生物材料在心脏修复和再生中的应用
2016-06-01ZhiCuiBaofengYangRenKeLi
Zhi Cui, Baofeng Yang, Ren-Ke Li,*
aInstitute of Medical Science & Department of Surgery, Division of Cardiovascular Surgery, University of Toronto, Toronto, ON M5G 2M9, CanadabDepartment of Pharmacology, College of Pharmacy, Harbin Medical University, Harbin 150001, China
cDivision of Cardiovascular Surgery, Toronto General Research Institute, University Health Network, Toronto, ON M5G 1L7, Canada
生物材料在心脏修复和再生中的应用
Zhi Cuia,c, Baofeng Yangb, Ren-Ke Lia,c,*
aInstitute of Medical Science & Department of Surgery, Division of Cardiovascular Surgery, University of Toronto, Toronto, ON M5G 2M9, CanadabDepartment of Pharmacology, College of Pharmacy, Harbin Medical University, Harbin 150001, China
cDivision of Cardiovascular Surgery, Toronto General Research Institute, University Health Network, Toronto, ON M5G 1L7, Canada
a r t i c l e i n f o
Article history:
Received 24 November 2015
Revised 7 March 2016
Accepted 9 March 2016
Available online 31 March 2016
心肌梗死
心脏再生
生物材料
组织工程
干细胞
心血管疾病是全世界主要致死原因之一。人们对新的干预性治疗手段的需求与日俱增。虽然药物和手术治疗极大地改善了心血管疾病患者的生活质量,但人们还是需要价格更便宜、副作用更小的治疗手段。天然和合成生物材料无论是作为给药载体,还是替代支架的细胞外基质,在心脏修复和再生中都展现出巨大的潜力。本文探讨了目前治疗心血管疾病的几种方式和应用于上述疾病干预性治疗的生物材料;着重研究了导电聚合物在纠正局部缺血性心脏病引发的传导异常及其在心脏起搏器中应用的可能性,以改善心肌梗死状态下的传导路径。
© 2016 THE AUTHORS.Published by Elsevier LTD on behalf of Chinese Academy of Engineering and Higher Education Press Limited Company.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.引言
根据加 拿大公共卫生局发布的报告《加拿大疾病经济负担》表明,2008年加拿大与心血管疾病相关的治疗、医院护理和药物成本为117亿加元,成为医疗系统最大的经济负担[1]。同样,心血管疾病在世界范围内也是主要的致死原因,严重影响了人们的生活质量[2]。因为心脏自身修复能力非常有限,所以一些常见的心血管疾病目前仍然无法得到完全治愈,如心肌梗死和心律失常。药物干预、冠状动脉旁路移植术和心室辅助装置等治疗方法可以显著改善患者的生活质量,延长他们的寿命[3-5]。然而,未来仍然需要新的、低成本且高效的治疗干预手段。
过去几十年里,再生医学治疗心血管疾病的研究逐渐兴起。此外,近几年基因和分子医学的发展为治疗心血管疾病确立了个性化治疗的概念[6]。人体诱导性多能干细胞(iPSC)的出现为再生医学的发展开启了新的篇章,因其可能成为心脏修复中心肌细胞的潜在来源[7]。事实上,人体iPSC源性心肌细胞已通过组织工程方法培养成功,并在植入患有心肌梗死的大鼠心脏组织后显著提升了心脏功能[8]。iPSC源性心肌细胞已被用于研究心脏离子通道病变和心律失常的体内发病机制[9]。然而,移植干细胞存活能力较差仍然是心脏再生的主要障碍。
生物材料能够被独立传输,也可作为支架、细胞或生长因子的载体[10-12],因此可以改善上述问题。天然和合成生物材料的治疗潜力均已在心肌梗死的动物模型和外周动脉疾病(PAD)的治疗中得到证实[13]。特别是可注射生物材料的应用,使左心室壁扩张减少、新生血管增多, 通过内源性干细胞募集增强组织修复,并达到保护心脏功能的效果[12,14]。
本文关注的是引发传导异常的心血管疾病及其目前的干预治疗手段,心血管研究中所应用的生物材料的类型,以及这些生物材料用于干预性治疗的潜力。目前的研究主要集中在如何提高心脏功能和心脏组织再生,而在评估导电聚合物用于纠正心脏活动相关的心律失常以及体内传导阻滞的潜力方面的研究相对较少。本文着重探讨将导电聚合物用于纠正心律失常和提高梗死心肌的传导能力,同时分析未来将导电聚合物从基础研究应用到临床治疗中所面临的挑战。
2.受损心脏再生
2.1.细胞疗法治疗缺血性心脏病
冠状动脉粥样硬化性心脏病(CAD)是最常见的心脏病之一,也是世界上主要的致死原因之一[2]。冠状动脉为心脏组织提供含氧血和营养。CAD的发生是由于在动脉内壁上含胆固醇的斑块沉积,导致血管壁增厚、血管腔变窄,进而导致血流量减少,造成心肌细胞缺血性损伤。心肌供血量减少的患 者会感到胸痛(心绞痛)、气短且活动能力受限。而血管完全阻塞会导致心肌梗死,造成心肌细胞坏死和大量损失[15]。目前针对局部缺血性心脏病的标准干预疗法主要是改变生活方式、药物干预和手术治疗[16]。所有可用治疗方案都无效的患者会逐步发展至心脏衰竭,这种情况需要进行心脏移植。
细胞疗法(向动物或患者体内注射细胞)成为治疗心肌梗死的一种潜在策略。研究者提出并研究了多种备选细胞类型,如骨髓单核细胞[17,18]、胚胎干细胞[19,20]、成肌细胞[21,22]、内皮祖细胞[23]和心脏干细胞[24,25]。虽然在动物和人类研究中都观察到细胞疗法有助于改善心脏功能[26-28],但仍然存在一些问题,如较低的细胞保留/移植率、细胞传输效率问题、机电一体化问题和长期安全问题[29-33]。改进这些问题对未来细胞治疗研究至关重要。生物材料也许是解决问题的答案,因为生物材料可作为细胞的载体,直接运送细胞至受损位置,或者在以生物材料为基础的支架上 培育细胞,用于移植[34,35]。
2.2.生物材料在心脏修复和再生中的应用
生物材料用于心脏修复的必备条件是生物相容性和生物降解性。它能降低局部微环境的排斥性,可长时间促进移植细胞融入自然组织,并作为生物活性分子缓慢释放的容器[36-38]。天然生物材料(明胶[39]、胶原[40]、藻酸盐[41,42]、壳聚糖[43]和纤维蛋白胶[44])和合成生物材料(聚乳酸-羟基乙酸共聚物(PLGA) [45]、碳纳米管[46]和聚氨酯[47])都是用于心脏再生的备选材料[37]。
2.3.天然生物材料
天然生物材料可通过注射水凝胶或补片的方式引入心脏[48]。胶原是细胞外基质(ECM)的一种主要成分,也是一种用于心脏修复的很受欢迎的天然材料。Miyagi等[10]研究发现在右心室缺陷大鼠模型中,胶原补片作为血管内皮生长因子(VEGF)-165的载体能够达到缓慢释放并促进血管形成的效果。在体外实验中,他们发现含固定化VEGF的胶原补片能够促进内皮和骨髓细胞的生长。VEGF-胶原补片植入缺损的右心室游离壁后与仅植入单独的胶原补片的对照组相比,促进了血管生成和心室壁增厚。这些发现说明固定化生长因子的胶原补片可通过促进细胞募集和增殖进而促进心脏修 复[10]。而单独的胶原补片可以保护梗死的心脏的收缩性,减少不利重构,提高心脏功能,因为胶原补片可以降低梗死区的纤维化,促进梗死区和补片之间供应血管的生成,吸引各种内源性细胞(如平滑肌细胞、心外膜细胞和未成熟的心肌细胞)填充到补片上[49]。胶原也可作为细胞的载体,在心肌梗死后将各种类型的细胞运输到梗死区。Frederick等[50]已经证实种植了内皮祖细胞的玻连蛋白/胶原支架可以募集血管生成素,并保护心肌梗死大鼠的心室功能。
壳聚糖是自然界第二丰富的多糖,被广泛用于农业、食品、营养、环境保护和材料科学等领域[51]。壳聚糖凝胶具有多孔的海绵状结构[52],可作为细胞支架[53]或载体,用于受控和局部给药[54]。Liu等[43]已经证实壳聚糖凝胶可以通过清除活性氧和募集趋化因子,提高缺血心肌的氧化应激,进而促进干细胞移植和存活。脱乙酰壳聚糖凝胶也被用来传输一种血管生成素-1类似物,增强内皮细胞功能和存活率。事实上 ,结合了血管生成素-1类似物的水凝胶减缓了内皮细胞的凋亡并刺激了细胞管状结构的形成[55]。同时,在患有心肌梗死的大鼠左心室中植入的壳聚糖透明质酸/丝纤蛋白补片可以减轻左心室扩张,增加室壁厚度,提高心脏功能[56]。
除了胶原和壳聚糖,其他天然生物材料也被用作心脏再生治疗的备选研究材料。藻酸盐是一种提取自海藻的多聚糖[57],已被广泛用于伤口愈合、给药和组织工程研究[58]。研究证实,藻酸盐凝胶注射进心肌后可完全被吸收,并在六周内被结缔组织取代。在心肌梗死模型中,注射藻酸盐7天和60天后,对疤痕厚度的增加、不利心室扩张的减缓和心脏功能的提升都是有效的[41]。这些结果对设定注射时间均有参考意义。在严重心肌梗死老鼠模型中,在梗死灶周围区域注射藻酸盐-壳聚糖凝胶后可以有效阻止左心室重构。其机制是通过减少细胞凋亡和增加新生血管促进组织修复[59]。纤维蛋白胶主要由纤维蛋白原和凝血酶构成,可用来形成纤维蛋白凝块。它经常用于严重心肌梗死继发破裂后的左心室壁修复。这种胶可以立刻封住破裂的心肌,并被自然吸收[60-62]。Christman等[44]研究了心肌梗死后种植纤维蛋白胶支架对心脏功能的保护作用。在缺血再灌注损伤的老鼠模型体内种植支架五周后,发现其能维持梗死室壁厚度和心脏功能[44]。
2.4.合成生物材料
合成生物材料通常由合成聚合物、金属或两者的组合构成。合成生物材料拥有很高的强度和耐久性,但毒性也较强,并且存在生物相容性等问题[48]。同天然生物材料一样,合成生物材料也是作为缓释和受控给药的容器以及支持细胞移植和融合的载体。
聚乳酸(PLA)、聚乙交酯(PLG)及其共聚物PLGA是常用的合成材料。Mukherjee等[63]采用了一种纳米结构的基质来模仿心肌的原生微环境,该基质由聚(L-乳酸)、聚(ε-己内酯)和胶原组成。在这项研究中,纳米级PLA-聚(ε-己内酯)共聚物/胶原生物复合材料支架被用来培养和支撑依附于支架上的单个兔心肌细胞。结果显示依附在支架上的成年兔心肌细胞的生长与原生心肌相当[63]。类胰岛素生长因子(IGF)-1结合在PLGA纳米粒子上,在心肌梗死后立即被传输至梗死周围区域。结果显示IGF-1合成PLGA纳米粒子延长了IGF-1在组织内的保留时间,减少了心肌细胞的凋亡,并提高了左心室功能[45]。
碳纳米纤维主要应用于心脏组织工程。Martins等[64]将传导性碳纳米管和壳聚糖混合,得到一种壳聚糖/碳支架,和原生心肌有相似的弹性。这种传导性支架不仅提高了新生老鼠心肌细胞在体外的存活率,而且增加了肌球蛋白重链、肌钙蛋白-T和连接蛋白-43的表达,这些蛋白的表达与肌肉收缩和电耦合相关,且对细胞电信号传输非常重要[64]。Zhou等[65]也研发出一种碳纳米纤维/动物凝胶支架用来支持新生老鼠心脏细胞的体外培育,且再植入心肌梗死的模型鼠心脏后可融入宿主心肌层。注入这种明胶后,心脏功能和射血分数均得到提高,同时抑制了病理恶化(如心室扩张) [65]。
人们也对合成多肽生物材料进行了研究。合成多肽可以自组装形成三维(3D)凝胶,具有调节纤维空间的性质和缩放比例的作用[66]。它们可直接注射进心肌,改善细胞募集和存活的微环境[67],也可以作为药物或细胞运输的载体[68]。Tokunaga等[69]利用自组装多肽凝胶肽在心肌梗死后将心脏祖细胞运输至心肌边界区,确保了有效的细胞传输并提高了细胞的存活率。除此之外,多肽纳米纤维也以一种可持续的方式被用于运输血小板源生长因子(PDGF),以防止心肌细胞死亡,在心肌梗死后保护心脏功能[70]。
聚氨酯是一种具有持久性和弹性的合成聚 合物,被称为弹性体薄膜,可以适应原生心脏组织的物理和机械性能,也被研究用作组织工程的支架。种植于聚氨酯薄膜上的心肌细胞可以随着薄膜的生长而生长,形成多层的收缩组织结构[71]。
尽管检验生物材料应用于心脏病治疗的临床试验还很 少,但最近的一项临床研究记录了随访6个月的治疗结果[72]。随访的结果证明,在标准药物治疗(SMT)中加入藻酸盐凝胶与单独使用SMT相比,可以更有效地提高老年慢性心力衰竭(HF)患者的运动能力。延长跟踪一年后的结果[73]显示,对于老年慢性HF患者,SMT加藻酸盐凝胶的疗法与单 独使用SMT相比,在改善患者运动能力、症状和临床状态方面更有效,且这种效果持续了一年。这些数据表明对这种 新颖疗法可以做进一步更广泛的评估。
3.传导异常和心律失常
3.1.室性心律失常
心律失常发病率升高通常与心肌梗死有关,特别是室性心律失常[74,75]。由于心脏的再生能力有限,心肌梗死后大量的心肌细胞死亡导致形成瘢痕组织,这种瘢痕组织主要由成纤维细胞和胶原蛋白组成。瘢痕组织的收缩性和电信号传导能力明显减弱,导致形成异常的传导通道或发展成传导阻滞。室性心律失常、室性心动过速(VT)或心室颤动(VF)在心肌梗死患者中很常见,也是心源性猝死(SCD)的主要原因[76]。对于严重的心肌梗死,梗死区周围的缺血性心肌细胞的电生理学性质已经被改变,有形成凹角回路(一种圆形电路)的潜在风险,该回路是导致持续室性心律失常的一种机制。随着心肌梗死度过急性 期,瘢痕开始稳定,瘢痕组织实际上成为一种结构块,促进了凹角回路的形成,触发SCD [76]。虽然医疗干预极大地降低了发病率和死亡率,但SCD仍然占据医院门诊CAD患者死亡人数的70 %,和所有患者死亡人数的50 % [77,78]。收缩不同步(心脏的心室收缩存在时间差或同步性差)导致患者从VF发展到血液动力降低、循环衰竭和脑功能损失。
心律失常触发机制主要包括电解质紊乱[79]、结构变化、局部缺血[74]、组织缺氧[80]和身体及精神波动[81]。现有疗法的主要目标是恢复正常心律。VF或无脉动持续性VT患者需要立即去心脏纤颤或恢复心律。早期使用β受体阻滞剂(一种药物,可稳定细胞膜并使心脏的整体代谢需求最小化)可以降低VF发病率[82,83]。另外一种临床干预是使用埋入式复律除颤器(ICD),这是一种电气装置,可停止纤维性颤动,恢复正常心律,通常用于心肌病患者和心脏骤停(SCA)患者 [78,84]。即使在临床上应用ICD来消除患者的心律失常,VT或VF仍然会发生,且会提高死亡率和HF风险[85]。
3.2.房室(AV)阻滞
心率和心脏收缩受右心房壁上窦房结的控制。窦房结是一种天然起搏器,可通过心房产生并发送电脉冲。在到达心室完成心房收缩前,这种信号会被位于心房间隔下部靠后区域的房室 (AV)结减慢。脉冲信号随后通过房室束到达心室,房室束分为左束支和右束支,且终止于浦肯野纤维系统,促进心室同步收缩[86]。此过程的任何一部分发生异常都会导致传导紊乱,影响心脏的泵血功能[87]。
房室传导阻滞是一种传导疾病,其发生机制是由于从心房发出的电信号被房室结部分或完全阻滞,进而导致室性心律减慢。肌肉神经紊乱(如肌肉萎缩症)[88,89]、系统性疾病(如心脏结节病[90]和淀粉样变性[91])、心肌缺血/梗死[92,93]、肿瘤疾病(如原发性心脏淋巴瘤[94])和导管消融[95,96]等疾病都可能导致AV传导异常[86]。电信号传导中断可能发生在AV结、AV结下部或AV结上部结构以及房室束和左、右束支的分叉点上[97-100]。结果导致心房电脉冲延迟或只是部分传导至心室,如果不进行治疗就有可能导致HF和SCD [101]。
目前无症状的AV阻滞不需要干预治疗,而有症状的AV阻滞(先天或运动中激发的)通过起搏器安置术治疗[100,101]。事实上,对于患有AV阻滞的患者,推荐采用永久性心脏起搏器安置术治疗,除非有原因导致AV阻滞可逆,或存在起搏器植入术禁忌[101]。
改善心脏功能和控制心律失常同等重要。但是目前治疗效果会因患者不同病症表现而有所限制[102]。因此需要 新的疗法来终止异常传导通路并提高患者存活率。AV阻滞和室性心律失常等传导紊乱患者可使用传导性生物材料——聚合物。这种材料有较高的导电性。初步研究证实传导性生物材料对改善缺血性心脏病和传导阻滞诱发的传导异常有巨大的潜力。
3.3.导电生物材料
1977年,Alan J.Heeger、Alan G.MacDiarmid和Hideki Shirakawa发现了一种有机导电高分子——聚乙炔,并在2000年获得诺贝尔奖[103]。人们对导电高分子感兴趣不仅是因为它们的导电性能,还因为它们很容易合成,且具有生物相容性和生物降解性[104]。导电高分子已成功被用作生物传感器、神经植入物、给药装置和组织工程支架的材料[105]。这一类高分子具有传导性是因为其独特的结构:一系列交替排列的单双键形成了一条共轭的主链。这些交替排列的键可以使电子在链之间和链内自由移动[106]。人们也对导电高分子促进生成电活性组织(如神经组织和心脏组织)的效果进行了研究[107,108]。
导电高分子有很多种,如聚吡咯(PPy)、聚苯胺(PANI)、聚(3,4-乙烯二氧噻吩) (PEDT, PEDOT)、聚薁(PAz)和聚噻吩衍生物[105]。其中PPy是一种性能较好的高分子,已在神经系统科学和心脏研究中得到广泛应用。George等[109]研究出一种含PPy的导电植入物,可引导神经干细胞(NSC)分化和神经突伸长,用于神经修复。他们发现含PPy的导电植入物植入大鼠的大脑皮层后,促进了植入物周围神经元和神经胶质细胞的生长[109]。一种导电性层粘连蛋白/PPy可以将人类胚胎干细胞引导至神经系统内,用于神经再生和修复[110]。另外,研究发现混合了十二烷基苯磺酸(DBS)的反荷离子可以支持NSC的存活,并诱导NSC向神经系统分化[111]。
人们也研究了PPy在心脏领域的应用。Kai等[112]制成了一种纳米纤维膜,由PPy、聚(ε-己内酯)和凝胶构成,用来模拟ECM来开展心脏组织工程。他们证明这种薄膜能够提高人体心肌细胞的附着性、扩散性、相互作用和心脏蛋白质的表达[112]。研究人员已制成一种3D PPy包覆的PLGA高分子支架,用于心肌梗死后进行干细胞移植。这种导电聚合物支架具有生物相容性,可支持心脏祖细胞和iPSC的生长与扩散。另外PPy包覆的PLGA支架在受到电刺激后可以调节细胞行为[113]。PPy也被证明在心肌梗死后可以刺激血管生成。Mihardja等[114]制成了一种藻酸盐-PPy聚合物,并将其注射进梗死区,和注射了生理盐水的动物进行对照,注射五周后,观察到前者血管生成增多。此外,梗死区的肌成纤维细胞渗透液也增多了[114]。
总而言之,PPy导电性聚合物不仅为心脏细胞提供了可持续的ECM,也影响了细胞的生物行为。在结合了细胞疗法的组织工程中,将导电性聚合物的心脏组织结构移植入心肌梗死后的心脏可以促进点信号传导,使植入细胞与宿主细胞融合。
导电聚合物的另外一项潜在应用是作为生物起搏器。患有传导阻滞或HF的患者采用植入电子起搏器治疗可以控制心律和心脏收缩,进而维持心脏的泵血功能[115]。虽然这种装置被证明有降低死亡率和患者住院治疗率的潜力,但因为非传导性组织或异常传导通道仍存在于心脏中,所以传导阻滞这一根本性问题仍然未解决。与生物起搏器开发相关的研究早在十年前就开始了,但主要是针对电子起搏器面临的限制,包括电池更换、慢性感染、高昂的手术成本和小儿患者的设备适应性(如胸腔和血管大小、儿童成长和先天性心脏缺陷) [116-118]。在正常心脏中,窦房结是一个触发器,发送电信号至基质——感受并接受该信号的细胞。触发器-基质的连接对组织的起搏和传导非常重要。生物起搏器能够与心脏结合,对内源性刺激冲动做出反应,增加或降低心脏活动性[1 19]。另外,植入生物起搏器会使侵入性最小化,很适合对电子起搏器有禁忌证的患者[116]。生物材料在 生物起搏器研究中发挥了重要作用,它能结合植入细胞,标记和追踪体内移植细胞,建立植入起搏细胞和其基质细胞之间的联系[120-122]。导电性聚合物在作为ECM支持细胞存活的同时能够建立细胞间的导电连接,是一种优秀的备选生物材料(图1)。同样地,导电性聚合物-起搏细胞结合的发展很可能成为未来制成用于治疗的生物起搏器的第一步。
4.未来的挑战和发展方向
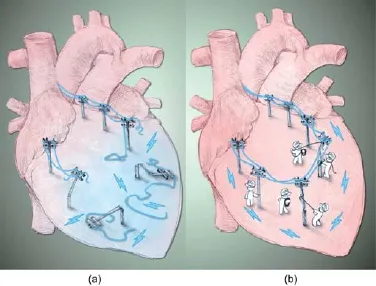
图1.重连梗死心脏内中断的电信号。(a) 心肌梗死引发心肌细胞大量损失,导致瘢痕组织形成。瘢痕组织中断了电信号传输,增加了患者心律失常的风险。(b) 导电聚合物的引入可利用现有的健康心肌重新连接受损区域,修复中断的电传导。
生物材料在心脏修复和再生方面有着巨大的潜力。大部分可用生物材料的生物相容性和生物降解性已被不同的团队通过不同的方式验证。生物材料和组织工程的研究方向正在转向寻找理想的生物材料-细胞类型组合。由于心脏是一种精确平衡的环境,由心肌细胞、成纤维细胞、内皮细胞和平滑肌细胞构成,目前仍然有很多问题没有得到解决。生物材料应用于心脏病的主要问题是创造一种用于心律失常的基质的可能性。尽管鲜有研究专门解决这一问题,但最近的一项研究证明,电生理变化的程度取决于生物材料的传播性能[123]。该研究发现,注入了高传导性生物材料的心脏不存在传导异常。然而,显示出最低间隙扩散的生物材料可在注入后短时间内通过引起左心室激活延迟和降低注射位置的间隙连接密度为心律失常构造基质。这项工作证明传输位置和间隙扩散特性是生物材料应用于心律失常的重要原因。另外,制备弹性和强度性能与原生心肌细胞相似的生物材料也非常重要[124]。将免疫反应最小化,防止生物材料或组织结构的包囊形成是另外一个问题,因为形成包囊会阻止移植细胞适时地融入原生环境[125]。由于心脏是身体最大的生物电来源[126],合成导电聚合物(可促进心肌细胞的同步跳动)将增强移植组织结构与原生心肌之间的信号传递。这一点非常重要,因为电场刺激会增加蛋白质组织,促进细胞极化,并增强电信号传播[107]。但是,引入生物材料或组织结构的最佳时间仍不清楚,该时间对在限制瘢痕组织形成的同时将炎症反应最小化是非常重要的。
尽管存在很多挑战,生物材料和组织工程研究仍然具有广阔前景,且在过去二十年间已取得了很大进展。这项研究最终目标是利用生物材料支架结合适当的细胞类型,用于部分或全部器官的生成或再生。这将最终降低器官移植的需求,提高生活质量。
致谢
笔者在此对Leigh Botly博士在原稿撰写和编辑方面提供的帮助表示感谢。图1中的说明由Ren-Ke Li教授设计,图形由大学健康网的研究通信员Benjamin Pakuts制作。本项工作由安大略心脏与卒中基金会向Ren-Ke Li提供的资金(G140005765)支持。Ren-Ke Li是心脏再生领域加拿大首席科学家。本项工作也由Eileen Mercier的慷慨捐款支持。
Compliance with ethics guidelines
Zhi Cui, Baofeng Yang, and Ren-Ke Li declare that they have no conflict of interest or financial conflicts to disclose.
[1] Public Health Agency of Canada [Internet].Economic burden of illness in Canada, 2005—2008.[2014-06-20].Available from: http://www.phac-aspc.gc.ca/publicat/ebic-femc/2005-2008/index-eng.php.
[2] Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association.Circulation 2014;129(3):399—410.
[3] Creaser JW, DePasquale EC, Vandenbogaart E, Rourke D, Chaker T, Fonarow GC.Team-based care for outpatients with heart failure.Heart Fail Clin 2015;11(3):379—405.
[4] Heidenreich P.Heart failure prevention and team-based interventions.Heart Fail Clin 2015;11(3):349—58.
[5] Larsen PM, Teerlink JR.Team-based care for patients hospitalized with heart failure.Heart Fail Clin 2015;11(3):359—70.
[6] Collins FS, Varmus H.A new initiative on precision medicine.N Engl J Med 2015;372(9):793—5.
[7] Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, et al.Functional cardiomyocytes derived from human induced pluripotent stem cells.Circ Res 2009;104(4):e30—41.
[8] Masumoto H, Ikuno T, Takeda M, Fukushima H, Marui A, Katayama S, et al.Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration.Sci Rep 2014;4:6716.
[9] Ivashchenko CY, Pipes GC, Lozinskaya IM, Lin ZJ, Xu XP, Needle S, et al.Human-induced pluripotent stem cell-derived cardiomyocytes exhibit temporal changes in phenotype.Am J Physiol Heart Circ Physiol 2013;305(6):H913—22.
[10] Miyagi Y, Chiu LL, Cimini M, Weisel RD, Radisic M, Li RK.Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair.Biomaterials 2011;32(5):1280—90.
[11] Segers VF, Lee RT.Biomaterials to enhance stem cell function in the heart.Circ Res 2011;109(8):910—22.
[12] Cheng K, Malliaras K, Shen DL, Tseliou E, Ionta V, Smith J, et al.Intramyocardial injection of platelet gel promotes endogenous repair and augments cardiac function in rats with myocardial infarction.J Am Coll Cardiol 2012;59(3):256—64.
[13] Ungerleider JL, Christman KL.Concise review: injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease: translational challenges and progress.Stem Cells Transl Med 2014;3(9):1090—9.
[14] Shen DL, Wang XF, Zhang L, Zhao XY, Li JY, Cheng K, et al.The amelioration of cardiac dysfunction after myocardial infarction by the injection of keratin biomaterials derived from human hair.Biomaterials 2011;32(35):9290—9.
[15] de Zwaan C, Daemen MJ, Hermens WT.Mechanisms of cell death in acute myocardial infarction: pathophysiological implications for treatment.Neth Heart J.2001;9(1):30—44.
[16] O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, et al.; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines.2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines.Circulation 2013;127(4):e362—425.
[17] Schächinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H; REPAIR-AMI Investigators.Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial.Eur Heart J 2006;27(23):2775—83.
[18] Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al.Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial.Lancet 2004;364(9429):141—8.
[19] Caspi O, Huber I, Habib M, Arbel G, Gepstein A, Yankelson L, et al.Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts.J Am Coll Cardiol 2007;50(19):1884—93.
[20] Laflamme MA, Chen KY, Naumova A, Muskheli V, Fugate JA, Dupras SK, et al.Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts.Nat Biotechnol 2007; 25(9):1015—24.
[21] Menasché P, Alfieri O, Janssens S, McKenna W, Reichenspumer H, Trinquart L, et al.The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation.Circulation 2008;117(9):1189—200.
[22] Menasché P.Skeletal myoblasts as a therapeutic agent.Prog Cardiovasc Dis 2007;50(1):7—17.
[23] Katritsis DG, Sotiropoulou PA, Karvouni E, Karabinos I, Korovesis S, Perez SA, et al.Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium.Catheter Cardiovasc Interv 2005;65(3):321—9.
[24] Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, et al.Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens.Circulation 2007;115(7):896—908.
[25] Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, et al.Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial.Lancet 2011;78(9806):1847—57.
[26] Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, et al.Comparison of allogeneic vs autologous bone marrow—derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial.JAMA 2012;308(22): 2369—79.
[27] Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, et al.Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts.Nature 2014;510(7504):273—77.
[28] Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, et al.Enhanced effect of human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and restore cardiac function after myocardial infarction.Circulation 2013;127(2):213—23.
[29] Segers VF, Lee RT.Stem-cell therapy for cardiac disease.Nature 2008;451(7181):937—42.
[30] Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, et al.Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials.Circulation 2005;112(9 Suppl):I150—6.
[31] Siminiak T, Kalawski R, Fiszer D, Jerzykowska O, Rzezniczak J, Rozwadowska N, et al.Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: Phase I clinical study with 12 months of follow-up.Am Heart J 2004;148(3):531—7.
[32] Chang MG, Tung L, Sekar RB, Chang CY, Cysyk J, Dong P, et al.Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model.Circulation 2006;113(15):1832—41.
[33] Macia E, Boyden PA.Stem cell therapy is proarrhythmic.Circulation 2009;119(13):1814—23.
[34] Seliktar D.Designing cell-compatible hydrogels for biomedical applications.Science 2012;336(6085):1124—8.
[35] Prestwich GD.Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine.J Control Release 2011;155(2):193—9.
[36] Pascual-Gil S, Garbayo E, Díaz-Herráez P, Prosper F, Blanco-Prieto MJ.Heart regeneration after myocardial infarction using synthetic biomaterials.J Control Release 2015;203:23—38.
[37] Zammaretti P, Jaconi M.Cardiac tissue engineering: regeneration of the wounded heart.Curr Opin Biotechnol 2004;15(5):430—4.
[38] O’Brien FJ.Biomaterials & Scaffolds for tissue engineering.Mater Today 2011;14(3):88—95.
[39] Akhyari P, Fedak PW, Weisel RD, Lee TY, Verma S, Mickle DA, et al.Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts.Circulation 2002;106(12 Suppl 1):I137—42.
[40] Zimmermann WH, Melnychenko I, Eschenhagen T.Engineered heart tissuefor regeneration of diseased hearts.Biomaterials 2004;25(9):1639—47.
[41] Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, et al.Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat.Circulation 2008;117(11):1388—96.
[42] Ruvinov E, Cohen S.Alginate biomaterial for the treatment of myocardial infarction: progress, translational strategies, and clinical outlook: from ocean algae to patient bedside.Adv Drug Deliv Rev 2016;96:54—76.
[43] Liu Z, Wang H, Wang Y, Lin Q, Yao A, Cao F, et al.The influence of chitosan hydrogel on stem cell engraftment, survival and homing in the ischemic myocardial microenvironment.Biomaterials 2012;33(11):3093—106.
[44] Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ.Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction.Tissue Eng 2004;10(3—4):403—9.
[45] Chang MY, Yang YJ, Chang CH, Tang AC, Liao WY, Cheng FY, et al.Functionalized nanoparticles provide early cardioprotection after acute myocardial infarction.J Control Release 2013;170(2):287—94.
[46] Meng X, Stout DA, Sun L, Beingessner RL, Fenniri H, Webster TJ.Novel injectable biomimetic hydrogels with carbon nanofibers and self assembled rosette nanotubes for myocardial applications.J Biomed Mater Res A 2013;101(4):1095—102.
[47] Lakshmanan R, Krishnan UM, Sethuraman S.Polymeric scaffold aided stem cell therapeutics for cardiac muscle repair and regeneration.Macromol Biosci 2013;13(9):1119—34.
[48] Lam MT, Wu JC.Biomaterial applications in cardiovascular tissue repair and regeneration.Expert Rev Cardiovasc Ther 2012;10(8):1039—49.
[49] Serpooshan V, Zhao M, Metzler SA, Wei K, Shah PB, Wang A, et al.The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction.Biomaterials 2013;34(36):9048—55.
[50] Frederick JR, Fitzpatrick JR 3rd, McCormick RC, Harris DA, Kim AY, Muenzer JR, et al.Stromal cell-derived factor-1 activation of tissue-engineered endothelial progenitor cell matrix enhances ventricular function after myocardial infarction by inducing neovasculogenesis.Circulation 2010;122(11 Suppl):S107—17.
[51] Anitha A, Sowmya S, Sudheesh Kumar PT, Deepthi S, Chennazhi KP, Ehrlich H, et al.Chitin and chitosan in selected biomedical applications.Prog Polym Sci 2014;39(9):1644—67.
[52] Song K, Qiao M, Liu T, Jiang B, Macedo HM, Ma X, et al.Preparation, fabrication and biocompatibility of novel injectable temperature-sensitive chitosan/glycerophosphate/collagen hydrogels.J Mater Sci Mater Med 2010;21(10):2835—42.
[53] Roughley P, Hoemann C, DesRosiers E, Mwale F, Antoniou J, Alini M.The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation.Biomaterials 2006;27(3):388—96.
[54] Bhattarai N, Gunn J, Zhang M.Chitosan-based hydrogels for controlled, localized drug delivery.Adv Drug Deliv Rev 2010;62(1):83—99.
[55] Miklas JW, Dallabrida SM, Reis LA, Ismail N, Rupnick M, Radisic M.QHREDGS enhances tube formation, metabolism and survival of endothelial cells in collagen-chitosan hydrogels.PLoS ONE 2013;8(8):e72956.
[56] Chi NH, Yang MC, Chung TW, Chou NK, Wang SS.Cardiac repair using chitosan-hyaluronan/silk fibroin patches in a rat heart model with myocardial infarction.Carbohydr Polym 2013;92(1):591—7.
[57] Wee S, Gombotz WR.Protein release from alginate matrices.Adv Drug Deliv Rev 1998;31(3):267—85.
[58] Lee KY, Mooney DJ.Alginate: properties and biomedical applications.Prog Polym Sci 2012;37(1):106—26.
[59] Deng B, Shen L, Wu Y, Shen Y, Ding X, Lu S, et al.Delivery of alginate-chitosan hydrogel promotes endogenous repair and preserves cardiac function in rats with myocardial infarction.J Biomed Mater Res A 2015;103(3):907—18.
[60] Terashima M, Fujiwara S, Yaginuma GY, Takizawa K, Kaneko U, Meguro T.Outcome of percutaneous intrapericardial fibrin-glue injection therapy for left ventricular free wall rupture secondary to acute myocardial infarction.Am J Cardiol 2008;101(4):419—21.
[61] Iemura J, Oku H, Otaki M, Kitayama H, Inoue T, Kaneda T.Surgical strategy for left ventricular free wall rupture after acute myocardial infarction.Ann Thorac Surg 2001;71(1):201—4.
[62] Okonogi T, Otsuka Y, Saito T.Repaired left ventricular free wall rupture after acute myocardial infarction by percutaneous intrapericardial fibrin-glue injection therapy.J Invasive Cardiol 2013;25(9):E186—7.
[63] Mukherjee S, Venugopal JR, Ravichandran R, Ramakrishna S, Raghunath M.Evaluation of the biocompatibility of PLACL/collagen nanostructured matrices with cardiomyocytes as a model for the regeneration of infarcted myocardium.Adv Funct Mater 2011;21(12):2291—300.
[64] Martins AM, Eng G, Caridade SG, Mano JF, Reis RL, Vunjak-Novakovic G.Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering.Biomacromolecules 2014;15(2):635—43.
[65] Zhou J, Chen J, Sun H, Qiu X, Mou Y, Liu Z, et al.Engineering the heart: evaluation of conductive nanomaterials for improving implant integration and cardiac function.Sci Rep 2014;4:3733.
[66] French KM, Somasuntharam I, Davis ME.Self-assembling peptide-based delivery of therapeutics for myocardial infarction.Adv Drug Deliv Rev 2016;96:40—53.
[67] Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, et al.Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells.Circulation 2005;111(4):442—50.
[68] Boopathy AV, Davis ME.Self-assembling peptide-based delivery of therapeutics for myocardial infarction.Methods Mol Bio 2014;1141:159—64.
[69] Tokunaga M, Liu ML, Nagai T, Iwanaga K, Matsuura K, Takahashi T, et al.Implantation of cardiac progenitor cells using self-assembling peptide improves cardiac function after myocardial infarction.J Mol Cell Cardiol 2010;49(6):972—83.
[70] Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT.Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofi bers.J Clin Invest 2006;116(1):237—48.
[71] McDevitt TC, Woodhouse KA, Hauschka SD, Murry CE, Stayton PS.Spatially organized layers of cardiomyocytes on biodegradable polyurethane films for myocardial repair.J Biomed Mater Res A 2003;66(3):586—95.
[72] Anker SD, Coats AJS, Cristian G, Dragomir D, Pusineri E, Piredda M, et al.A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial).Eur Heart J 2015;36(34):2297—309.
[73] Mann DL, Lee RJ, Coats AJS, Neagoe G, Dragomir D, Pusineri E, et al.One-year follow-up results from AUGMENT-HF: a multicentre randomized controlled clinical trial of the efficacy of left ventricular augmentation with Algisyl in the treatment of heart failure.Eur J Heart Fail 2015;18(3):314—25.
[74] Ghuran AV, Camm AJ.Ischaemic heart disease presenting as arrhythmias.Br Med Bull 2001;59(1):193—210.
[75] Benito B, Josephson ME.Ventricular tachycardia in coronary artery disease.Rev Esp Cardiol (Engl Ed) 2012;65(10):939—55.[English Version]
[76] Myerburg RJ, Junttila MJ.Sudden cardiac death caused by coronary heart disease.Circulation 2012;125(8):1043—52.
[77] Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al.; WRITING GROUP MEMBERS; American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Heart disease and stroke statistics—2010 update: a report from the American Heart Association.Circulation 2010;121(7):e46—215.
[78] Yousuf O, Chrispin J, Tomaselli GF, Berger RD.Clinical management and prevention of sudden cardiac death.Circ Res 2015;116(12):2020—40.
[79] Khan IA.Clinical and therapeutic aspects of congenital and acquired long QT syndrome.Am J Med 2002;112(1):58—66.
[80] Noakes TD, Higginson L, Opie LH.Physical training increases ventricular fibrillation thresholds of isolated rat hearts during normoxia, hypoxia and regional ischemia.Circulation 1983;67(1):24—30.
[81] Lampert R, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D.Emotional and physical precipitants of ventricular arrhythmia.Circulation 2002;106(14):1800—5.
[82] The Norwegian Multicenter Study Group.Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction.N Engl J Med 1981;304(14):801—7.
[83] Chen ZM, Pan HC, Chen YP, Peto R, Collins R, Jiang LX, et al.; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group.Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial.Lancet 2005;366(9497):1622—32.
[84] Goldenberg I, Gillespie J, Moss AJ, Hall WJ, Klein H, McNitt S, et al.; Executive Committee of the Multicenter Automatic Defibrillator Implantation Trial II.Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: an extended 8-year follow-up study of the Multicenter Automatic Defi brillator Implantation Trial II.Circulation 2010;122(13):1265—71.
[85] Dorian P, Hohnloser SH, Thorpe KE, Roberts RS, Kuck KH, Gent M, et al.Mechanisms underlying the lack of effect of implantable cardioverter-defibrillator therapy on mortality in high-risk patients with recent myocardial infarction: insights from the Defibrillation in Acute Myocardial Infarction Trial (DINAMIT).Circulation 2010;122(25):2645—52.
[86] Vogler J, Breithardt G, Eckardt L.Bradyarrhythmias and conduction blocks.Rev Esp Cardiol (Engl Ed) 2012;65(7):656—67.[English Version]
[87] Laske T, Iaizzo P.The cardiac conduction system.In: Iaizzo PA, editors Handbook of cardiac anatomy, physiology, and devices.Totowa: Humana Press; 2005.p.123—36.
[88] Finsterer J, Stöllberger C.Cardiac involvement in Becker muscular dystrophy.Can J Cardiol 2008;24(10):786—92.
[89] Altekin RE, Yanikoglu A, Ucar M, Ermis C.Complete AV block and cardiac syncope in a patient with Duchenne muscular dystrophy.J Cardiol Cases 2011;3(2):e68—70.
[90] Lee JC, Seiler J, Blankstein R, Padera RF, Baughman KL, Tedrow UB.Images in cardiovascular medicine.Cardiac sarcoidosis presenting as heart block.Circulation 2009;120(15):1550—1.
[91] Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD.Updates in cardiac amyloidosis: a review.J Am Heart Assoc 2012;1(2):e000364.
[92] Singh SM, FitzGerald G, Yan AT, Brieger D, Fox KA, López-Sendón J, et al.High-grade atrioventricular block in acute coronary syndromes: insights from the Global Registry of Acute Coronary Events.Eur Heart J 2015;36(16):976—83.
[93] Hreybe H, Saba S.Location of acute myocardial infarction and associated arrhythmias and outcome.Clin Cardiol 2009;32(5):274—7.
[94] Cho SW, Kang YJ, Kim TH, Cho SK, Hwang MW, Chang W, et al.Primary cardiac lymphoma presenting with atrioventricular block.Korean Circ J 2010;40(2):94—8.
[95] Schaffer MS, Silka MJ, Ross BA, Kugler JD; Pediatric Electrophysiology Society.Inadvertent atrioventricular block during radiofrequency catheter ablation.Results of the Pediatric Radiofrequency Ablation Registry.Circulation 1996;94(12):3214—20.
[96] Belhassen B, Glick A, Rosso R, Michowitz Y, Viskin S.Atrioventricular block during radiofrequency catheter ablation of atrial flutter: incidence, mechanism, and clinical implications.Europace 2011;13(7):1009—14.
[97] Rardon DP, Miles WM, Zipes DP.Atrioventricular block and dissociation.In: Zipes DP, Jalife J, editors Cardiac electrophysiology: from cells to bedside.2nd ed.Philadelphia: WB Saunders; 1995.p.485—9.
[98] Issa Z, Miller JM, Zipes DP .Atrioventricular conduction abnormalities.In: Clinical arrhythmology and electrophysiology: a companion to Braunwald’s heart disease.Philadelphia: WB Saunders; 2008.p.127—42.
[99] Barold SS, Hayes DL.Second-degree atrioventricular block: a reappraisal.Mayo Clin Proc 2001;76(1):44—57.
[100] Barold SS, Ilercil A, Leonelli F, Herweg B.First-degree atrioventricular block.Clinical manifestations, indications for pacing, pacemaker management & consequences during cardiac resynchronization.J Interv Card Electrophysiol 2006;17(2):139—52.
[101] Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, et al.; ESC Committee for Practice Guidelines (CPG); Document Reviewers.2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC).Developed in collaboration with the European Heart Rhythm Association (EHRA).Eur Heart J 2013;34(29):2281—329.
[102] Stevenson WG, John RM.Ventricular arrhythmias in patients with implanted defi brillators.Circulation 2011;124(16):e411—4.
[103] Miller JS.The 2000 Nobel Prize in Chemistry—a personal accolade.Chemphyschem 2000;1(4):229—30.
[104] Rivers TJ, Hudson TW, Schmidt CE.Synthesis of a novel, biodegradable electrically conducting polymer for biomedical applications.Adv Funct Mater 2002;12(1):33—7.
[105] Balint R, Cassidy NJ, Cartmell SH.Conductive polymers: towards a smart biomaterial for tissue engineering.Acta Biomater 2014;10(6):2341—53.
[106] Guiseppi-Elie A.Electroconductive hydrogels: synthesis, characterization and biomedical applications.Biomaterials 2010;31(10):2701—16.
[107] Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, et al.Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaff olds.Proc Natl Acad Sci USA 2004;101(52):18129—34.
[108] Nakashima T, Ohkusa T, Okamoto Y, Yoshida M, Lee JK, Mizukami Y, et al.Rapid electrical stimulation causes alterations in cardiac intercellular junction proteins of cardiomyocytes.Am J Physiol Heart Circ Physiol 2014;306(9):H1324—33.
[109] George PM, Lyckman AW, LaVan DA, Hegde A, Leung Y, Avasare R, et al.Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics.Biomaterials 2005;26(17):3511—9.
[110] Zhang L, Stauffer WR, Jane EP, Sammak PJ, Cui XT.Enhanced differentiation of embryonic and neural stem cells to neuronal fates on laminin peptides doped polypyrrole.Macromol Biosci 2010;10(12):1456—64.
[111] Lundin V, Herland A, Berggren M, Jager EW, Teixeira AI.Control of neural stem cell survival by electroactive polymer substrates.PLoS ONE 2011;6(4):e18624.
[112] Kai D, Prabhakaran MP, Jin G, Ramakrishna S.Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering.J Biomed Mater Res A 2011;99(3):376—85.
[113] Gelmi A, Zhang JB, Cieslar-Pobuda A, Ljunngren MK, Los MJ, Rafat M, et al.Electroactive 3D materials for cardiac tissue engineering.In: Bar-Cohen Y, editor Proceedings of SPIE Volume 9430: Electroactive Polymer Actuators and Devices (EAPAD) 2015; 2 015 Mar 9—12; San Diego, CA, USA.Bellingham: SPIE; 2015.p.94301T.
[114] Mihardja SS, Sievers RE, Lee RJ.The effect of polypyrrole on arteriogenesis in an acute rat infarct model.Biomaterials 2008;29(31):4205—10.
[115] Witte KK, Pipes RR, Nanthakumar K, Parker JD.Biventricular pacemaker upgrade in previously paced heart failure patients—improvements in ventricular dyssynchrony.J Card Fail 2006;12(3):199—204.
[116] Cho HC, Marbán E.Biological therapies for cardiac arrhythmias: can genes and cells replace drugs and devices? Circ Res 2010;106(4):674—85.
[117] Berul CI, Cecchin F; American Heart Association; American College of Cardiology.Indications and techniques of pediatric cardiac pacing.Expert Rev Cardiovasc Ther 2003;1(2):165—76.
[118] Rosen MR, Brink PR, Cohen IS, Robinson RB.Cardiac pacing: from biological to electronic ...to biological? Circ Arrhythm Electrophysiol 2008;1(1):54—61.
[119] Munshi NV, Olson EN.Translational medicine.Improving cardiac rhythm with a biological pacemaker.Science 2014;345(6194):268—9.
[120] Rosen MR, Robinson RB, Brink PR, Cohen IS.The road to biological pacing.Nat Rev Cardiol 2011;8(11):656—6.
[121] Rosen AB, Kelly DJ, Schuldt AJ, Lu J, Potapova IA, Doronin SV, et al.Finding fluorescent needles in the cardiac haystack: tracking human mesenchymal stem cells labeled with quantum dots for quantitative in vivo three-dimensional fl uorescence analysis.Stem Cells 2007;25(8):2128—38.
[122] Proulx MK, Carey SP, Ditroia LM, Jones CM, Fakharzadeh M, Guyette JP, et al.Fibrin microthreads support mesenchymal stem cell growth while maintaining diff erentiation potential.J Biomed Mater Res A 2011;96(2):301—12.
[123] Suarez SL, Rane AA, Muñoz A, Wright AT, Zhang SX, Braden RL, et al.Intramyocardial injection of hydrogel with high interstitial spread does not impact action potential propagation.Acta Biomater 2015;26:13—22.
[124] Reis LA, Chiu LLY , Feric N, Fu L, Radisic M.Biomaterials in myocardial tissue engineering.J Tissue Eng Regen Med 2016;10(1):11—28.
[125] Griffith LG, Naughton G.Tissue engineering—current challenges and expanding opportunities.Science 2002;295(5557):1009—14.
[126] Tandon N, Cannizzaro C, Chao PH, Maidhof R, Marsano A, Au HT, et al.Electrical stimulation systems for cardiac tissue engineering.Nat Protoc 2009;4(2):155—73.
* Corresponding author.
E-mail address: renkeli@uhnres.utoronto.ca
2095-8099/© 2016 THE AUTHORS.Published by Elsevier LTD on behalf of Chinese Academy of Engineering and Higher Education Press Limited Company.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
英文原文: Engineering 2016, 2(1): 141—148
Zhi Cui, Baofeng Yang, Ren-Ke Li.Application of Biomaterials in Cardiac Repair and Regeneration.Engineering,
http://dx.doi.org/10.1016/J.ENG.2016.01.028
