Evaluation of entomopathogenic Bacillus sphaericus isolated from Lombok beach area against mosquito larvae
2016-04-12BambangFajarSuryadiBagyoYanuwiadiTriArdyatiSuharjonoSuharjono1DepartmentofBiologyFacultyofMathematicsNaturalSciencesMataramUniversityJalanMajapahitNoMataram8315WestNusaTenggaraIndonesiaDepartmentofBiologyFacultyofMathematics
Bambang Fajar Suryadi, Bagyo Yanuwiadi, Tri Ardyati, Suharjono Suharjono1Department of Biology, Faculty of Mathematics Natural Sciences, Mataram University, Jalan Majapahit No. 6, Mataram 8315, West Nusa Tenggara, IndonesiaDepartment of Biology, Faculty of Mathematics Natural Sciences, Brawijaya University, Jalan Veteran, Malang 65145, East Java, Indonesia
Evaluation of entomopathogenic Bacillus sphaericus isolated from Lombok beach area against mosquito larvae
Bambang Fajar Suryadi1,2*, Bagyo Yanuwiadi2, Tri Ardyati2, Suharjono Suharjono21Department of Biology, Faculty of Mathematics Natural Sciences, Mataram University, Jalan Majapahit No. 62, Mataram 83125, West Nusa Tenggara, Indonesia
2Department of Biology, Faculty of Mathematics Natural Sciences, Brawijaya University, Jalan Veteran, Malang 65145, East Java, Indonesia
Original article http://dx.doi.org/10.1016/j.apjtb.2015.10.013
Tel: +62 81339530390
E-mail: bambangfs_mail@yahoo.com
PeerreviewunderresponsibilityofHainanMedicalUniversity.Thejournalimplementsdouble-blindpeerreviewpracticedbyspeciallyinvitedinternationaleditorialboardmembers.
Foundation Project: Supported by Ministry of Education and Culture Republic of Indonesia, Directorate General of Higher Education (DGHE) scholarship batch 2012 (Grant No. 2625/E4.4/2012).
2221-1691/Copyright©2016 Hainan Medical University. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http:// creativecommons.org/licenses/by-nc-nd/4.0/).
ARTICLE INFO
Article history:
Received 7 Sep 2015
Received in revised form 22 Sep, 2nd revisedform23 Sep,3rdrevisedform 29 Sep 2015
Accepted 23 Oct 2015
Available online 20 Dec 2015
Keywords:
Bacillus sphaericus
Lombok Island
Mosquito larvae
Culex quinquefasciatus
Anopheles aconitus
Aedes aegypti
ABSTRACT
Objective: To isolate, characterize and evaluate toxicity of Bacillus sphaericus (B. sphaericus) from beach area of Lombok Island.
Methods: Soil was collected from determined locations and suspended in sterile physiological saline water. After heat shock was applied, suspension was spread on NYSM agar medium. Colonies grown were then observed and isolated. Colony, cell morphology, and biochemical/physiological characteristics were tested and compared to B. sphaericus 2362 as standard. Initial toxicity testing was done against three species of mosquito larvae (Culex quinquefasciatus, Anopheles aconitus and Aedes aegypti) and isolates that showed more than 50% larvae killing will be assayed to obtain LC50and LC90values within 48 h. PCR technique were conducted to obtain 16s rDNA amplicon for sequencing and to detect toxin-expressing genes (using multiplex PCR).
Results: Twenty isolates of B. sphaericus have been collected from 20 determined locations and their characteristics were in agreement with standard B. sphaericus characteristics. Bioassay testing showed that four isolates (namely isolate MNT, SLG, TJL2 and PLG)weremildlytoxicagainstalllarvae.Therestswereeitherlowtoxicornon-toxicatall. Phylogenetic analysis showed that all four isolates were clustered with other known mildly and highly toxic strains. The multiplex PCR result showed four toxic isolates owned 1–2 bandsfrom Bintoxingenesandthreebandsfrom Mtxtoxingenes,whereas16isolateswith low to non-toxic characteristics showed only three bands from Mtx toxin genes.
Conclusions: Four toxic isolates of B. sphaericus were isolated from beach area of Lombok Island. They showed mild toxicity against larvae of three mosquito species.
1. Introduction
Lombok Island is one of the popular tourism destinations in Indonesia after Bali. In Lombok Island, some mosquito-borne diseases are still becoming health problems. The most popular mosquitoes are Anopheles and Aedes. Anopheles is known as the vector of malaria, whereas Aedes is the vector of dengue hemorrhagic fever. In Lombok Island there are 6400 people infected by malaria, while 760 people are reported suffering from dengue hemorrhagic fever, respectively [1]. Other mosquito genus, Culex is known for spreading filariasis and Japan encephalitis virus. Diseases spread by Culex is less common in this island[2].
In many countries mosquito control relies on chemical pesticides. Although showing high efficacy, the usage of chemical pesticides in longtermwill resultinmalicious effectonhumanand environment.Therearemanyhealthproblemsrelatedtopesticides, from abdominal pain, dizziness, headaches, nausea, vomiting, as well as skin and eye problems to cancer and developmental defect aswell[3].Sideeffectsonenvironmentarerangingfromnon-target organism killing (non-harmless insects, birds, amphibians and fishes) to increasing resistance to mosquito [4]. To prevent unwanted effects of chemical pesticides, biological-agent-based pesticides (biopesticide) should be used as an alternative. This includes microorganisms or natural products[5].
4月14日下午,青海省水利厅副厅长宋玉龙紧急召集湟中、湟源、大通、互助四县水务局主要负责同志,就保障灾区城乡供水安全作出部署,要求四县水务局分别组建15人的应急抢险小分队,由一名局领导带队,以最快速度投入到灾区救援。同时青海省防汛机动抢险队调集20t吊车1台、1m3吊车1台以及必备的抢险物资抵达灾区展开救援,另有320挖掘机2台、装载机2台、运输车辆6台以及工程抢险救援人员25人集结待命。
One popular biocontrol agent is Bacillus sphaericus (B. sphaericus) (besides Bacillus thuringiensis that was previously popular). This rod-formed Gram positive bacteria that has terminalbulgingendosporeshowshightoxicityagainst Culexand Anopheles,whileitshows thelowest susceptibility against Aedes. The bacteria has been used extensively in some countries. Many strainsandtheirpotentialshavebeenstudiedaswell[6].However, little is known about B. sphaericus isolated from Lombok Island and its potential against Culex, Anopheles and Aedes larvae.
Development of local strain-based biopesticide/bacterial agent is very important to suppress cost from importing from foreign countries and also to promote local industry capability. Some researchers have developed medium formulation and production system to propagate potential bacterial agent using local and low-cost ingredient [7]. This capability will enable biopesticide production on community and low industrial level, without sacrificing effectiveness of the bacterial agent in controlling mosquito larvae.
In this study, we collected B. sphaericus from 20 locations near beach area around Lombok Island. We also evaluated toxicity of isolated B. sphaericus against Culex, Anopheles and Culex larvae. Toxin gene detection was also performed to support the toxicity attribute of isolated B. sphaericus.
2. Materials and methods
2.1. Bacterial isolation and characterization
Twenty locations near beach area around Lombok Island were determined for soil collection. Locations were chosen based on closeness to village (Kampung) and predictive mosquito breeding habitat (small ditch, river opening, etc.). The sampling locations are presented in Figure 1.
Five hundred grams of soil from those areas were collected compositely and stored at sterile screw capped container. The isolation procedure was done as follows. Suspension was made from 25 g of soil with 225 mL sterile physiological salt solution. Then it was heated at 80°C for 30 min and diluted into 10−1–10−5dilutions. The dilution was then spread on NYSM agar (nutrient agar with 0.5 g/L yeast extract, 0.2 g/L MgCl2, 0.01 g/L MnCl2, and 0.1 g/L CaCl2). Antibiotic was also added to the medium (streptomycin, 100 μg/mL) to selectively inhibit other bacteria[8].
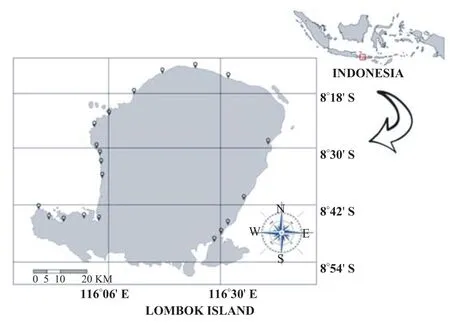
Figure 1. Sampling locations around Lombok Island, Indonesia.
Characterizationofthebacteriawasdonemorphologicallyand biochemically/physiologically. Morphological characteristics observed were colony morphology, cell structure, Gram staining and endospore form and position. Biochemical/physiological characteristics tested were catalase, starch hydrolysis, nitrate reduction, sugar utilization, indole, H2S, urease, oxidase, casein hydrolysis and aerobicity. Sensitivity against antibiotics (mainly streptomycin and chloramphenicol) were tested as well[9].
2.2. Bioassay
2.2.1. Larva preparation
A loop of single colony from chosen isolate(s) and B. sphaericus 2362 as standard were taken and inoculated on 100 mL NYSM liquid medium supplemented with 0.2 g/L MgCl2, 0.01 g/L MnCl2and 0.1 g/L CaCl2[8]. The cultures were incubated at 30°C, shaken at 170 r/min for 72 h.
2.2.3. Initial toxicity testing
This testing was based on procedures mentioned by Dulmage et al. [10]. Cx. quinquefasciatus, An. aconitus and Ae. aegypti larvae were prepared in containers (20 larvae per container in three replications)filled with well water (untreated water) and mixed with bacterial culture (B. sphaericus isolates) reached 10% (v/v) in 200 mL total volume. Larvae death was observed in 48 h after application. Average larvae death was calculate for each B. sphaericus isolates tested.
2.2.4. Bioassay
Third instarlarvaeof Cx.quinquefasciatus, An.aconitusand Ae. aegypti were prepared in 63 containers (21 containers for each species). Each container was filled with 200 mL untreated water mixed with liquid culture of isolated B. sphaericus (in 7 dilutions with 10-fold different concentrations in 3 replications)[10]. This procedure was done for every isolate that showed more than 50% larvae killing in initial toxicity testing. Dead larvae observation from each dilution was made at 48 h after application. Probit analysis was made from the observation and lethal values (LC50and LC90on 48 h observation) were calculated with 0.05 of significance level using Minitab statistical software version 16 for Windows.
2.3. Phylogenetic analysis and toxin gene detection
2.3.1. DNA extraction
The method was described by Ausubel et al. [11] with slight modification. A full loop of B. sphaericus isolates from solid NYSM agar was added into 560 μL of TE buffer (pH 7.6). Ten microliters of lysozyme (concentration of 30 mg/mL) was added and the mixture was then incubated at 37°C for 1 h. Thirty microliters of 10% SDS and 10 μL of 20 mg/mL Proteinase K were added. The mixture was then incubated at 37°C for 1 h. One hundred microliters of 5% NaCl and 80 μL of cetyl trimethyl ammonium bromide (concentration of 10% in NaCl) were then added to the mixture and incubated at 65°C for 1 h. Extraction with phenol: chloroform (1:1 v/v) was done and the mixture was stirred at 10000 r/min for 5 min. Upper phase was collected and mixed with 2×volume of absolute ethanol. After being incubated at−20°C overnight, the mixturewas then stirred at 12000 r/min for 20 min and supernatant was carefully discarded. Pellet obtained was washed with 75% ethanol. After being stirred at 12000 r/min for 30 min, DNA pellet was dissolved with 50 μL of TE buffer (pH 7.6).
2.3.2. Phylogenetic analysis
Genomic DNA of isolated B. sphaericus was amplified using 16s rDNA primers (namely 27f and 1492r). The sequence of 27f primer was 5'-AGA GTT TGA TCM TGG CTC AG-3'and the sequence of 1492r primer was 5'-GGT TAC CTT GTT ACG ACT T-3'[12]. The amplification parameter was as follows. Predenaturation was at 94°C for 5 min, denaturation was at 94°C for 20 s, primer annealing was at 52°C for 30 s, and elongation was at 72°C for 90 min. Cycle number was 35 cycles and postelongation was at 72°C for 5 min. Amplicon resulted was then sequenced and contigs were developed from the sequences using BioEdit Program for Windows. Contig alignment and tree construction were performed using MEGA V5 for Windows[13].
2.3.3. Toxin gene identification
The existence of toxin genes owned by B. sphaericus was detected with multiplex PCR applying bin and mtx gene primer pairs [14] presented in Table 1. Amplification parameter was as follows. Pre-denaturation was at 94°C for 5 min, followed by denaturation at 94°C for 30 s, primer annealing at 45°C for 30 s and elongation at 72°C for 60 s. The cycle were 35 cycles with final elongation at 72°C for 5 min. Amplicon was analyzed using agarose gel electrophoresis in 2% gel.
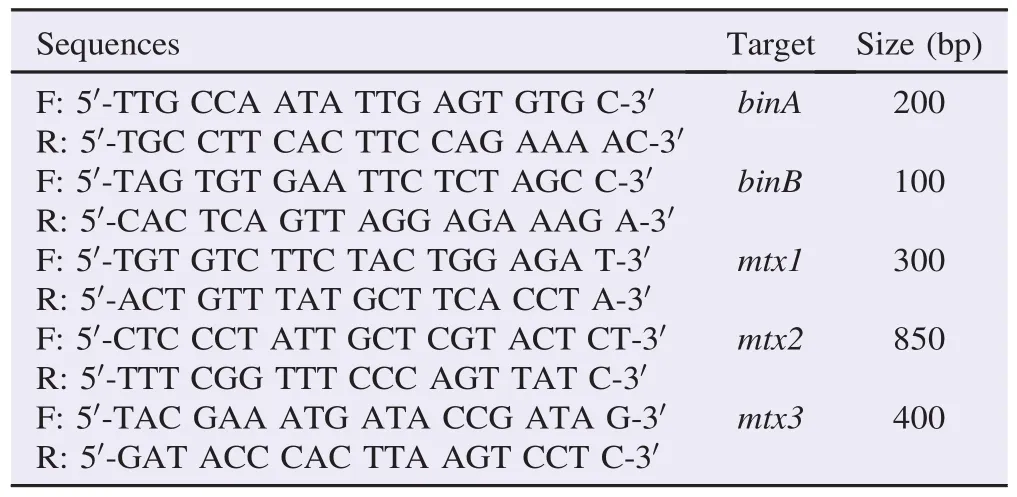
Table 1Sequence of primers used in multiplex toxin detection PCR.
3. Results
3.1. Bacterial isolation and characterization
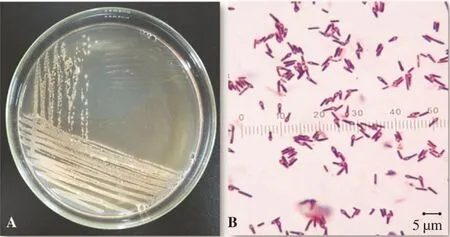
Figure 2. Colony and cell morphology.A: Colony on NYSM agar 72 h later; B: Gram staining of isolated B. sphaericus (1000×).
From all sampling locations, 20 isolates of B. sphaericus were isolated.Thecharacteristicsof B.sphaericusisolatedarepresented in Figure 2. Detail characterization (colony, cell and biochemical/ physiological)ofthe B.sphaericusisolatesispresentedin Table2.
All characteristics of isolated B. sphaericus have showed similarity to those mentioned in Bargey's Manual of Determinative Bacteriology[9], except for some characteristics that were varied. All isolates were collected from area that was unexposed directly and indirectly by sea water (in form of salt dam/pool or rip-tide). Sampling points were sand/soil covered/shaded by foliage and rich in organic matters.
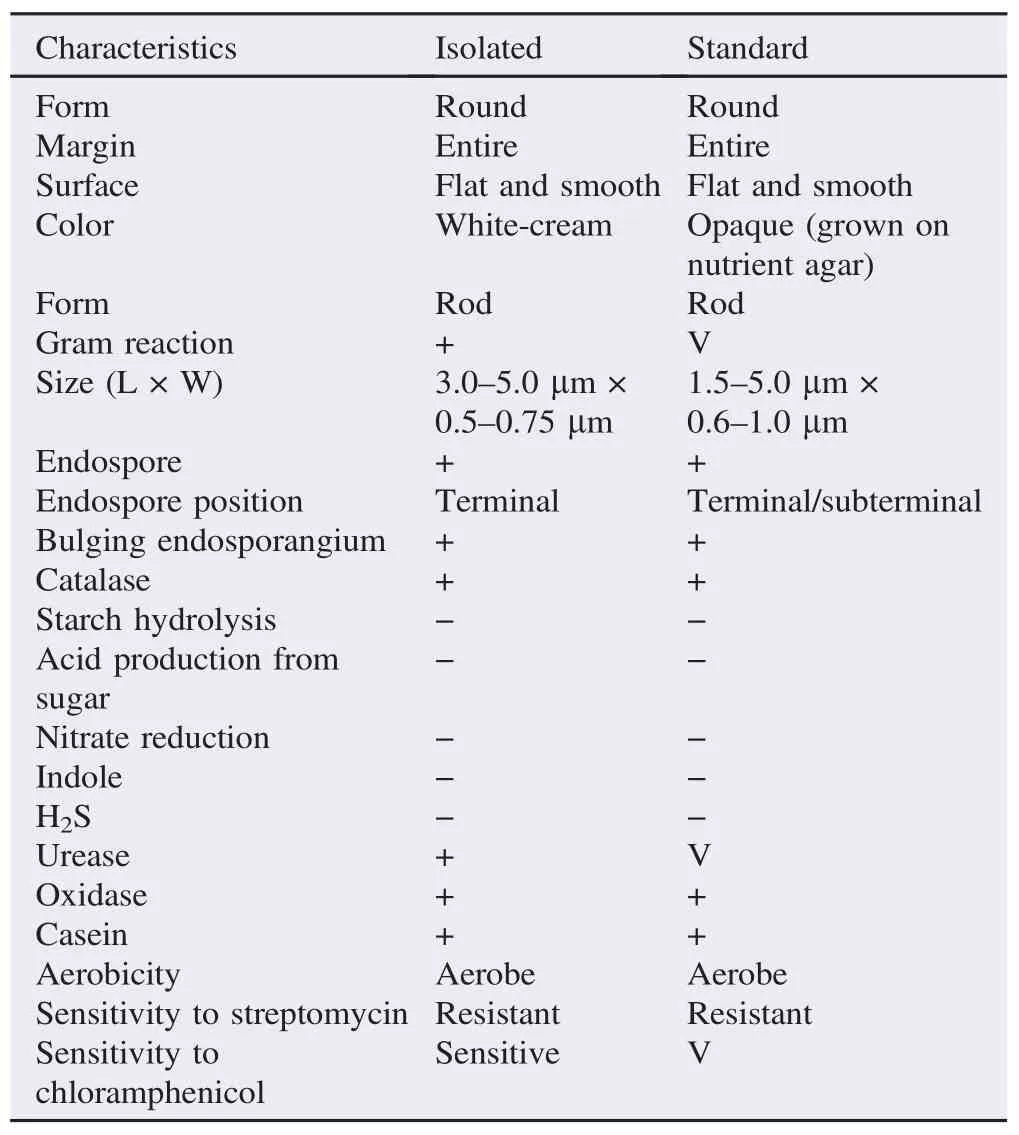
Table 2Culture characteristics of the isolated B. sphaericus.
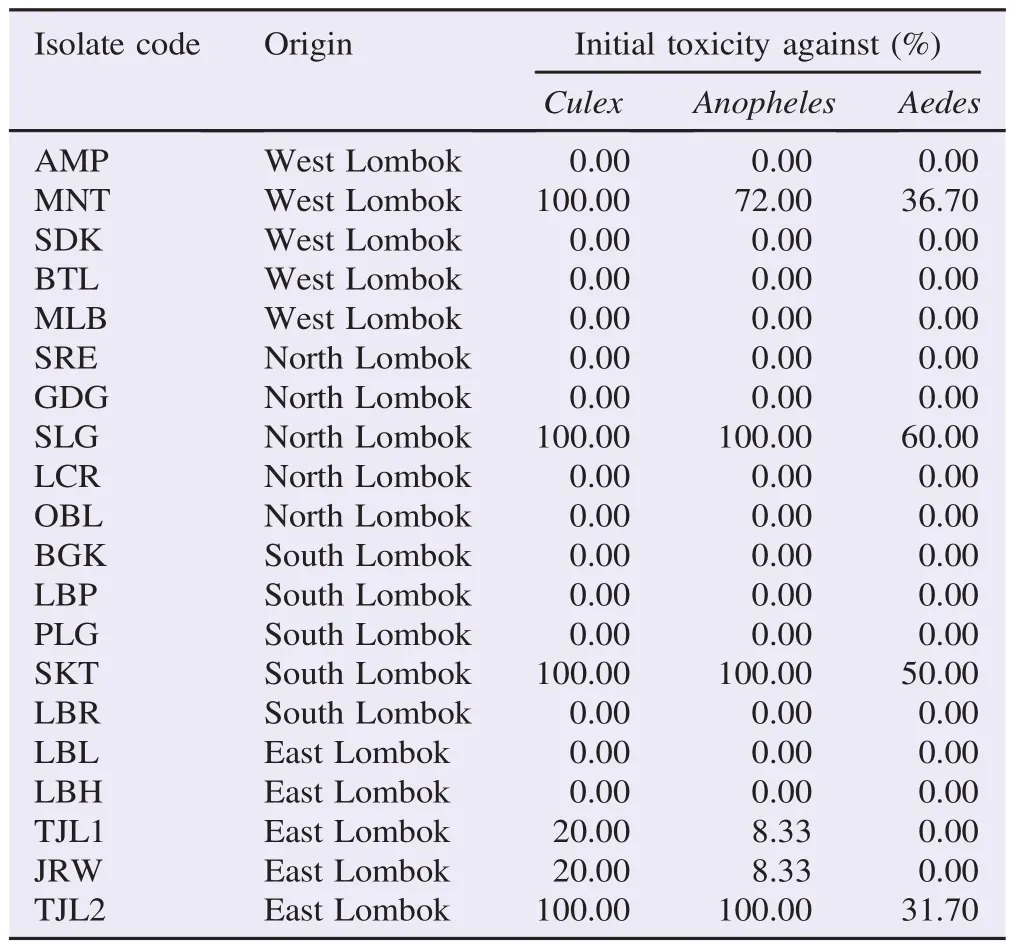
Table 3Isolates of B. sphaericus from many location/origin with their toxicity against 3 species of mosquitoes.
3.2. Toxicity of isolated B. sphaericus
Table 3 presents toxicity testing results of isolated B. sphaericus [in 10−1final whole culture (FWC) dilution] against 3 species of mosquitoes.
From 20 locations sampled, there were 4 locations gave isolates that were toxic against 3 kinds of mosquitoes' larvae. Others showed very low to none toxicity. The values of LC50and LC90of the isolates from the four locations in 48 h observation are presented in Table 4.
Both toxic and low toxic isolates showed similar pattern, Culex was the most susceptible to all isolates and Anopheles was the second after Culex. Toxic isolates and low toxic isolates only killed Aedes larvae in small percentages. Compared to 3 other isolates, isolate SLG was the most toxic isolates. This was followed by isolate TJL2, SKT and MNT, respectively.
3.3. Phylogenetic analysis and toxin gene detection
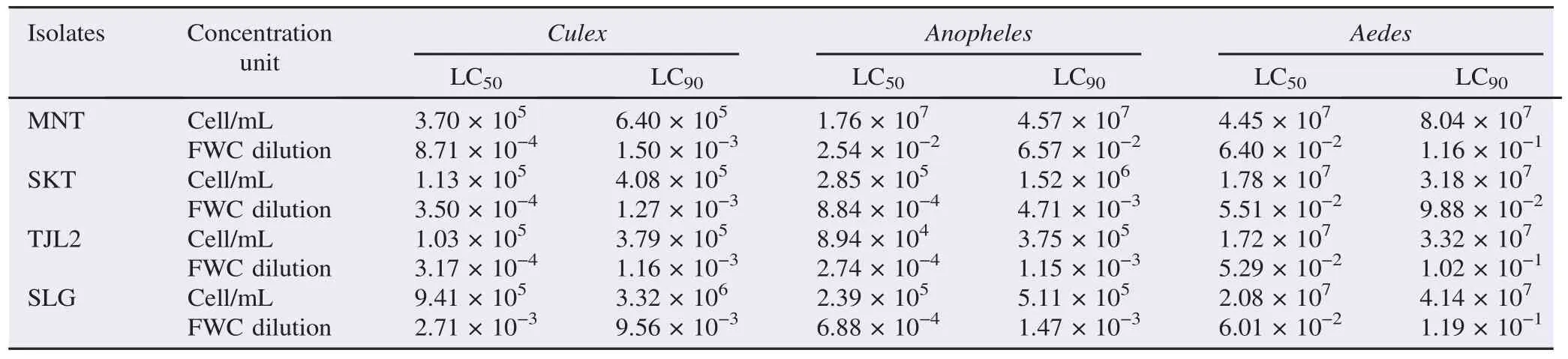
Table 4LC values of isolated B. sphaericus in 48 h.
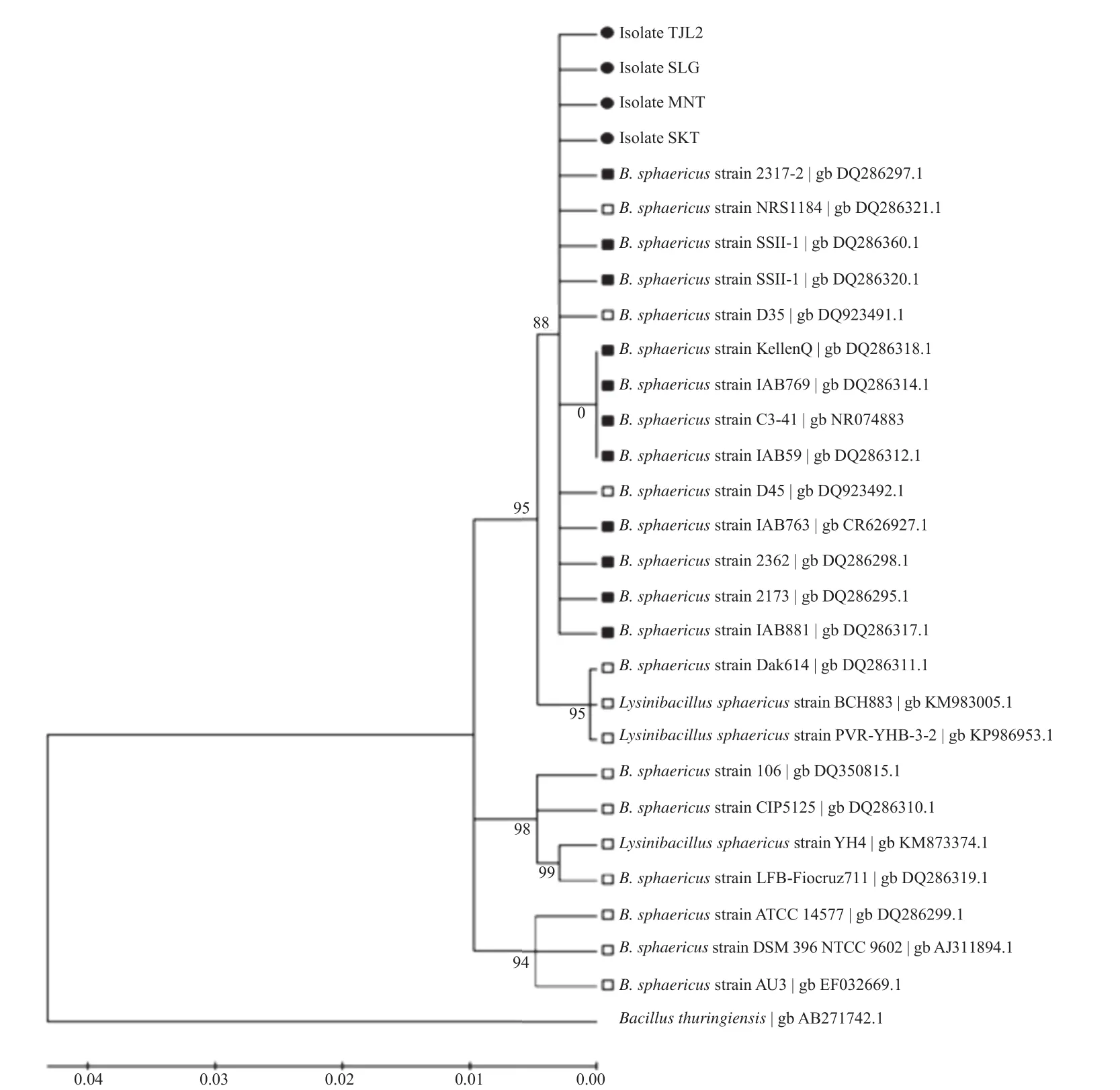
Figure 3. Neighbor-joining cladogram of isolated B. sphaericus (marked with dot symbol) with known strains of toxic B. sphaericus (marked with solid square symbol) and non-toxic B. sphaericus (marked with hollow square symbol) and with B. thuringiensis as outgroup species.
Phylogenetic tree where isolated B. sphaericus and some strains of known B. sphaericus as comparison is presented in Figure 3. Isolates PLG and MNT were in the same cluster with some known highly and mildly toxic strains of B. sphaericus. Isolates TJL2 and SLG were close to eachother, but not in the same cluster with first two isolates. Grouping B. sphaericus using 16s rRNA approach only showed strain closeness by 16s rRNA sequence similarity, but it cannot group their toxicity.
Toxin gene detection PCR result is presented in Figure 4. The existence of binary toxin components (represented by 100 bp for binB and 200 bp for binA PCR product) can be related to the high anti-larval effect of B. sphaericus strain (shown by B. sphaericus 2362, SLG, TJL2 and PLG). Both Bin components showed higher anti-larval effect compared to single component (showed by isolate MNT). Mosquitocidal toxin (represented by 300 bp for mtx1, 400 bp for mtx3 and 850 bp for mtx2) showed very low anti-larval effect (or none at all).
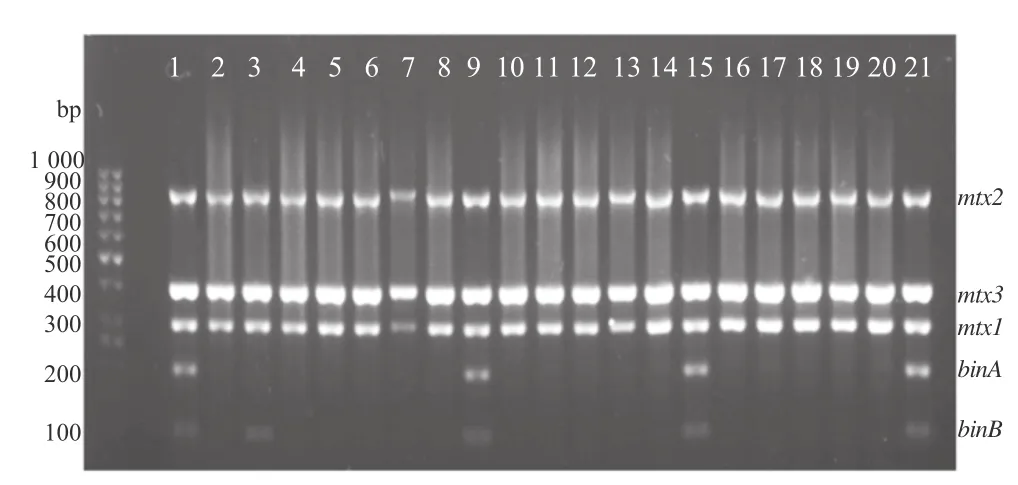
Figure 4. Toxin genes (bin and mtx) multiplex amplification result in 2% agarose gel.Lane 1: B. sphaericus 2362; Lane 2: Isolate AMP; Lane 3: Isolate MNT; Lane 4: Isolate SDK; Lane 5: Isolate BTL; Lane 6: Isolate MLB; Lane 7: Isolate SRE; Lane 8: Isolate GDG; Lane 9: SLG; Lane 10: Isolate LCR; Lane 11: Isolate OBL; Lane 12: Isolate BGK; Lane 13: Isolate LBP; Lane 14: Isolate PLG; Lane 15: Isolate SKT; Lane 16: Isolate LBR; Lane 17: Isolate LBL; Lane 18: Isolate LBH; Lane 19: Isolate TJL1; Lane 20: Isolate JRW; Lane 21: Isolate TJL2.
4. Discussion
This report was the first report of B. sphaericus isolated from Lombok Island, Indonesia. The most-frequently used strain of B. sphaericus that has been applied in some countries is B. sphaericus 2362 that was firstly isolated in Nigeria. This strain was isolated from black fly [15]. This strain becomes one standard for comparison with newly isolated B. sphaericus from many countries.
Since providing all nutrients and growing factors for bacteria that live in it, soil becomes a potential habitat for B. sphaericus. However, this study showed that area that was rich in organic matters did not always become habitat for toxic B. sphaericus. From all locations that were explored, toxic isolates of B. sphaericus were obtained only from 4 locations. Other isolates from the rest 16 locations were low toxic on no toxic at all. This demonstrated that soil rich in organic matters was not the key factor to obtain toxic B. sphaericus. Any chances that enable bacteria contact with mosquito larvae should be considered to gain toxicity. That was described in some reports in the discovery of Bacillus thuringiensis and B. sphaericus [16,17].
It was mentioned by de-Barjac [18], that toxicity of B. sphaericus can be categorized into 3 classes. The highest toxicity showed LC50value of 10−6–10−8(based on final whole concentration/FWC dilution). The examples of high toxicity strains are B. sphaericus strain 2362, 1593 and LB24. While mild toxicity showed LC50value of 10−4–10−5of FWC dilution. The examples of strains having this toxicity are B. sphaericus strain SSII-1, ISPC5 and LB29. The lowest value is ranging from 10−2to 10−3(of FWC dilution), and B. sphaericus strain K and strain Q are low toxic strains discovered in the United States. All those LC values were against Culex larvae. If compared to that category, B. sphaericus isolated from Lombok Island can be grouped into lowly to mildly toxic isolates.
Larvae death occurred within some hours after B. sphaericus endospores have been ingested by a larval. After ingested, protease released in the midgut will process the endospores into toxins subunit components. Toxin related to sporulated B. sphaericus is binary toxin. The binary toxin will be broken into two components, namely BinA (51 kDa) and BinB (42 kDa). This toxin will make interaction with specific receptor along Culex and Anopheles midgut. However, in Aedes midgut, there are no interaction between toxin and toxin receptor. Hence, binary toxin will give the highest anti-larvicidal activity only against Culex and Anopheles. On the other hand, Aedes showed the least susceptibility against B. sphaericus [19].
Besides binary toxin, B. sphaericus also synthesizes nonsporulation related toxin, called mosquitocidal toxin (Mtx toxin). The toxin is produced when the bacteria is on vegetative life stage. This toxin comprises of Mtx1 (100 kDa), Mtx2 (32 kDa) and Mtx3 (36 kDa) subunits [20,21]. In contrast with binary toxin, mosquitocidal toxin does not interact with receptor inside larvae midgut and only give low antilarvicidal effect to B. sphaericus [22]. The low toxicity of mosquitocidal toxin was caused by proteolytic degradation on Mtx toxin [23]. When intact, Mtx toxin (mainly Mtx1 subunit) shows ability to kill larvae better than binary toxin [24]. This was shown that when mtx genes were cloned into protease-free non-toxic B. sphaericus strain, mtx1 gene expression in protease-free B. sphaericus could kill mosquito larvae as good as B. sphaericus strain harboring Bin toxin protein and naturally toxic B. sphaericus strain [25]. B. sphaericus toxins activities will cause collapse of larvae nervous and muscle system. In turn, this will make the larvae loss their ability to move along water surface and undergo asphyxia by drowning [26].
Grouping B. sphaericus using 16s rRNA could not cluster this species based on strain toxicity, so other approaches should be made. Grouping using flagella agglutination by de-Barjac et al. [27] has clustered B. sphaericus in serotype H1A; H2; H5; H5A, 5B; H2 and H5. This grouping clustered B. sphaericus toxicity very well. Other approach using phage typing [28], resulted in four phagetypes groups, which was able to cluster B. sphaericus based on their toxicity as well. This phylogenetic analysis, besides showing closeness of these toxic B. sphaericus isolates to other known B. sphaericus strains, also supports identification of the bacteria, since there are some species show similar characteristics to B. sphaericus. The other technique, such as multiplex PCR which we used in this study, was able to detect toxin-expressing genes (binary and mosquitocidal toxins).
Multiplex PCRisvariantof PCRtechniqueapplyingmorethan one primer pairs to amplify more than one locus/positions simultaneously [29]. In this study, multiplex PCR succeeded to detect the existence of Bin and Mtx toxins altogether. This supports toxicity testing and bioassay results. Mild to high toxicity effect of B. sphaericus can be expected if two PCRproducts present (100 and 200 bp). PCR products of 100 bp and 200 bp are related to existence of binB and binA genes, respectively. These genes express binary toxin in non-vegetative stage of B. sphaericus that are most active in killing mosquito larvae.OtherPCRproducts,300,400and850bpwhicharerelated to mtx1, mtx3 and mtx2 genes, showed very little effect of killing mosquito larvae (or none at all). If all PCR products exist altogether, high toxicity effect can be expected.
Many strains of B. sphaericus (either toxic or not toxic) have been studied and collected in some countries, but local strain is still promising for local-ingredient-based biopesticide candidates. Along with more toxic B. sphaericus strain search, growth and production medium formulation should be explored as well.
Twenty B. sphaericus isolates from beach areas around Lombok Island have been isolated and 4 isolates showed low to mild toxicity against 3 species of mosquito larvae. The rest 16 isolates have showed either very low toxicity or non-toxicity at all.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors thank for the financial support from Ministry of Education and Culture Republic of Indonesia, Directorate General of Higher Education (DGHE) scholarship batch 2012 (Grant No. 2625/E4.4/2012).
We also thank to Head of IVRCRD, Salatiga, Central Java, for giving us permission to utilize mosquito rearing and testing facilities and Mrs. Esti Rahadiningtyas of IVRCRD for providing us B. sphaericus 2362 used for comparison in this study.
References
[1] Ministry of Health of Indonesia. [Health profiles of West Nusa Tenggara Province in 2012]. Jakarta: Ministry of Health of Indonesia; 2013. Indonisia. [Online] Available from: http://dinkes. ntbprov.go.id/sistem/data-dinkes/uploads/2013/10/Data-Kesehatan-Provinsi-NTB-2012.pdf [Accessed on 23rd January, 2015]
[2] World Health Organization. WHO campaign: about vector-borne diseases. Geneva: World Health Organization; 2015. [Online] Available from: http://www.who.int/campaigns/world-healthday/2014/vector-borne-diseases/en/ [Accessed on 7th August, 2015]
[3] Lorenz ES. Potential health effects of pesticides. Pennsylvania: Penn State's College of Agricultural Sciences; 2009. [Online] Available from: http://extension.psu.edu/pests/pesticide-education/ applicators/fact-sheets/pesticide-safety/potential-health-effects-ofpesticides/extension_publication_file [Accessed on 7th August, 2015]
[4] Denholm I, Devine GJ, Williamson MS. Evolutionary genetics. Insecticide resistance on the move. Science 2002; 297(5590): 2222-3.
[5] United States Environmental Protection Agency. What are biopesticides. Washington: United States Environmental Protection Agency; 2015. [Online] Available from: http://www2. epa.gov/ingredients-used-pesticide-products/what-are-biopesticides [Accessed on 20th November, 2015]
[6] Poopathi S, Abidha S. Mosquitocidal bacterial toxins (Bacillus sphaericus and Bacillus thuringiensis serovar israelensis): mode of action, cytopathological effects and mechanism of resistance. Glob Sci Res J 2013; 1(1): 53-69.
[7] Yadav K, Dhiman S, Baruah I, Singh L. Development of cost effective medium for production of Bacillus sphaericus strain isolated from Assam, India. Microbiol J 2011; 1(2): 65-70.
[8] Vanlalhruaia N, Kumar S, Gurusubramanian G. Bacillus sphaericus in the biological control of mosquito vector complex. Sci Vis 2011; 11(2): 61-71.
[9] Holt JG. Bergey's manual of determinative bacteriology. 9th ed. Baltimore: The Williams & Wilkins Company; 1994.
[10] Dulmage T, Yousten AA, Singer S, Lacey LA. Guidelines of production of Bacillus thuringiensis H-14 and Bacillus sphaericus. Geneva: World Health Organization; 1990. [Online] Available from: https://extranet.who.int/iris/restricted/handle/10665/61645[Accessed on 23rd January, 2015]
[11] Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, et al. Current protocols in molecular biology. New York: John Wiley & Sons Inc; 1995.
[12] James G. Universal bacterial identification by PCR and DNA sequencing of 16S rRNA gene. In: Carter IWJ, Schuller M, James GS, Sloots TP, Halliday CL, editors. PCR for clinical microbiology. Netherlands: Springer; 2010, p. 209-14.
[13] Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011; 28(10): 2731-9.
[14] PrabhuDI,Sankar SG,VasanPT,PiriyaPS,SelvanKB,VennisonSJ. Molecular characterization of mosquitocidal Bacillus sphaericus isolated from Tamil Nadu, India. Acta Trop 2013; 127(3): 158-64.
[15] Weiser J. A mosquito-virulent Bacillus sphaericus in adult Simulium damnosum from northern Nigeria. Zentralbl Mikrobiol 1984; 139(1): 57-60.
[16] Goldberg LH, Margalit J. A bacterial spore demonstrating rapid larvicidal activity against Anopheles sergentii, Uranotaenia unguiculata, Culex univitatus, Aedes aegypti and Culex pipiens. Mosq News 1977; 37(3): 355-8.
[17] Kellen WR, Clark TB, Lindegren JE, Ho BC, Rogoff MH, Singer S. Bacillus sphaericus Neide as a pathogen of mosquitoes. J Invertebr Pathol 1965; 7(4): 442-8.
[18] de-Barjac H. Classification of B. sphaericus strains and comparative toxicity to mosquito larvae. In: de Barjac H, Sutherland DJ, editors. Bacterial control of mosquitoes & black flies. Netherlands: Springer; 1990, p. 228-36.
[19] Poopathi S, Tyagi BK. The challenge of mosquito control strategies: from primordial to molecular approaches. Biotechnol Mol Biol Rev 2006; 1(2): 51-65.
[20] Arapinis C, de la Torre F, Szulmajster J. Nucleotide and deduced amino acid sequence of Bacillus sphaericus 1593M gene encoding a 51.4 kD polypeptide which act synergistically with the 42 kD protein for the expression of the larvicidal toxin. Nucleic Acid Res 1988; 16(15): 7731-9.
[21] Baumann L, Broadwell AH, Baumann P. Sequence analysis of the mosquitocidal toxin genes encoding 51.4- and 41.9-kilodalton protein of Bacillus sphaericus 2362 and 2297. J Bacteriol 1988; 170(5): 2045-50.
[22] Nielsen-LeRoux C, Raghunatha Rao D, Murphy JR, Carron A, Mani TR, Hamon S, et al. Various levels of cross-resistance to Bacillus sphaericus strains in Culex pipiens (Diptera: Culicidae) colonies resistant to B. sphaericus strain 2362. Appl Environ Microbiol 2001; 67(11): 5049-54.
[23] Yang Y, Wang L, Gaviria A, Yuan Z, Berry C. Proteolytic stability of insecticidal toxins expressed in recombinant bacilli. Appl Environ Microbiol 2007; 73(1): 218-25.
[24] Wei S, Cai Q, Yuan Z. Mosquitocidal effect of Bacillus sphaericus induces stronger delayed effect than binary toxin on Culex quinquefasciatus (Diptera: Culicidae). J Med Entomol 2006; 43(4): 726-30.
[25] Thanabalu T, Porter AG. A Bacillus sphaericus gene encoding a novel type of mosquitocidal toxin of 31.8 kDa. Gene 1996; 170(1): 85-9.
[26] Litaiff EC, Tadei WP, Porto JIR, Oliveira IMA. Analysis of toxicity on Bacillus sphaericus from amazonian soils to Anopheles darlingi and Culex quinquefasciatus larvae. Acta Amazon 2008; 38(2): 255-62.
[27] de-Barjac H, Larget-Thi´ery I, Cosmao Dumanoir V, Ripouteau H. SerologicalclassificationofBacillussphaericusonthebasisoftoxicity to mosquito larvae. Appl Microbiol Biotechnol 1985; 21: 85-90.
[28] Yousten AA, de-Barjac H, Hendrick J, Cosmao Dumanoir V, Myers P. Comparison between bacteriophage typing and serotyping for differentiation of Bacillus sphaericus strains. Ann Microbiol (Paris) 1980; 131B(3): 297-308.
[29] Hayden MJ, Nguyen TM, Waterman A, Chalmers KJ. Multiplexready PCR: a new method for multiplexed SSR and SNP genotyping. BMC Genomics 2008; 9: 80.
*Corresponding author:Bambang Fajar Suryadi, Department of Biology, Faculty of Mathematics and Natural Sciences, Mataram University, Jalan Majapahit No. 62, Mataram 83125, West Nusa Tenggara, Indonesia.
猜你喜欢
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Inhibitory actions of Pseuderanthemum palatiferum (Nees) Radlk. leaf ethanolic extract and its phytochemicals against carbohydrate-digesting enzymes
- Feasibility of using melatonin as a new treatment agent for Ebola virus infection: A gene ontology study
- Prevalence of refractive errors among primary school children in a tropical area, Southeastern Iran
- Inhibitory effect of gold nanoparticles conjugated with interferon gamma and methionine on breast cancer cell line
- Step-by-step external fixation of unstable pelvis with separate anterior and posterior modules
- Evaluation of imatinib mesylate (Gleevec) on KAI1/CD82 gene expression in breast cancer MCF-7 cells using quantitative real-time PCR
