.Soc.Rev.,2011,40(7):3496-3508
[34]Burd S D,Ma S,Perman J A,et al.J.Am.Chem.Soc.,2012,134(8):3663-3666
[35]Zhou X P,Li D,Zheng S L,et al.Inorg.Chem.,2006,45 (18):7119-7125
[36]Zhao H,Qu Z R,Ye Q,et al.Inorg.Chem.,2004,43(6): 1813-1815
[37]Winter M.WebElements Periodic Table(Professional Edition),http://www.webelements.com
[38]Du M,Wang Q,Li C P,et al.Cryst.Growth Des.,2010,10 (7):3285-3296
[39]Crestani M G,Manbeck G F,Brennessel W W,et al.Inorg. Chem.,2011,50(15):7172-7188
中图分类号:O614.121
文献标识码:A
文章编号:1001-4861(2016)05-0871-08
DOI:10.11862/CJIC.2016.115
收稿日期:2015-11-27。收修改稿日期:2016-04-06。
Syntheses,Structural Characterization and Fluorescent Properties of 3D Copper(Ⅱ)Coordination Polymers with Extended π…π Interactions
HUANG Ting-Hong*ZHU Sheng-LanYANG HuZHAO BinYANG Yan
(College of Materials and Chemical Engineering,Sichuan University of Science&Engineering,Zigong,Sichuan 643000,China)
Abstract:Two copper(Ⅱ) coordination polymers,{[Cu4(4-bpo)4(CH3CN)4](BF4)4}n(1)and{[Cu3(4-bpo)2(4,4′-bipy (CH3CN)6](BF4)3}n(2)(4,4′-bipy=4,4′-bipyridine,4-bpo=2,5-bis(4-pyridyl)-1,3,4-oxadiazole),have been synthesized and characterized by IR,1H NMR,19F NMR,11B NMR and X-ray crystal structure analysis.Structural analysi shows that complex 1 contains repeat“8”-shape building units that are linked to each other by the bridgin coordination action of 4-bpo,forming a 3D network.The introduction of 4,4′-bipyridine results in the siz variance of complex 2,which consists of 1D meso-helical chain,2D multilayer architecture and 3D network formed by intermolecular π…π interactions and hydrogen bonds.All these indicate that the change of secondary ligand might be the key of the extended supramolecular networks of the lower-dimensional coordination polymers Moreover,solid-state emission spectrum of complex 1 displays the existence of ILCT excited states.CCDC 1043969,1;1043970,2.
Keywords:copper(Ⅱ)coordination polymers;crystal structure;π-π stacking;fluorescent properties
