β-Conglycinin对不同发育时期鲤鱼消化酶活力的影响
2016-06-03邢秀苹赖红娥杨欢欢吴莉芳
邢秀苹,赖红娥,赵 晗,杨欢欢,吴莉芳,闫 磊
(1 吉林农业大学 动物科技学院,吉林 长春 130118;2 厦门利洋水产科技有限公司,福建 厦门 361012)
β-Conglycinin对不同发育时期鲤鱼消化酶活力的影响
邢秀苹1,赖红娥1,赵晗1,杨欢欢2,吴莉芳1,闫磊1
(1 吉林农业大学 动物科技学院,吉林 长春 130118;2 厦门利洋水产科技有限公司,福建 厦门 361012)
[摘要]【目的】 研究β-伴大豆球蛋白(β-Conglycinin)对鲤幼鱼、稚鱼蛋白酶和淀粉酶活力的影响。【方法】 以初始体质量为(10.06±0.14) g/尾的鲤稚鱼和(110.23±0.23) g/尾的鲤幼鱼为研究对象,以鱼粉为动物蛋白源,面粉、糊精为糖源,混合油脂(m(鱼油)∶m(玉米油)=1∶1)为脂肪源,分别配制5种等氮(鲤幼鱼和稚鱼粗蛋白质量分数分别为36%和40%)、等能(鲤幼鱼和稚鱼总能分别是15.2和16.9 MJ/kg) 的半精制饲料,其β-Conglycinin的添加量(质量分数)分别为0(CK),2.0%,4.0%,6.0%和8.0%,每组饲料设3个重复,在控温单循环养殖系统中进行为期8周的饲养试验,试验结束后,取鲤幼鱼、稚鱼的前、中、后肠道和肝胰脏,分别用福林-酚试剂法和淀粉酶试剂盒法,测定肠道和肝胰脏蛋白酶及淀粉酶的活力。【结果】 鲤幼鱼肝胰脏蛋白酶活力各组之间差异不显著(P>0.05);β-Conglycinin 添加量为6.0%和8.0%组的前肠、中肠蛋白酶活力显著低于对照组(P<0.05);而β-Conglycinin添加量为8.0%组后肠蛋白酶活力显著低于对照组(P<0.05)。在鲤稚鱼肝胰脏和后肠,β-Conglycinin添加量为8.0%组的蛋白酶活力显著低于对照组(P<0.05);前肠蛋白酶活力则以2.0%,4.0%,6.0%和8.0%添加组显著低于对照组(P<0.05);中肠蛋白酶活力为4.0%,6.0%和8.0%添加组显著低于对照组(P<0.05)。 β-Conglycinin对鲤幼鱼和稚鱼肝胰脏、前肠、中肠及后肠淀粉酶活力均无显著影响(P>0.05)。【结论】 鲤幼鱼配合饲料中β-Conglycinin的添加量不应超过6.0%;鲤稚鱼配合饲料中β-Conglycinin的添加量不应超过2.0%。
[关键词]β-伴大豆球蛋白;鲤;幼鱼;稚鱼;蛋白酶;淀粉酶
随着集约化水产养殖的发展,鱼粉资源短缺,寻求鱼粉蛋白源替代品已成为国际性研究课题。大豆蛋白源是水产饲料应用最多的植物蛋白源之一。目前,国内外学者在大豆蛋白源替代鱼粉方面做了许多研究,涉及的鱼类主要有虹鳟(Oncorhynchusmykiss)[1]、金头鳟(SparusaurataL.)[2]、大西洋鲑(SalmosalarL.)[3]、杂交罗非鱼(Oreochromisniloticus×O.aureus)[4]、草鱼(Ctenopharyngondonidellus)[5]、大黄鱼(PseudoscjaenacroceaR.)[6]、异育银鲫 (Carassiusauratusgibelio)[7]、齐口裂腹鱼(Schizothoraxprenanti)[8]、埃及胡子鲇(Clariaslazera)[9]、鲤鱼(Cyprinuscarpio)[10]等,研究的内容多集中在大豆蛋白源替代鱼粉蛋白后对鱼类摄食、消化、生长、健康等的影响。这些研究表明,以过量的大豆蛋白替代鱼粉蛋白,不仅会影响鱼类肠上皮细胞增生及肠道组织形态,而且会影响鱼类对饲料的消化及生长性能。其主要原因是大豆蛋白中含有多种抗营养因子,其中大豆抗原蛋白是大豆中主要的抗营养因子之一。β-伴大豆球蛋白(β-Conglycinin)比大豆球蛋白(Glycinin)具有更强的抗原性[11],普通的热处理不能灭活β-伴大豆球蛋白的免疫活性,其能够引起鱼类消化道过敏,造成胃、肠道损伤,进而引起消化吸收障碍,甚至死亡。但由于大豆抗原蛋白具有蛋白含量高、价格低廉、来源丰富等优点,因此国内外学者就大豆抗原蛋白对动物的影响进行了广泛研究。目前,关于大豆抗原蛋白的研究主要集中在猪[12]、犊牛[13]、鼠[14]、羔羊[15]等陆生动物上,而对水产动物研究报道较少,仅见郭林英[16]研究了大豆β-伴球蛋白提取物对鲤鱼肠上皮细胞增殖及其功能的影响。鱼类消化道的消化酶是影响饲料消化吸收的主要因素,消化酶受体内外多种因素的影响。本研究分别以鲤幼鱼和稚鱼为供试动物,研究了β-Conglycinin对不同发育时期鲤鱼消化酶活力的影响,旨在为合理开发利用大豆蛋白源及大豆抗原蛋白的去除提供依据。
1材料与方法
1.1β-Conglycinin的分离纯化
β-Conglycinin采用简化膜中间试验方法[17]获得。
1.2试验饲料
以鱼粉为动物蛋白源,面粉、糊精为糖源,混合油脂(m(鱼油)∶m(玉米油)=1∶1)为脂肪源,分别配制5种等氮(鲤幼鱼和稚鱼的蛋白质量分数分别为36%和40%)、等能(鲤幼鱼和稚鱼总能分别为15.2和16.9 MJ/kg) 的半精制饲料,β-Conglycinin的添加量(质量分数)分别为0(对照,CK),2.0%,4.0%,6.0%和8.0%。各原料粉碎后过孔径0.246 mm的筛,按配方准确称其质量,在吉林农业大学动物水产实验室用电动绞肉机制成粒径1.5 和2.5 mm颗粒饲料。晒干后置于-20 ℃冰箱中保存备用。鲤幼鱼和稚鱼的试验饲料组成及营养成分见表1和表2。

表 1 鲤幼鱼饲料配方及营养水平(风干基础)

表 2 鲤稚鱼饲料配方及营养水平(风干基础)
1.3饲养条件及管理
养殖试验在吉林农业大学控温单循环系统中进行,试验期间连续充气,水中氨氮质量浓度低于0.5 mg/L,溶解氧高于5.0 mg/L,温度为25~27 ℃,养殖试验持续8周。
试验鱼来源于吉林省九台市渔场,试验前饱食投喂对照组饲料,预饲15 d,预饲试验结束后,饥饿24 h,挑选鳍鳞完整、规格整齐、体质健壮的鲤幼鱼((110.23±0.23) g/尾) 300尾和鲤稚鱼((10.06±0.14) g/尾) 450尾,分别随机放养在15个玻璃缸中,鲤幼鱼每缸放养20尾,鲤稚鱼每缸放养30尾。放养前用质量浓度为20 mg/L的高锰酸钾溶液药浴10 min,随机安排3个玻璃缸为一个试验组。在试验过程中,每天称取足量饲料,分2次投喂(09:00,16:00),投饵方式为人工手撒,直至鱼不再到水面摄食为止,日投饵率为体质量的3%~5%,每天记录每缸鱼的摄食饲料质量。
1.4样品的收集与粗酶液的制备
参照吴莉芳等[18]的方法进行样品收集与粗酶液制备。(1)样品的收集。饲养试验结束前停食24 h后,每缸活体解剖10尾鱼,取出肝胰脏和其他内脏,称其质量(精确到0.01 g)。取出肠道和肝胰脏,剔除附着物,用去离子水冲洗肠道内容物,滤纸吸干,-20 ℃冰柜保存待测。肠道从第一个回折点以前为前肠,最后一个回折点以后为后肠,其间为中肠。(2)粗酶液的制备。称样品质量,加入10倍体积的高纯水匀浆,在4 ℃冰箱中静置过夜,5 000 r/min离心10 min,取上清液即为粗酶液,4 ℃冰箱保存、待测。粗酶液需在24 h内测定完毕。
1.5消化酶活力的测定
蛋白酶活力采用福林-酚试剂法(Folin-phenol)测定[18];淀粉酶活力采用淀粉酶试剂盒(南京建成科技有限公司)测定[18]。
1.6数据统计分析
采用SPSS17.5软件对鲤幼鱼、稚鱼蛋白酶及淀粉酶活力进行方差分析,若差异显著,进一步进行LSD和Duncan’s多重比较,分析组间差异显著性。试验数据用“平均值±标准差”(Mean±SD)表示。显著性水平设定为P<0.05。
2结果与分析
2.1β-Conglycinin对鲤幼鱼和稚鱼蛋白酶活力的影响
β-Conglycinin对鲤幼鱼、稚鱼蛋白酶活力的影响分别见表3和表4。

表 3 β-Conglycinin对鲤幼鱼蛋白酶活力的影响
注:同列数据后标不同小写字母表示差异显著(P<0.05)。下表同。
Note:Different lowercase letters in each column indicate significant difference(P<0.05).The same below.
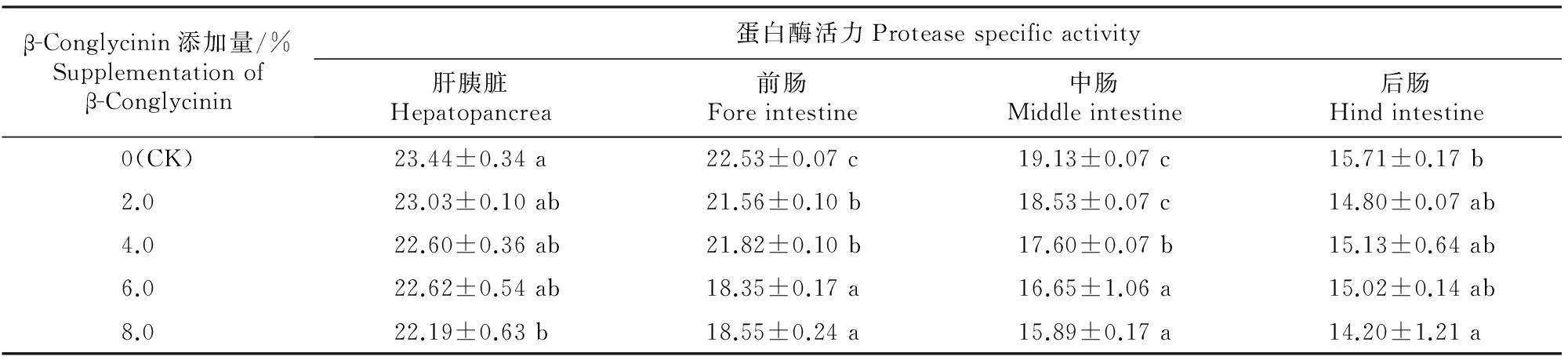
表 4 β-Conglycinin对鲤稚鱼蛋白酶活力的影响
由表3可以看出,鲤幼鱼肝胰脏蛋白酶活力各组之间差异不显著(P>0.05)。2.0%和4.0% β-Conglycinin 添加组前肠、中肠蛋白酶活力与对照组差异不显著(P>0.05),6.0%和8.0% β-Conglycinin添加组前肠、中肠蛋白酶活力显著低于对照组(P<0.05);8.0% β-Conglycinin添加组前肠蛋白酶活力显著低于2.0%,4.0%和6.0%添加组(P<0.05),6.0% β-Conglycinin添加组前肠蛋白酶活力显著低于2.0%和4.0%添加组(P<0.05);6.0%和8.0%β-Conglycinin添加组中肠蛋白酶活力显著低于2.0%及4.0%添加组(P<0.05)。2.0%,4.0%,6.0%β-Conglycinin添加组后肠蛋白酶活力与对照组差异不显著(P>0.05),8.0%添加组后肠蛋白酶活力显著低于对照组(P<0.05);2.0%,4.0%,6.0%和8.0%添加组之间后肠蛋白酶活力无显著差异(P>0.05)。
由表4可知,在鲤稚鱼肝胰脏和后肠中,2.0%, 4.0%和6.0% β-Conglycinin添加组蛋白酶活力与对照组差异不显著(P>0.05),8.0%β-Conglycinin添加组蛋白酶活力显著低于对照组(P<0.05),2.0%,4.0%,6.0%和8.0%β-Conglycinin添加组之间蛋白酶活力无显著差异(P>0.05);在鲤稚鱼前肠中,2.0%, 4.0%,6.0%和8.0% β-Conglycinin添加组蛋白酶活力显著低于对照组(P<0.05),2.0%和4.0% β-Conglycinin添加组蛋白酶活力显著高于6.0%及8.0%添加组(P<0.05);在鲤稚鱼中肠中,2.0% β-Conglycinin添加组蛋白酶活力与对照组差异不显著(P>0.05) , 而4.0%,6.0%和8.0%β-Conglycinin添加组蛋白酶活力显著低于对照组(P<0.05),2.0%β-Conglycinin添加组蛋白酶活力显著高于4.0%,6.0%和8.0%添加组(P<0.05),4.0%β-Conglycinin添加组蛋白酶活力显著高于6.0%和8.0%添加组(P<0.05)。
2.2β-Conglycinin对鲤幼鱼和稚鱼淀粉酶活力的影响
表5和表6表明,在本试验条件下,2.0%, 4.0%,6.0%和8.0%β-Conglycinin添加组鲤幼鱼与稚鱼肝胰脏、前肠、中肠、后肠淀粉酶活力与对照组差异均不显著(P>0.05)。

表 5 β-Conglycinin对鲤幼鱼淀粉酶活力的影响

表 6 β-Conglycinin对鲤稚鱼淀粉酶活力的影响
3讨论
3.1β-Conglycinin对鲤幼鱼和稚鱼蛋白酶活力的影响
本研究结果表明,β-Conglycinin对鲤幼鱼和稚鱼的前肠、中肠、后肠蛋白酶活力的影响存在一定的差异。在鲤幼鱼的配合饲料中,β-Conglycinin添加量为6.0%和8.0%组前肠、中肠蛋白酶活力显著低于对照组(P<0.05);在鲤稚鱼的配合饲料中,β-Conglycinin 添加量为2.0%,4.0%,6.0%和 8.0% 组前肠蛋白酶活力显著低于对照组(P<0.05),而中肠蛋白酶活力则以4.0%,6.0%和8.0% β-Conglycinin添加组显著低于对照组(P<0.05)。这可能是由于鲤幼鱼和稚鱼消化道结构发育程度不同,对β-Conglycinin的敏感性不同所致。鲤稚鱼消化系统发育尚不成熟,消化器官不发达,消化机能不完善,消化道中酶的分泌量不足,使大量未消化的营养物质进入了肠道。因此,β-Conglycinin也可大量进入肠道,引起肠道损伤,从而导致消化酶活力降低。张帆等[6]研究了饲料中豆粕替代鱼粉对大黄鱼消化酶活性的影响,结果表明,大黄鱼肠道胰蛋白酶的活性随豆粕替代水平的升高而显著降低。Burrells等[19]研究表明,在饲料中添加一定量的大豆蛋白,会引起虹鳟的后肠结构形态变化,并降低刷状缘的酶活性。Krogdahl等[20]研究发现,豆粕能够引起虹鳟中肠与后肠上皮刷状缘胞外酶碱性磷酸酶、亮氨酸氨肽酶以及麦芽糖酶、乳糖酶、蔗糖酶活性下降。吴莉芳等[9]研究了去皮豆粕替代鱼粉对埃及胡子鲇消化酶活力的影响,结果表明,当去皮豆粕替代鱼粉蛋白的45%和60%时,埃及胡子鲇前肠和后肠的蛋白酶活力显著下降。Ksudhik 等[21]在大西洋鲑的饲料中添加一定量的大豆蛋白,引起其后肠结构发生形态变化,刷状缘的酶活性降低。关于不同添加量的β-Conglycinin引起不同发育时期鲤鱼肠道组织结构的变化,需进一步通过组织学方法进行研究。
3.2β-Conglycinin对鲤幼鱼和稚鱼淀粉酶活力的影响
鱼类的淀粉酶是碳水化合物水解酶类的一种,活性较低,同种鱼类不同消化器官淀粉酶的活力不同,另外随着鱼类年龄的增加,其淀粉酶活力也发生改变,淀粉酶活性在同一消化器官不同部位也会有所差异。彭翔等[22]在黑鲷鱼饲料中用发酵豆粕替代0~50%的鱼粉蛋白质,研究结果表明,饲料中各组淀粉酶的活性差异不显著。吴莉芳等[23]研究了不同大豆蛋白源替代鱼粉对鲤鱼蛋白酶和淀粉酶活力的影响,结果表明,不同大豆蛋白源对鲤鱼淀粉酶活力影响不显著。钱曦等[24]研究报道,在翘嘴红鲌的饲料中,当豆粕替代鱼粉蛋白的13%和27%时,对其肝胰脏和肠道淀粉酶活力影响不显著。在本试验条件下,β-Conglycinin 的添加量对鲤幼鱼和稚鱼肝胰脏、前肠、中肠、后肠淀粉酶活力影响均不显著。这主要是由于鱼类的淀粉酶对食物类型和饲料组成有明显的适应性[25]。鲤鱼属于杂食性鱼类,在天然的食谱中存在一定量的植物蛋白源,而β-Conglycinin就属于植物蛋白源。因此,鲤鱼肝胰脏和肠道淀粉酶对β-Conglycinin具有一定的适应性。
[参考文献]
[1]Venold F F,Penn M H,Krogdahl Å,et al.Severity of soybean meal induced distal intestinal inflammation,enterocyte proliferation rate,and fatty acid binding protein (Fabp2) level differ between strains of rainbow trout (Oncorhynchusmykiss) [J].Aquaculture,2012,264:281-292.
[2]Kokou F,Rigos G,Henry M,et al.Growth performance,feed utilization and non-specific immune response of gilthead sea bream (SparusaurataL.) fed graded levels of a bioprocessed soybean meal [J].Aquaculture,2012,364:74-81.
[3]Bakke-Mckellep A M,Sanden M, Danieli A,et al.Atlantic sal-mon (SalmosalarL.)Parr fed genetically modified soybeans and maize:Histological,digestive,metabolic,and immunological investigations [J].Research in Veterinary Science,2008,84:395-408.
[4]Lin S M,Luo L.Effects of different levels of soybean meal inclusion in replacement forfish meal on growth,digestive enzymes and transaminase activities inpractical diets for juvenile tilapia,Oreochromisniloticus×O.aureus[J].Animal Feed Science and Technology,2011,168:80-87 .
[5]吴莉芳,王洪鹤,张东鸣,等.饲料中大豆蛋白对草鱼生长及饲料利用的影响 [J].华南农业大学学报,2009,30(2):78-81.
Wu L F,Wang H H,Zhang D M,et al.Effects of different levels of dietary soybean protein on growth performance and feed utilization in grass carp (Ctenopharyngondonidellus) [J].Journal of South China Agricultural University,2009,30(2):78-81.(in Chinese)
[6]张帆,张文兵,麦康森,等.饲料中豆粕替代鱼粉对大黄鱼生长、消化酶活性和消化道组织学的影响 [J].中国海洋大学学报,2012,42(Sup.):75-82.
Zhang F,Zhang W B,Mai K S,et al.Effects of replacement of dietary fish meal by soybean meal on growth,digestive enzyme activity and digestive tract histology of Juvenile Large Yellow Croaker,PseudosciaenacroceaR. [J].Periodical of Ocean University of China,2012,42(Sup.):75-82.(in Chinese)
[7]王崇,雷武,解绶启,等.饲料中豆粕替代鱼粉蛋白对异育银鲫生长、代谢及免疫功能的影响 [J].水生生物学报,2009,33(4):740-747.
Wang C,Lei W,Xie S Q,et al.Effect of dietary replacement of fishmeal protein by soybean meal protein on growth performance,metabolism and immuniny of gibel Carp (Carassiusauratusgielio) [J].Acti Hydrobiologica Sinica,2009,33(4):740-747.(in Chinese)
[8]向枭,周兴华,陈建,等.饲料豆粕蛋白替代鱼粉蛋白对齐口裂腹鱼生长性能、体成分及血液生化指标的影响 [J].水产学报,2012,36(5):723-731.
Xiang X,Zhou X H,Chen J,et al.Effect of dietary replacement of fish meal protein with soybean meal protein on the growth,body composition and hematology indices ofSchizothoraxprenanti[J].Journal of Fisheries of China,2012,36(5):723-731.(in Chinese)
[9]吴莉芳,秦贵信,孙泽威,等.饲料中去皮豆粕替代鱼粉对埃及胡子鲇消化酶活力和肠道组织的影响 [J].中山大学学报:自然科学版,2010,49(4):99-105.
Wu L F,Qin G X,Sun Z W,et al.Effect of dietary dehulled soyabean meal replacing fish meal on the activity of digestive enzyme and the ineestinal tissue ofClariaslazera[J].Acta Scientiarum Naturalium Universitatis Sunyatseni,2010,49(4):99-105.(in Chinese)
[10]张锦秀,周小秋,倪学勤,等.分离大豆蛋白对幼建鲤生长及肠道的影响 [J].水产学报,2008,32(1):84-89.
Zhang J X,Zhou X Q,Ni X Q,et al.Effects of soybean protein isolate on growth performance and intestine ofCyprinuscarpiovar.jian juveniles [J].Journal of Fisheries of China,2008,32(1):84-89.(in Chinese)
[11]Ogawa T,Bando N,Tsuji H,et al.Alpha-subunit of beta-conglycinin,an allergenicprotein recognized by IgE antibodies of soybean-sensitive patients with atopic dermatitis [J].Biosci Biotechnol Biochem,1995,59:831-833.
[12]Zhao Y,Qin G X,Sun Z W,et al.Effects of glycinin and β-conglycinin on enterocyte apoptosis,proliferation and migration of piglets [J].Food and Agricultural Immunology,2012,21(3):209-218.
[13]Seegraber J,Morrill J L.Effect of protein source in calf milk replacers on morphology and absorptive ability of the small intestine [J].Journal of Dairy Science,1986,69(2):460-469.
[14]Christensen H R,Susanne W B,Frokiaer H.Antigenic specificity of serum antibodies in mice fed soy protein [J].International Archives of Allergy and Applied Immunology,2003,132(1):58-67.
[15]Johnston C.Effect of injecting lambs with soy flour extract on serum soy protein antibody concentration and rate of gain [J].Small Ruminant Research,1996,21(2):149-154.
[16]郭林英.大豆β-伴球蛋白提取物对鲤鱼肠上皮细胞增殖及其功能的影响 [D].四川雅安:四川农业大学,2006.
Guo L Y.Effects of soybean β-conglycinin extract on the proliferation and function of carp intestinal-epithelial cells in primary culture [D].Ya’an, Sichuan:Sichuan Agricultural University,2006.(in Chinese)
[17]Wu S W,Murphy P A,Johnsno L A,et al.Simplified process for soybean glycinin and β-conglycinin fractionation [J].J Agric Food Chem,2000,48:2702-2708.
[18]吴莉芳,赖红娥,杨婳,等.大豆抗原蛋白Glycinin对鲤稚鱼、幼鱼蛋白酶和淀粉酶的影响 [J].西北农林科技大学学报:自然科学版,2013,41(12):30-36.
Wu L F,Lai H E,Yang H,et al.Effects of soybean antigen protein glycinin on the activities of protease and amylase in the larval and juvenile common carp [J].Journal of Northwest A&F University:Natural Science Edition,2013,41(12):30-36.(in Chinese)
[19]Burrells C P D,Williams P J,Southgate V O,et al.Immunological,physiological and pathological response of rainbow trout (Oncorhynchusmykiss)to increasing dietary concentrations of soybean proteins [J].Vet Immunol Immunopathol,1999,72:277-288.
[20]Krogdahl Å,Bakke-mckellep A M,Baeverfjord G.Effects of graded levels of standard soybean meal on intestinal structure mucosal enzyme activities,and pancreatic response in atlantic salmon(SalmosalarL.) [J].Aquaculture Nutr,2003,9:361-371.
[21]Ksudhik S J,Coves D,Dutto G.Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost,the European seabass,Dicentearchuslabrax[J].Aquaculture,2004,230:391-404.
[22]彭翔,宋文新,周凡,等.发酵豆粕替代鱼粉对黑鲷胃肠道和血清指标的影响 [J].江苏农业学报,2012,28(5):1096-1103.
Peng X,Song W X,Zhou F,et al.Effects of fermented soybean meal replacing fish meal on gastrointestinal tract and serum indexes in black sea bream [J].Jiangsu J of Agr Sci,2012,28(5):1096-1103.(in Chinese)
[23]吴莉芳,赵晗,秦贵信,等.2种大豆蛋白替代鱼粉蛋白对鲤鱼蛋白酶和淀粉酶活力的影响 [J].吉林农业大学学报,2011,33(2):222-226.
Wu L F,Zhao H,Qin G X,et al.Effects of replacement of fish meal with two soybean protein sources on the activities of protease and amylase inCyprinuscarpio[J].Journal of Jilin Agricultural University,2011,33(2):222-226.(in Chinese)
[24]钱曦,王桂芹,周洪琪,等.饲料蛋白水平及豆粕替代鱼粉比例对翘嘴红鲌消化酶活力的影响 [J].动物营养学报,2007,19(2):182-187.
Qian X,Wang G Q,Zhou H Q,et al.Effect of dietary protein on the activities of digestive enzymes of topmouth culter(ErythroculterIliishaeformisBleeker) [J].Chinese Journal of Animal Nutrition,2007,19(2):182-187.(in Chinese)
[25]Das K M,Tripathi S D.Studies on the digestive enzymes of grasscarp,Ctenopharyngodonidella(Val) [J].Aquaculture,1991,92:21-32.
Effects of β-Conglycinin on activities of protease and amylase in juvenile and larval common carps
XING Xiu-ping1,LAI Hong-e1,ZHAO Han1,YANG Huan-huan2,WU Li-fang1,YAN Lei1
(1FacultyofAnimalScienceandTechnology,JilinAgriculturalUniversity,Changchun,Jilin130118,China;2LiyangXiamenAquaticTechnologyCompanyLimited,Xiamen,Fujian361012,China)
Abstract:【Objective】 The research investigated the effects of β-Conglycinin on activities of protease and amylase in juvenile and larval common carps.【Method】 Larval and juvenile common carps with the initial weights of (10.06±0.14) g/tail and (110.23±0.23) g/tail were used as experimental objects for eight-week feeding trial at controlled temperature in single recirculating system.Five diets with identical nitrogen (total crude protein contents for juvenile and larval were 36% and 40%,respectively) and energy (total energies for juvenile and larval were 15.2 and 16.9 MJ/kg,respectively) as well as different β-Conglycinin contents (0(CK),2.0%,4.0%,6.0%,and 8.0%) were provided.Fish meal was animal protein source,dextrin and flour were carbohydrate source,and mixed oil (m(corn oil)∶m(fish oil)=1∶1) was fat source.Each group had three repetitions.The activities of protease and amylase in foregut,midgut and hepatopancreas were detected using Folin-phenol method and Amylase kit.【Result】 No significant effects of β-Conglycinin on activities of protease in hepatopancreas of juvenile common carps were observed (P>0.05).The activities of protease in foregut and midgut of juvenile common carps in 6.0% and 8.0% groups were significantly lower than that of the control group (P<0.05),and that in hindgut of 8.0% group were significantly lower than that of the control group (P<0.05).The activities of protease in hepatopancreas and hindgut of larval common carps in 8.0% groups were significantly lower than that of the control group (P<0.05),that of 2.0%,4.0%,6.0%,and 8.0% groups were significantly lower (P<0.05) in foregut,and that of 4.0%,6.0%,and 8.0% groups were significantly lower (P<0.05) in midgut.In addition,no significant effects of β-Conglycinin on activities of amylase in hepatopancreas and tract of both juvenile and larval common carps were observed (P>0.05).【Conclusion】 The amount of β-Conglycinin should be less than 6.0% and 2.0% in the diet of juvenile and larval common carp,respectively.
Key words:β-Conglycinin;Cyprinus carpio;juvenile common carp;larval common carp;protease;amylase
[文章编号]1671-9387(2016)01-0019-06
[中图分类号]S965.116.31+2
[文献标志码]A
[作者简介]邢秀苹(1989-),女,吉林农安人,在读硕士,主要从事水产动物营养与饲料研究。E-mail:461934406@qq.com[通信作者]吴莉芳(1970-),女,吉林农安人,教授,博士,主要从事水产动物营养与饲料研究。
[基金项目]吉林省教育厅项目(2012043)
[收稿日期]2014-04-10
DOI:网络出版时间:2015-12-0214:2510.13207/j.cnki.jnwafu.2016.01.004
网络出版地址:http://www.cnki.net/kcms/detail/61.1390.S.20151202.1425.008.html
