人HNF4α基因真核表达载体的构建与鉴定*
2016-04-24丁宁张明香
丁宁,张明香
人HNF4α基因真核表达载体的构建与鉴定*
丁宁,张明香
目的体外构建人肝细胞核因子4α(HNF4α)基因真核表达载体,并初步鉴定其在HepG2细胞的表达。方法从肝癌手术患者获得肝组织,分离得到肝组织总RNA,将其逆转录合成cDNA,经特异性引物扩增HNF4α基因片段,将其定向连接到pcDNA3.1(+)真核表达载体,经抗生素筛选后,酶切及测序鉴定其序列正确。提取质粒,转染HepG2细胞,48 h后裂解细胞,应用抗HNF4α行Western blot检测目的蛋白。结果从临床肝癌患者肝组织中成功分离得到总RNA,扩增出HNF4α基因,经筛选、酶切鉴定和测序,确认pcDNA3.1-4α真核表达载体构建成功。在转染HepG2细胞48 h后,检测显示53 kDa位置有明显融合蛋白条带。结论我们成功构建了HNF4α基因真核表达载体,体外转染HepG2细胞成功表达HNF4α蛋白,为后续研究奠定了基础。
HepG2细胞;肝细胞核因子4α; 真核表达; 细胞因子
肝细胞核因子4α(Hepatocyte nuclear factor 4α,HNF4α)是核激素受体超家族HNF4 的成员之一,高表达于肝脏组织,在胰腺、肾脏和小肠等组织内亦有少量的表达。在成人肝脏,其可与约12%基因的启动子结合[1]。HNF4α处于转录因子调控网络的上游,以同源二聚体的形式与DNA 结合,可与四十多种靶基因的启动子和增强子相互作用,进而调控与肝细胞功能相关基因的表达,参与糖、脂和胆固醇的代谢,具有解毒作用,通过调节胆汁酸代谢和蛋白质合成等途径,也具有激活编码细胞外粘附分子、细胞外基质、细胞骨架蛋白基因表达的功能[2~4]。在胚胎发育过程中,最先在胚泡的原始内胚层中发现HNF4αmRNA的存在,其表达水平随着肝细胞分化成熟而逐渐增高[5]。本研究构建了人HNF4α基因的真核表达载体,通过转染HepG2细胞鉴定载体正常表达,为进一步研究提供了良好的研究对象。
1 材料与方法
1.1细胞和试剂HepG2细胞为本科保存。PCReasyMIx (全式金生物有限公司,北京),DNA胶回收试剂盒(Qiagen,德国),T-A克隆试剂盒(Promega,美国),RNA逆转录试剂盒(Invitrogen,美国),EcoR I/BamH I核酸内切酶(NEB公司,美国),pcDNA3.1(+)真核表达载体(Invitrogen,美国),DMEM和胎牛血清(Gibico,美国),转染试剂X-geme HD(Roche,美国),Western blot套装(康为世纪,北京),抗HNF4a单克隆抗体(Santa Cruz,美国),β-actin(康为世纪,北京),HRP标记的兔抗鼠IgG(中杉金桥,北京),引物序列由上海生工生物公司合成。
1.2 HNF4α基因的引物设计及cDNA获取自NCBI获得HNF4αcDNA(GenBank Number:NM_000545)提交序列,按照序列信息合成HNF4α扩增引物。上游引物4αF:5'-AGGATCCATGCGACTCTCCAAAACCCTCG-3'(BamH I);下游引物4αR:5'-AGAATTCCCTAGATAACTTCCTGCTTGGTG-3'(EcoR I)。从我院肝胆外科2例男性(41岁和58岁)原发性肝癌患者手术获得的癌旁新鲜肝组织,立即加入液氮,在研钵内研碎。严格按照Qiagen组织RNA提取试剂盒说明书提取总RNA。按照逆转录试剂盒说明书以oligdT引物在42℃作用90 min,70℃15 min灭活逆转录酶,将mRNA逆转录为cDNA。PCR扩增体系:2×PCR Mix 25μl, 4αF 0.5μl,4αR 0.5μl,cDNA产物5μl,加ddH2O补足至50μl。PCR扩增条件:94℃3 min,94℃15 s,55℃20 s,72℃40 s×35循环;72℃10 min,4℃保存。将全部PCR产物加入1%TAE琼脂糖凝胶中,110 v,25 min,电泳鉴定,对约1500 bp位置的阳性条带进行切胶,按照试剂盒说明,回收目的PCR产物片段,以溴乙锭(EB)30μl重溶产物DNA,置于-20℃保存。
1.3 HNF4αcDNA克隆首先进行连接实验,即应用连接体系:T4 DNA连接酶Buffer 1μl,pGEMT Easy载体(50 ng)1μl,胶回收DNA 3μl,T4 DNA连接酶(3 U/μl)1μl,加ddH2O补足至10μl,轻柔混匀,短暂离心,置于4℃过夜;转化:将自-70℃冰箱中取出的JM109感受态细胞置于冰上5 min,待菌液完全融化后,轻柔吹打混合,取感受态细胞50 μl,加入到连接体系的EP管中,静置于冰上20 min,在42℃水浴中热击1 min,确保转化EP管不震动。随后,迅速将EP管移到冰浴中,使细胞冷却2 min。在每管连接反应转化细胞中加入平衡至室温的SOC培养基500μl。将转化管在恒温振荡器中振荡培养,37℃,180 r/m摇菌1 h。按照300μl转化培养基/板的比例将培养基均匀涂布于含有XGal/(异丙基-β-d-硫代半乳糖苷,IPTG)/氨苄青霉素的固体平板培养皿表面。将培养皿置于37℃过夜。次日,挑取单克隆白色菌斑进行鉴定;菌落PCR鉴定:将双蒸水10μl加入PCR反应管中,采用无菌牙签挑取白色单克隆菌落,在上述反应管中冲涮数次,再接种于新的复制板上,将PCR管置于PCR仪中95℃作用10 min,再冰浴3 min,裂菌。将复制板置于37℃孵箱中过夜培养。以裂解菌液产物为模板,PCR反应条件和体系同前,进行目的片段扩增。将扩增产物经1%TAE琼脂糖凝胶电泳鉴定,发现目的片段,则为阳性克隆。取阳性克隆菌液送测序鉴定。
1.4 pcDNA3.1-4α真核表达载体的构建将测序正确的HNF4αcDNA克隆菌液和转化了pcDNA3.1(+)空质粒的菌液,在37℃,220 r/m摇菌、过夜。按照试剂盒提取质粒,分别以EcoR I/BamH I双酶切质粒,酶切体系为:NEB Buffer 3.1 3μl,EcoR I 1μl,BamH I 1μl,HNF4αcDNA质粒/pcDNA3.1(+)空质粒5μl,加ddH2O补足至30μl,37℃孵育过夜。酶切产物经1%TAE琼脂糖凝胶电泳鉴定后,胶回收5.3 kb和1.5 kb目的片段。取洗脱液15μl,分别溶解回收目的片段。连接体系为:T4 DNA连接酶Buffer 1μl,酶切pcDNA3.1(+)空质粒产物1 μl,酶切HNF4αcDNA产物3μl,T4 DNA连接酶(3 U/μl)1μl,加ddH2O补足至10μl,置于4℃过夜。转化和菌落PCR实验条件与1.3条件相同。取阳性克隆,送测序、鉴定。
1.5 pcDNA3.1-4α载体质粒转染HepG2细胞提取转染级的pcDNA3.1-4α载体质粒,并测定其浓度。取HepG2细胞铺板,按3×105个细胞/孔接种于6孔细胞培养板中。1 d后,按载体质粒1μg/孔转染细胞,取pcDNA3.1-4α与转染试剂X-geme HD按1:2.5混合于Optim-MEM培养基,室温孵育30 min,缓慢滴加转染混合物至无血清培养基孵育的HepG2细胞培养板中,37℃,5%CO2孵育4~5 h,弃去上清,以PBS清洗细胞2遍,加入含2%胎牛血清(FBS)的DMEM培养基1 ml,37℃,5%CO2孵育48 h,留取HepG2细胞。
1.6 pcDNA3.1-4α载体转染细胞HNF4α蛋白表达检测在细胞裂解液中按100:1加入蛋白酶抑制剂,在6孔板,每孔加入细胞裂解液100 μL,冰上充分裂解细胞,1000 g离心5 min,将上清转移到新的EP管中。将裂解的蛋白按5:1加入PAGE胶上样缓冲液,混匀,置于沸水中10 min,室温冷却后,在预制的10% SDS-PAGE胶上样,35 mA电流,作用2~3 h,按海绵-滤纸-胶-膜-滤纸-海绵的顺序叠放夹好,其间不留气泡,置于转膜槽中,200 mA电流转膜2 h,5%脱脂牛奶封闭膜,加入抗HNF4α(1:200),β-actin (1:3000),4℃过夜。次日,洗涤膜3~5次,加入HRP标记的兔抗鼠IgG,室温孵育2~4 h,洗涤膜3~5次,置ECL发光试剂盒显影、拍照,观察蛋白条带。
2 结果
2.1 HNF4α基因cDNA扩增结果自2例肝癌患者癌旁肝组织提取总RNA,应用特异性引物扩增出HNF4αcDNA 1.5 kb 产物,经1%TAE电泳鉴定,条带清晰单一(图1)。

图1 癌旁肝组织HNF4αcDNA扩增产物电泳鉴定A1,A2:患者1;B1,B2:患者2
2.2 HNF4α基因cDNA 克隆情况将PCR产物自胶回收目的片段,经蓝白斑(图2A)筛选,挑取白色阳性克隆菌落,行PCR鉴定(图2B)后,菌液送测序鉴定。

图2 目的片段克隆及鉴定A :蓝白斑筛选;B:PCR鉴定HNF4α基因cDNA菌落A1~A4:患者1;B1~B4:患者2
2.3 pcDNA3.1-4α真核表达载体的构建及鉴定HNF4αcDNA 克隆质粒与pcDNA3.1(+)空载体质粒经EcoRI/BamHI双酶切(图3A),胶回收1.5 kb的目的片段,将回收的pcDNA3.1(+)载体与HNF4αcDNA片段通过T4DNA连接酶连接过夜,转化,涂板,挑菌落,提取质粒,经EcoRI/BamHI双酶切鉴定(图3B),在5.3 kb和1.5 kb各有单一条带。送条带正确的质粒测序。

图3 pcDNA3.1-4α真核表达载体的构建及鉴定A:EcoRI/BamHI双酶切HNF4αcDNA 克隆质粒电泳鉴定;B:EcoRI/BamHI双酶切pcDNA3.1-4α载体质粒电泳鉴定A1~A3:患者1;B1,B4:患者2
2.4 pcDNA3.1-4α载体转染细胞HNF4α蛋白表达情况取鉴定正确的pcDNA3.1-4α重组表达载体,提取转染级质粒,转染HepG2细胞48 h后收集细胞,裂解提取蛋白,经Western blot检测,发现在15 KDa位置有一单一清晰的HNF4α蛋白条带表达(图4)。
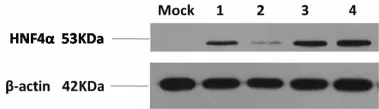
图4 Western blot检测pcDNA3.1-4α载体转染HepG2细胞HNF4α蛋白表达1~3:患者1;4:患者2
3 讨论
肝脏合成白蛋白和载脂蛋白等一系列血浆蛋白,合成和储存糖原,调节胆固醇的合成和运输以及解毒功能,负责代谢调控等[6]。然而,肝脏疾病不仅种类繁多,而且具有较高的发病率和死亡率,如肝硬化、肝癌、病毒性肝炎、急性肝功能衰竭,成为当今社会威胁人类健康的主要疾病之一[7,8]。目前,肝细胞移植为肝脏疾病的治疗开辟了一条新的途径,可用于治疗代谢性肝病和急、慢性肝衰竭等[9~11]。然而,肝细胞的来源短缺成为这些应用与研究推进的一大挑战。
间充质干细胞(MSCs)是一类起始于发育早期中胚层的成体干细胞,具有自我更新、多向分化和免疫调控的能力,在组织工程和再生医学领域具有巨大的应用前景[12,13]。MSCs在体内及体外都能够跨胚层分化为肝细胞,为解决肝细胞来源问题带来了新的希望。近年来,肝细胞核因子被认为在肝脏发育和分化中起重要作用。维持肝细胞分化和控制肝脏特异性基因的表达在很大程度上归因于肝细胞核因子。研究显示,HNF4α在维持肝细胞形态、功能发挥和分化过程中必不可少[14,15]。Nagy et al[16]发现在成熟肝脏中,HNF4α在卵圆细胞分化为肝细胞时即开始表达,据此认为其在卵圆细胞向肝细胞分化过程中发挥重要作用。体外实验显示应用RNAi干扰HNF4α表达后肝细胞分化被抑制,在肝细胞中过表达HNF4α可使肝细胞分化相关基因恢复表达,肝功能恢复正常[17,18]。此外,国外一些研究表明,上调HNF4α表达可以促进多能干细胞、骨髓间充质干细胞分化为肝细胞[19,20]。
本文从肝组织中提取总RNA,经特异性引物扩增HNF4α基因cDNA,克隆成功,预留EcoRI/BamHI双酶切cDNA克隆质粒后,将目的片段定向连接入pcDNA3.1(+)真核表达载体中,载体鉴定正确后,提取质粒,转染肝癌细胞系HepG2细胞,经Western blot检测细胞内有HNF4α蛋白的表达,说明HNF4α真核表达载体构建成功。成功构建HNF4α真核表达载体为后续转染脐带间充质干细胞(hUC-MSCs),使其诱导分化为肝样细胞研究奠定了基础。
[1] Odom DT,Zizlsperger N,Gordon DB,et al. Control of pancreas and liver gene expression by HNF transcription factors. Science,2004,303(5662):1378-1381.
[2] Bagwell AM,Bailly A,Mychaleckyj JC,et al. Comparative genomic analysis of the HNF-4αtranscription factor gene. Mol Genet Metab,2004,81(2):112-121.
[3] Watt AJ,Garrison WD,Duncan SA. HNF4:a central regulator of hepatocyte differentiation and function. Hepatology,2003,37(6): 1249-1253.
[4] Chiang JY. Hepatocyte nuclear factor 4αregulation of bile acid and drug metabolism.Expert Opin Drug Metab Toxicol,2009,5(2):137-147.
[5] Torres-Padilla ME,Fougere-Deschatrette C,Weiss MC. Expression o f HNF4alpha isoforms in mouse liver development is regulated by sequential promoter usage and constitutive 3’end splicing. Mech Dev,2001,109(2):183-193.
[6] Si-Tayeb K,L emaigre FP,Duncan SA. Organogenesis and development of the liver.Devel Cell,2010,18(2):175-189.
[7] Rautou PE,Mansouri A,L ebrec D,et al. Autophagy in liver diseases. J Hepatol,2010,53(6):1123-1134.
[8] Tillmann H,Wiese M,Braun Y,et al. Q uality of life in patients with various liver diseases. J V iral Hepat,2011,18(4):252-261.
[9] Bilir BM,Guinette D,K arrer F,et al. Hepatocyte transplantation in acute liver failure.Liver Transplant,2000,6(1):32-40.
[10] Dhawan A,Mitry RR,Hughes RD. Hepatocyte transplantation for liver-based metabolic disorders. J Inherit Metab Dis,2006,29(2-3):431-435.
[11] Dhawan A,Puppi J,Hughes RD,et al. Human hepatocyte transplantation:current experience and future challenges.Nat Rev G astro Hepat,2010,7(5):288-298.
[12] Pittenger MF,Mackay AM,Beck SC,et al. Multilineage potential of adult human mesenchymal stem cells. Science,1999,284(5411):143-147.
[13] Ratcliffe E,Glen K E,Naing MW,et al. Current status and perspectives on stem cell-based therapies undergoing clinical trials for regenerative medicine:case studies.Br Med Bull,2013,108:73-94.
[14] L i J,Ning G,Duncan SA. Mammalian hepatocyte differentiation requires the transcription factor HNF-4alpha.Genes Dev,2000,14(4):464-474.
[15] Hayhurst GP,L ee YH,L ambert G,et al. Hepatocyte nuclear factor 4 alpha(nuclear receptor 2A1)is essential for maintenance of hepatic gene expression and lipid home ostasis.Mol Cell Biol,2001,21(4):1393-1403.
[16] Nagy P,Bisgaard HC,Thorgeirsson SS. Expression of hepatic transcription factors during liver development and oval cell differentiation.J Cell Biol,1994,126 (1):223-233.
[17] K imata T,Nagaki M,Tsukada Y,et al. Hepatocyte nuclear factor-4 alpha and-1 small interfering RNA inhibits hepatocyte differentiation induced by extracellular matrix.Hepatol Res,2006,35(1):3-9.
[18] Naiki T,Nagaki M,Asano T,et al. Adenovirus-mediated hepatocyte nuclea r factor-4alpha overexpression maintains liver phenotype in cultured rat hepatocytes.Biochem Biophys Res Commun,2005,335(2):496-500.
[19] Chen M L,L ee K D,Huang HC,et al. HNF-4alpha determines hepatic differentiation of human mesenchymal stem cells from bone marrow.World J Gastroenterol,2010,16(40):5092-5103.
[20] Delaforest A,Nagaoka M,Si-Tayeb K,et al. HNF4A is essential for specification of hepatic progenitors from human pluripotent stem cells.Development,2011,138(19):4143-4153.
(收稿:2015-09-21)
(本文编辑:陈从新)
Construction and identification of eukaryotic expression vector of human hepatocyte nuclear factor 4α geneinvitro
Ding Ning,Zhang Mingxiang.
Department of Liver Diseases,Sixth People’s Hospital,Shenyang 110066,Liaoning Province,China
Objective To construct eukaryotic expression vector of human hepatocyte nuclear factor(HNF)-4αgene and identify its expression in vitro.Methods The total RNA was isolated from the liver tissues of two patients with hepatocellular carcinoma(HCC)who had underwent surgical operation,and HNF4αcDNA was synthesized by reverse transcription and amplified with the existence of specific primers.Then,the HNF4α fragment was directionally linked to pcDNA3.1 positive-eukaryotic expression vector.After antibiotic screening,the sequence analysis was conducted.The vector was transfected into HepG2 cells,and the expression of target protein after 48-hour incubation was detected by Western blot.Results Enzyme digestion and sequencing illustrated that pcDNA3.1-4αpositive-eukaryotic expression vector was successfully constructed,and the results of Western blot revealed that obvious band of fusion protein was present at 53 kDa position.Conclusion The eukaryotic expression vector of HNF4αgene is successfully constructed,and it works well in HepG2 cells in vitro,which is good for further study of HNF4αprotein.
HepG2 cells;Hepatocyte nuclear factor-4α;Eukaryotic expression;Cytokines
国家传染病重大专项分课题:慢性病毒性肝炎
中西医结合治疗方案优化研究(2012ZX1005004-001)
110006 沈阳市第六人民医院
,丁宁,女,39岁,副主任医师,主要从事慢性肝炎、肝硬化,肝衰竭等疾病的治疗研究E-Mail:dingningwz_1976@sina.com
,张明香,E-Mail:zmx6511@126.com
10.3969/j.issn.1672-5069.2016.02.008
