Female preference promotes asynchronous sex evolution in Elephantiformes
2016-03-29WANGShiQiDENGTao
WANG Shi-QiDENG Tao
(1Key Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences,Institute of Vertebrate Paleontology and Paleoanthropology,Chinese Academy of SciencesBeijing 100044 wangshiqi@ivpp.ac.cn)
(2CAS Center for Excellence in Tibetan Plateau Earth SciencesBeijing 100101)
(3Key Laboratory of Economic Stratigraphy and Palaeogeography,Chinese Academy of Sciences(Nanjing Institute of Geology and Palaeontology) Nanjing 210008)
Female preference promotes asynchronous sex evolution in Elephantiformes
WANG Shi-Qi1,2,3DENG Tao1,2
(1Key Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences,Institute of Vertebrate Paleontology and Paleoanthropology,Chinese Academy of SciencesBeijing 100044 wangshiqi@ivpp.ac.cn)
(2CAS Center for Excellence in Tibetan Plateau Earth SciencesBeijing 100101)
(3Key Laboratory of Economic Stratigraphy and Palaeogeography,Chinese Academy of Sciences(Nanjing Institute of Geology and Palaeontology) Nanjing 210008)
Sexually dimorphic characters are usually thought to enhance copulatory success by intraspeci fi c competition; for example, larger body size and stronger tusks are sexually dimorphic characters in fossil and extant male proboscideans. Here, we show that some sexually dimorphic characters in fossil Elephantiformes, the largest group of proboscideans, are strongly correlated with the evolution of this group rather than direct sexual competition. In MiocenePlatybelodon grangeriandGomphotherium angustidens, males tended to initially possess evolutionarily more derived characters than females, and females then evolved similar variation. This phenomenon may have occurred as a result of female preference. During the early evolutionary stage (thriving stage) of Elephantiformes, sexual selection pressure promoted development of more prominent derived characters in males than females. However, during their late evolutionary stage (declining stage), sexual selection pressure seems to have weakened; thus, the asynchrony between the two sexes diminished. This new discovery may help explain a common mechanism of large ungulate evolution and extinction, because substantial sexual dimorphism is often displayed in thriving groups, such as Cervidae and Bovidae, in contrast to little sexual dimorphism in declining groups, such as extant taxa of Equidae, Rhinoceridae, and Giraf fi dae.
Elephantiformes, sexual dimorphism, female preference, evolution
1 Introduction
Since Darwin, sexual selection has been considered the key factor that influences secondary sexually dimorphic character development within a species (Darwin, 1874; Fisher, 1930; Andersson, 1994). As a result, these characters are thought to improve male copulatory success by intraspeci fi c competition. However, the relationship between sexual dimorphismand evolution is not fully understood. For example, in Elephantiformes Tassy, 1988, sexual dimorphism has been observed in OligocenePhiomia, MiocenePlatybelodon grangeri,Gomphotherium angustidens, ?Stegotetrabelodonsp. (Matsumoto, 1924; Osborn and Granger, 1932; Lambert, 1992; Tassy, 1996, 2013; Bibi et al., 2012; Wang et al., 2013), and extant elephants (Nowak, 1999; Roth and Shoshani, 1988; Kurt et al., 1995; Douglas-Hamilton et al., 2006; Lee and Poole, 2011). InG. angustidensthere is sexual dimorphism not only of body size and tusk characteristics but also of other characters that have not been fully understood (Tassy, 1996, 2013). Furthermore, in large ungulates, substantial sexual dimorphism is often exhibited in early evolutionary stage (thriving) groups, such as Cervidae and Bovidae; however, little sexual dimorphism in late evolutionary stage (declining) groups, such as extant taxa of Equidae, Rhinoceridae, and Giraf fi dae. Therefore, the mechanism underlying sexual dimorphism and evolution in this group should be studied further.
Recently, a population of fossilP. grangeriwas discovered from the Middle Miocene Zengjia locality, Guanghe County, Gansu Province, China (Fig. 1). This population included a large number of crania and mandibles (Wang et al., 2013), and therefore provided a good model to study sexual dimorphism in Miocene Elephantiformes and evaluate the relationship between elephantiforme sexual dimorphism and evolution.
Fig. 1 Map showing the Zengjia locality (N 35°26'23.1", E 103°26'37.6", H 2150 m) yielding the population ofPlatybelodon grangeri
Institutional abbreviations AMNH, American Museum of Natural History, New York, USA; BPV, Beijing Natural History Museum, Beijing, China; HMV, Paleozoological Museum, Hezheng, Gansu, China; MNHN, Muséum National d’Histoire Naturelle, Paris, France.
2 Materials and methods
Specimens All of thePlatybelodon grangerispecimens from the Zengjia locality are housed in HMV, and a total of 32 specimens were analyzed: HMV 0014-0020, 0022-0039, 0042, 0043, 0939, 0940, 1836, 1841, and 1842. The ontogenetic ages of the specimens range from Dental Age XVIII to XXI (Tassy, 2013), which means all of the specimens were adults (as determined based on functioning m3 and/or M3) with similar ontogenetic ages.P. grangerifrom Tunggur are AMNH 26408, 26460, 26462, 26469, 26472, and 26490 (Dental Ages from XVIII to XX). These specimens are from thePlatybelodonQuarry and Wolf Camp localities because they were found in the same horizon (Wang et al., 2003).Loxodonta africanaspecimens are four adult females (AMNH 32732, 42469, 51949, and 88404) and four adult males (AMNH 32734, 39083, 51939, and 113819). TwoGomphotherium angustidenscrania are MNHN Si37 (male, Dental Age XIX) and MNHN SEP185 (female, Dental Age XXI). An adult skull with an associated mandible (probably a male) ofPlatybelodondanoviis BPV 2000 (Dental Age XX). All of the specimens belong to the museum collections and are open to scienti fi c research.
Locality and age All of theP. grangerispecimens that were considered part of the same population were collected from the Zengjia locality (N 35°26′23.1′′, E 103°26′37.6′′, H 2150 m, No. LX200002, Fig. 1) of the Linxia Basin, Gansu Province, China. The fossil bearing strata belong to the Middle Miocene Hujialiang Formation, which consists of grayish-yellow fine conglomerates and sandstone rocks, which indicate fluvial strata (Deng et al., 2013). The fauna is also composed of a typical Middle Miocene mammalian community, includingCastorsp.,Alloptoxsp.,Pseudaelurussp.,Gomphotheriumcf.G. subtapiroideum,G. wimani,Zygolophodoncf.Z. gobiensis,Chalicotheriumsp.,Anchitherium gobiensis,Hispanotherium matritense,Listriodonsp.,Kubanochoerussp., and Cervidae indet. (Deng et al., 2013).
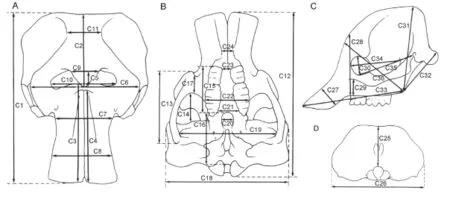
Fig. 2 The cranial measurements of Elephantiformes, after Tassy (1996: fi g. 11.1, slightly revised), not scaled A. dorsal view; B. ventral view; C. lateral view; D. posterior view
Measurements Cranial and mandibular measurements of elephantiforme specimens were based on those described by Tassy (2013); a total of 36 cranial measurements (Fig. 2, indicated by “C”) and 24 mandibular measurements (Fig. 3, indicated by “M”) were taken. There was one minor change to the measurement protocol: M1 was taken from the anterior border of the incisive alveolus instead of from the lower tusk, which we did not analyze in the present article, to the mandibular condyles.
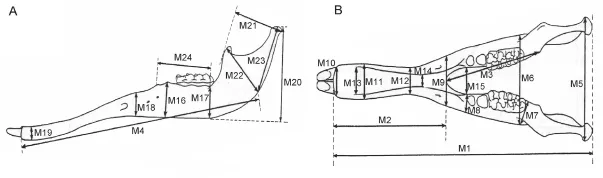
Fig. 3 The mandibular measurements of Elephantiformes in lateral (A) and dorsal (B) views After Tassy (1996: fi g. 11.1, slightly revised), not scaled
Measurement inclusion In multivariate sex assessment, measurements that re fl ect little sexual dimorphism and have substantial variation may affect the results. Therefore, the only measurements used in analysis are those for which the coef fi cient of variation is less than 15. The included measurements consist of a total of 21 cranial measurements, C1-4, 6-8, 10-12, 14, 15, 18, 19, 22, 30, 31, and 33-36, and 18 mandibular measurements, M1-10, 12, 13, 17, 20-24.
Data prediction and modification In the multivariate sex assessment analysis, a complete data set is ideal but not available because of the incompleteness of specimens. Therefore, we used a linear regression method (Wang and Deng, 2010) to predict the missing measurements.
For the analyzed material, some measurements were questionable because of substantial deformation or unreliable restoration. In these cases, the questionable data were considered missing. Then, the data were weighted based on the mean of the raw data and the predicted data. The weighted measurement values were calculated as follows:

wherewis the weight,pis the predicted datum, andmis the original measured value (1 -wis assigned as the weight for predicted data). Complete pseudo data sets were constructed using this procedure.
Data normalization We estimated the total length, or size factor (SF), of each specimen based on the C1 and M1 values. In the complete pseudo data set, data were divided by each SF to produce a size-normalized pseudo (SNP) data set to obtain shape information. Then, the SNP data set was logarithmically transformed, because each measurement was of equal importance for morphological discrimination and should be analyzed on the same scale. The following formula was used:
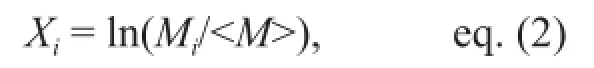
whereMiwas an SNP value of a certain measurement of specimeniand〈M〉 was the mean of the SNP values for all specimens. This new data set was defined as the size-normalized logarithmic pseudo (SNLP) data set for multivariate analyses.
Sex assessment Before sex assessment, only the length of upper incisor could be directly used for sexual discrimination (〈ca. 300 mm for females and 〉ca.300 mm for males, Fig. 4). These typical adult crania were fi rst selected to form the SNLP data sets (males and females were considered two different groups) and examined by principal component analysis (PCA). The resulting principal components (PCs), of which the cumulative variance contribution rate exceeded 95%, were selected as new variables for Fisher’s discriminant function analysis (DFA), and the fi rst discriminant function (DF1) was calculated. Then, the remaining crania of uncertain sex were examined using the DF1 (Appendix 1) and we preliminarily determined the sex for each cranium. Then, these crania were divided into two groups based on inferred sex, and two more SNLP data sets were constructed then re-examined in a new PCA-DFA cycle. If a cranium clustered into the opposite sex group in DFA, we changed the cranium’s sex assignment and began another PCA-DFA cycle. Cycles did not stop until the data clustered appropriately based on sex assignment. After several PCA-DFA cycles, an optimal sex assessment was obtained for each cranium.
No sex assignment was proposed based on mandibular characters or measurements. The specimens with long symphyses were considered male, because some were associated with male crania (HMV 0939 and 0940). Consequently, those mandibles with short symphyses were considered female. The same PCA-DFA cycles were performed on the mandibles as on the crania until all of the mandibles had optimal sex assessments.
Univariate analyses Univariate analyses were carried out on individual characters in both sexes. Only the originally measured or modi fi ed values were used in analyses, and those specimens missing original measurements were not included. For each measurement (including M3 and m3 measurements), the mean, standard deviation (s.d.), coef fi cient of variation (c.v.), and maximal and minimal values were calculated. Two-tailed Student’s t-tests were performed between the two sexes, and p-values less than 0.05 were considered signi fi cant.
Bivariate scatter plots and histograms DF1 from the last PCA-DFA cycle and normalized length from crania and mandibles, length and width from M3 and m3, and length and maximal width of mandibular symphyses were depicted in bivariate scatter plots; the 95% con fi dential eclipses for both sexes were calculated and also included in the plots. C2/C1, C34/C31, and M11/M2 measurements of each specimen were calculated and their statistics were performed. The mean + s.d. of the results were shown in histograms. Two-tailed Student’s t-tests were performed between the two groups, and p-values less than 0.2 were considered signi fi cant.
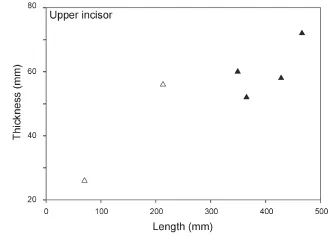
Fig. 4 Bivariate scatter plot of upper tusks in male and femalePlatybelodon grangeriSolid triangles, male; open triangles, female
Simpson’s ratio diagrams The formula for Simpson’s ratio is as follows:

wheremis a measured value in an object group,sis the corresponding value in the standard group, andris the resultant Simpson’s ratio. Bothmandsare available from individual specimens and the mean of the specimens. Simpson’s ratio diagrams were used to compare both sexes ofPlatybelodon grangeri,Gomphotherium angustidens, andLoxodonta africana.
3 Results
The results show that there is signi fi cant sexual dimorphism in the population of fossilP. grangeri(Figs. 5A, B, and Appendix 1). In addition to larger and more robust upper tusks, which are bene fi cial for direct combat, other craniomandibular sexually dimorphic characters in males include: 1) a higher-arched but shorter temporal fossa (C34/C31); 2) more posteriorly positioned nasal bones, which indicates a more developed trunk (C2/C1) (Figs. 6A, B, and I); and 3) a longer mandibular symphysis (M11/M2) (Figs. 6C, D, I, and Fig. 7). The p-values of C34/C31 (0.1775) and C2/C1 (0.1233) indicate that these ratios are signi fi cant because they are under the signi fi cance level of 0.2. This relatively high signi fi cance level is acceptable for two reasons. First, male and female individuals are in the same population of the same species. Therefore, the measurement ratios should not substantially differ. Second, the sample size is relatively small. However, the p-value of M11/M2 (0.0058) is under the significance level of 0.05 (as in the univariate analysis); this is important because we used symphysis length to initially determine sex.
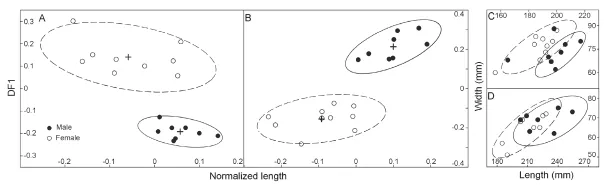
Fig. 5 Bivariate scatter plots showing sexual dimorphism inPlatybelodon grangerifrom the Zengjia locality Cranium (A) and mandible (B) are examined in PCA-DFA cycles for sex-assessment and the fi nal results are shown in normalized length-DF1 plane. As a result of sex-assessment, measurements from M3 (C) and m3 (D) are also shown on length-width plane. Two sexes are represented by solid (male) and open cycles (female), respectively, with 95% con fi dential ellipses denoting their distributions (solid-lined ellipses for male and dashlined ellipses for female)
In addition to the above craniomandibular sexually dimorphic characters, males also possess narrower and longer M3 and m3. Note that the molar measurements, which were not used for sex assessment, are independent variables; thus, this is an independent veri fi cation of our sex assessment (Figs. 5C and D). In the Simpson’s ratio diagrams, the slopes of C1 & C2, C31 & C34, and M2 & M11 differ between male and femaleP. grangeri, which also indicates sexual dimorphism in these characters (Fig. 8). However, Student’s t-tests for single measurements primarily produce large, non-significant p-values (Tables 1 and 2), which indicates a relatively large amount of overlap in variation of these characters between the two sexes. These sexually dimorphic characters are often obscured by difficulty in sex determination and the incompleteness of specimens unless more elaborate methods are used, such as the one presented in this article.
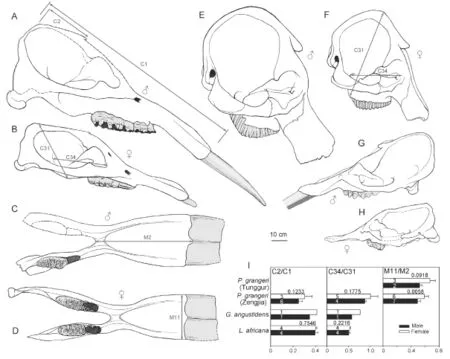
Fig. 6 Sketches showing sexual dimorphism in crania (A, B, E-H) and mandibles (C, D) ofPlatybelodon grangeri(A-D),Loxodonta africana(E, F), andGomphotherium angustidens(G, H) A. HMV 0940; B. HMV 0023; C. HMV 0031; D. HMV 0042; E. AMNH 51939; F. AMNH 88404; G. MNHN Si37; H. MNHN SEP185; I. histograms show sexual dimorphism in three evolutionary signi fi cant characters (C2/C1, C34/C31, M11/M2) with the form mean + s.d. ♂, male; ♀, female Measurements are illustrated on the sketches. The number in each bar represents the sample size n and the number near each pair of bars represents the p-value of the t-test
The above sexually dimorphic characters were also observed inP. grangerifromTunggur. In a female cranium (AMNH 26462), the upper border of the nostril aperture is located in line with postorbital processes (Osborn and Granger, 1932). In contrast, a possible male (AMNH 26480) has more posteriorly positioned nostril aperture, although the incisorswere broken. There are also two types of mandibular symphyses, short and long, which may represent females and males, respectively (Fig. 7). However, juvenile individuals exhibit width/ length ratios that are intermediate between adult males and females (Fig. 7). Furthermore, these sexually dimorphic characters were also observed in Middle MioceneG. angustidens. In male crania, the temporal fossa is higher but short, and the nasal bones are more posteriorly positioned (Figs. 6G, H, I, and Fig. 8; Tassy, 1996: fi g. 11.4).
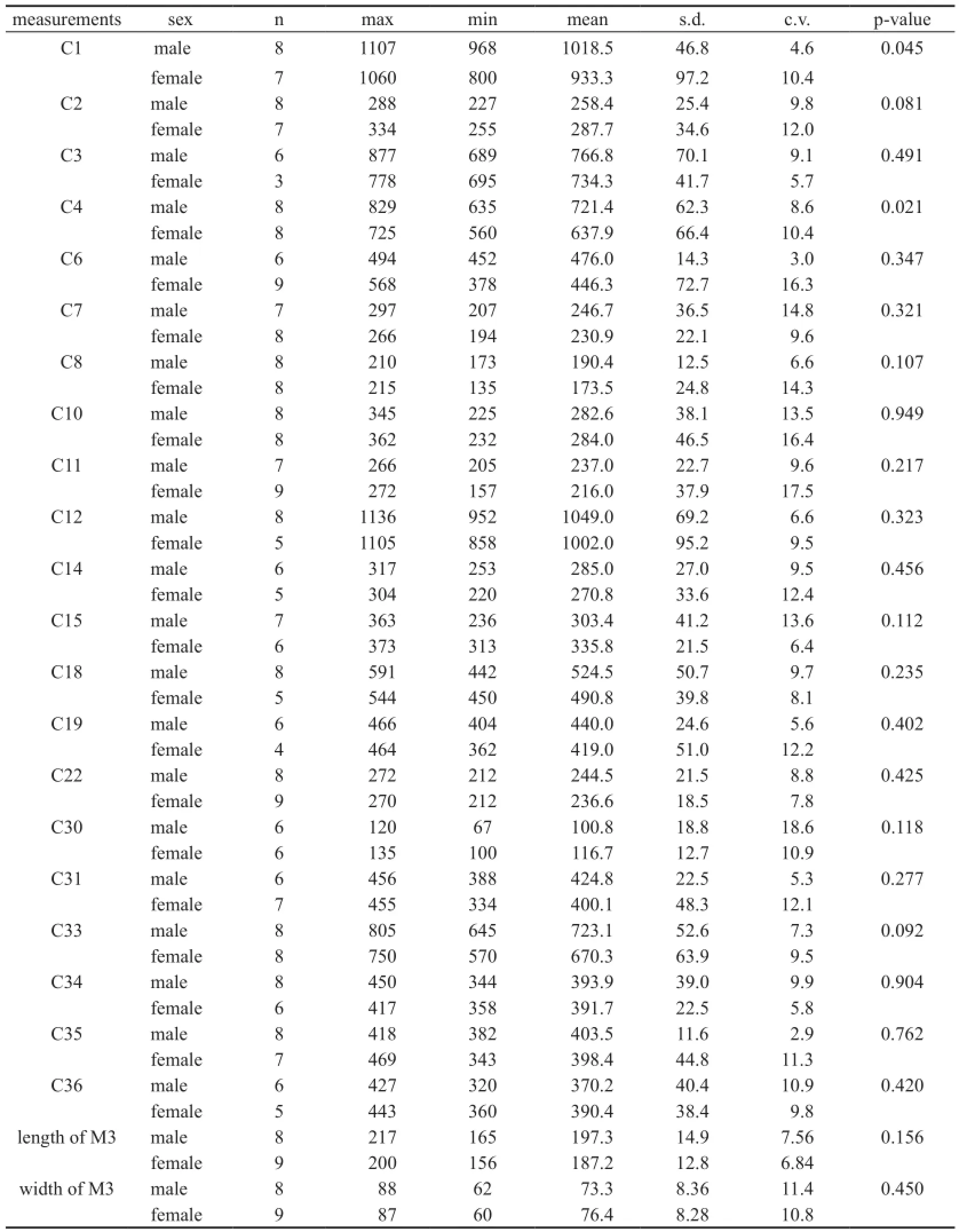
Table 1 Univariate analyses between male and female crania inPlatybelodon grangeri(mm)
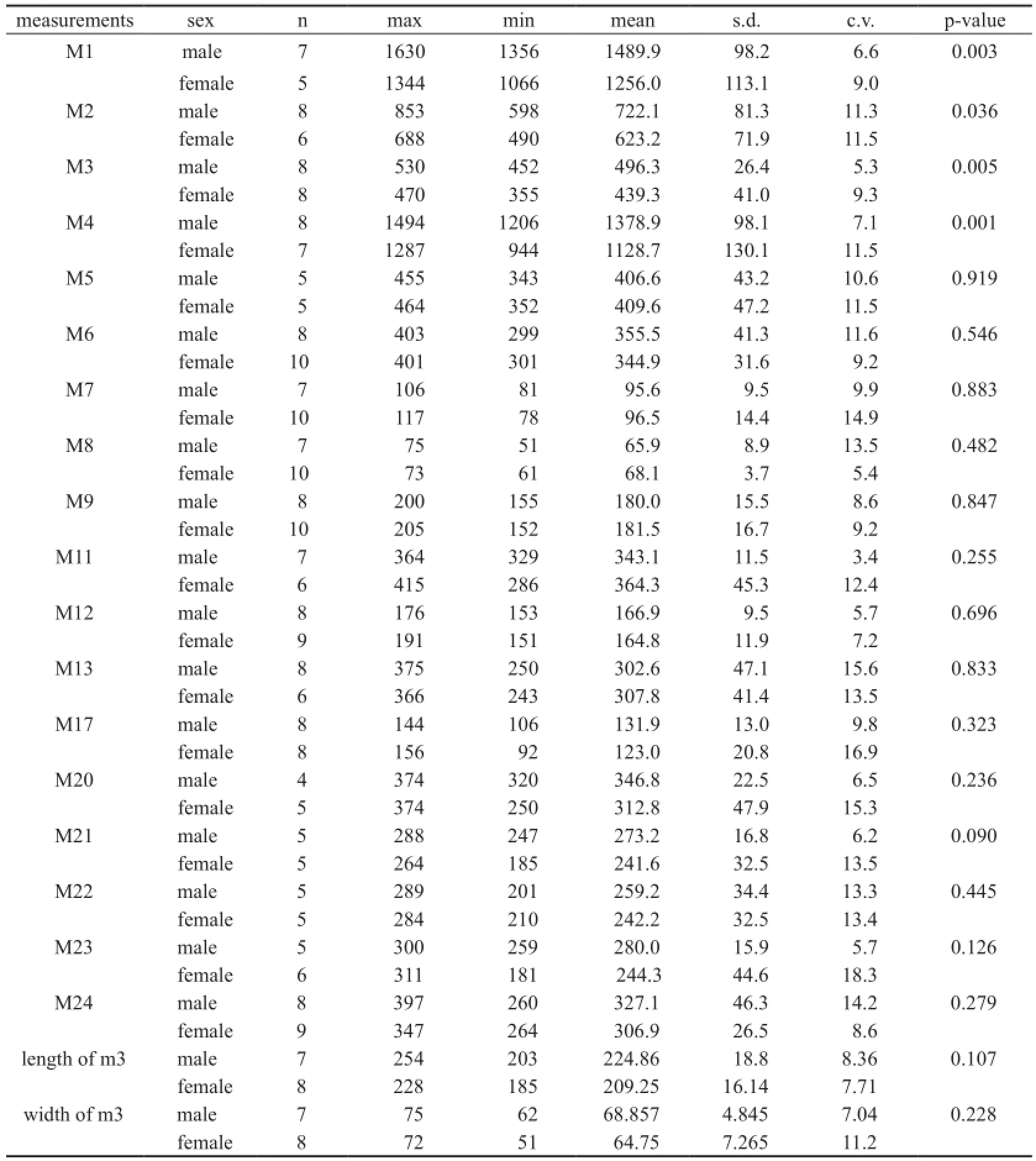
Table 2 Univariate analyses between male and female mandibles inPlatybelodon grangeri(mm)
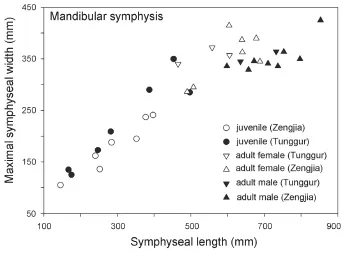
Fig. 7 Bivariate scatter plot of length vs. maximal width of mandibular symphysis inPlatybelodon grangeri♂, male; ♀, female
Sexual dimorphism in ancestralPhiomiaserridensand derivedLoxodonta africanahas also been studied (Matsumoto, 1924; Roth and Shoshani, 1988; Lee and Poole, 2011). InP. serridens, there appears to have sexual dimorphism with regard to size but only have slight sexual dimorphism with regard to symphyseal morphology. For instance,P.“osborni”Matsumoto, 1922 differs fromP.“wintoni” (Andrews, 1905) in more posteriorly positioned proximal edge of mandibular symphysis (Matsumoto, 1924). However, these two species now have been considered junior synonyms ofP. serridens(Shoshani and Tassy, 1996: appendix C2). However, in extantL. africana, although size difference is still prominent (Roth and Shoshani, 1988; Lee and Poole, 2011), the high-arched cranium, extremely posteriorly positioned nostril aperture, and stout mandibles are highly homomorphic in both sexes (Figs. 6E, F). In the Simpson’s ratio diagram, the shapes of polylines that represent the two sexes ofL. africanaare almost identical, which means that individuals of the two sexes display highly similar cranial shapes (Fig. 8).
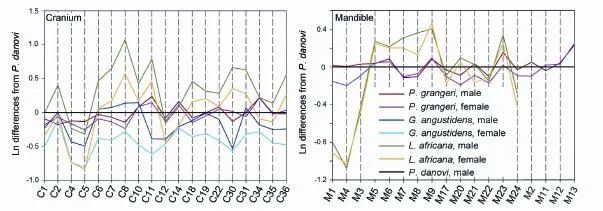
Fig. 8 Simpson’s ratio diagrams showing the cranial and madibular sexual dimorphsim between two sexes in Elephantiformes A skull ofPlatybelodon danovi(BPV 2000) is shown as the standard (the black lines) Measurements see Figs. 2 and 3
4 Discussion
We analyzed sexual dimorphism in four different elephantiforme taxa covering its entire known evolutionary range (OligocenePhiomia, MioceneGomphotherium,Platybelodon, and extantLoxodonta). These taxa are closely related and represent key nodes in the evolution of Elephantiformes. Previous phylogenetic analyses revealed thatPhiomiais the sister groupof all other taxa of Elephantiformes, except the contemporaryPalaeomastodon(Shoshani and Tassy, 2005; Gheerbrant and Tassy, 2009). Therefore,Phiomiais an ideal primitive elephantiforme model.Gomphotheriumis considered the most important representative of the group trilophodont gomphotheres s. s., which is derived fromPhiomiaand sister to Elephantoidea; thus,Gomphotheriumrepresents an intermediate stage between primitivePhiomiaand derived elephants.Platybelodon(a member of Amebelodontinae), which is also derived fromPhiomia, is a close relative of trilophodont gomphotheres s. s. and includes the group trilophodont gomphotheres s. l.. Moreover, as one of the two representatives of extant elephant genera,Loxodontais representative of elephantiforme terminal taxa.
Many sexually dimorphic characters differ among these taxa. However, larger and stronger upper tusks in males were observed in all taxa. These sexually dimorphic characters are clearly associated with male social behavior, such as combat. However, it is difficult to interpret the role of other sexually dimorphic characters in sexual competition, such as C34/C31, C2/C1, M11/M2, and length/width of M3 and m3 inPlatybelodonand the sexually dimorphic characters C34/C31 and C2/C1 inGomphotherium. Nevertheless, these characters have strong evolutionary signi fi cance in Elephantiformes (Andrews, 1906; Maglio, 1972). The nostril aperture is anterior to the orbit inPhiomia, the same level as the orbit inGomphotheriumandPlatybelodon, and posterior to the orbit inLoxodonta, which indicates gradual elongation of the trunk in the evolutionary history of Elephantiformes. The brain case is flat and anteroposteriorly elongated inPhiomia, slightly arched and shortened inGomphotheriumandPlatybelodon, and strongly arched and shortened inLoxodonta; this represents gradual vertical orientation of m. temporalis, which indicates that masticatory function shifted from grinding shearing to horizontal shearing (Maglio, 1972). Therefore, an arched brain case and posteriorly positioned nostril aperture are derived characters in Elephantiformes. Furthermore, the mandibular symphysis is short inPhiomiabut long inPlatybelodon, and m3 and M3 are wide inPhiomiabut narrow inPlatybelodon(narrowness of m3 and M3 is a diagnostic feature in Amebelodontinae; Tassy, 1986). Therefore, at least in Amebelodontinae, a long symphysis and narrow m3 and M3 are derived characters.
Based on the sexual dimorphism analysis, we can draw two conclusions. First, four evolutionarily signi fi cant characters were sexually dimorphic in MioceneGomphotheriumorPlatybelodonand perhaps other members of the trilophodont gomphotheres s. l. group, but were not sexually dimorphic in the primitive taxonPhiomiaand terminal taxonLoxodonta. Second, inGomphotherium,Platybelodon, and perhaps other members of the trilophodont gomphotheres s. l. group, if a character was sexually dimorphic, males always possessed evolutionary more derived character states than females. This issue is of interest with regard to elephantiforme evolutionary history, because the two sexes show asynchronous evolution. This asynchrony might have evolved in primitivePhiomia, become exaggerated in the Miocene trilophodont gomphotheres, and ceased in extant elephants. It appears as though character variation in males slightly preceded similar variation in females, and eventually the females evolved similar character variation (Fig. 9). However, at present, it is not entirely clear whythis phenomenon occurred.
It is difficult to determine the mechanism underlying the production of the sexually asynchronous morphological variations in the evolution of Elephantiformes at genetic and ontogenetic levels. We hypothesize that this asynchrony may re fl ect female preference; that is, in the evolution of a sex-related character in a group, female choice plays a key role (Fisher, 1930; Anderson, 1994). For example, in a population ofGomphotheriumorPlatybelodon, genetic variation (possibly sex-linked gene(s)) produces variation in a sexually dimorphic character, such as trunk length variation in males. The individuals with a longer-than-average trunk, which was determined when there was a more posteriorly positioned nostril aperture (values less than C2/C1), were more attractive to females; this could be because a longer trunk enhances survival of both the males and their offsprings. Similar cases could also be made for the characters C34/C31 and M11/M2 . Therefore, this sexual selection pressure may have promoted the prevalence of the sex-linked allele or alleles that code for a longer trunk in the population and produced the sexual dimorphism we observed inGomphotheriumandPlatybelodon.
This “runaway selection” process (Fisher, 1930) represents the first step of the asynchronous sex evolution; the derived male character variation slightly preceded similar variation in females. Because the newly developed character in males was also advantageous in females, the longer trunk allele(s) were gradually fi xed as non-sex-linked gene(s). Finally, as determined by similar C2/C1 values for both sexes, extant female Elephantiformes also have longer trunks, as can be observed in extantLoxodonta. Moreover, sexual selection pressure also weakened, and it was not necessary for males to have longer trunks than females. This process represents the second step of the asynchronous sex evolution: the females evolved similar traits to those of the males. This two-step hypothesis is plausible for explaining the sexual asynchrony observed in elephantiforme evolution and describing the development of this newly derived character within Elephantiformes.
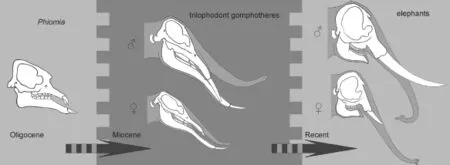
Fig. 9 Schematics showing asynchronous evolution between two sexes in Elephantiformes The gray scales in the background represent relatively greater (relatively darker) or smaller (relatively lighter) sexual-dimorphism, not to scale. ♂, male; ♀, female. Sketches ofPhiomiaafter Andrews (1906)
This asynchronous sex evolution in Elephantiformes appears to be correlated with thriving and declining groups. MioceneGomphotheriumandPlatybelodonshow more prominent sexual dimorphism than primitivePhiomiaand terminalLoxodonta. The Miocene is a thriving period for Elephantiformes, who had a broad geological distribution and diverse taxa; in contrast, there are only three extant species of elephants, which are con fi ned to tropical areas in Asia and Africa (Shoshani and Tassy, 1996). One possible reason for this correlation with asynchronous sex evolution is that females became less selective of these traits in males and sexual selection pressure consequently decreased, which led to extant elephantiforme females and males possessing similar derived characters. For example, females seem very picky in copulation and often refuse to mate with males they dislike (Douglas-Hamilton et al., 2006). Furthermore, relationships between sexual dimorphism and evolution were also observed in other groups of large ungulates. For example, thriving groups, such as Cervidae and Bovidae, often exhibit substantial sexual dimorphism, whereas declining groups, such as extant members of Equidae, Rhinoceridae, and Giraffidae (Janis, 1982), often exhibit little sexual dimorphism. However, some extinct taxa of Equidae and Rhinoceridae, such asHyracotheriumandChilotherium, have strong sexual dimorphism during their thriving period (Gingerich, 1981; Chen et al., 2010).
The patterns observed in this study may be related to the relationship between male competition and female preference. As Darwin (1874) and Fisher (1930) have discussed, both factors contribute to animals’ mating behaviors. In most cases (especially in the thriving groups), the two factors do not contradict each other. A male elephant does compete and defeat competitors to gain copulatory priority, because female elephants are often attracted to and want to mate with the winner. However, in some cases (appears more frequently being in declining groups), even the winner in male competitions may not successfully attract females. A previous study showed that female elephants may refuse males’ mating attempts (Douglas-Hamilton et al., 2006). As in humans, males can attract females by various direct or indirect competitions with other males, but the ultimate decision is often up to the female.
The second issue is that the variation in males is less than that in females. The 95% confidential eclipses of females take up a larger area in Fig. 5A, B, and most standard deviations from female measurements are greater than those of male measurements (Tables 1 and 2). This phenomenon has also been noticed in other larger ungulates, such as extantEquus hemionusand fossilChilotherium wimani(Chen et al., 2010; Wang, 2010), and even observed in some birds, reptiles, anurans, and invertebrates (Johnston, 1966; Stamps and Gon III, 1983). This phenomenon could occur if males are opportunists and would be willing to mate with a wide range of females, however females are picky because of their greater sexual investment in fewer gametes (Fisher, 1930; Anderson, 1994). This mechanism may also have been involved in the observed asynchronous sex evolution and would be helpful for preserving potential variation in the genomes of the species to maintain evolutionary potential, which facilitates rapid response to dramatic environmental changes.
5 Conclusions
In this paper, we studied sexual dimorphism in a fossil population ofPlatybelodon grangerifrom the Middle Miocene Zengjia locality in China. In this population, males possess larger and stronger upper tusks than females. However, several other morphological characters also slightly differ between the sexes, and males have: 1) slightly more arched brain cases, 2) slightly more posteriorly positioned nostril apertures; 3) longer mandibular symphyses; and 4) narrower m3 and M3. The slightly more arched brain cases and slightly more posteriorly positioned nostril apertures are also observed in MioceneGomphotheriumbut not in primitivePhiomiaand terminalLoxodonta. By comparing these data with previously determined elephantiforme evolutionary data, we observed asynchronous evolution between males and females. In the thriving period of Elephantiformes, males possessed more derived characters than females; however, in the declining period, females and males possessed similar derived characters. This type of asynchronous sex evolution should be studied further because it might also have occurred in other large ungulates.
Acknowledgments We thank P. Tassy, U. Göhlich, M. Pickford, Z. X. Qiu, J. Ye, Y. G. Zhang, Q. Q. Shi, S. K. Chen, S. K. Hou, H. B. Lü and Y. N. Zhang for discussions and advice on this work; and W. He, S. Q. Chen, P. Tassy, J. Meng, J. Galkin, E. Westwig, Y. G. Zhang, and Z. H. Zeng for preparing the specimens.
Andersson M, 1994. Sexual Selection. Princeton: Princeton University Press. 1-599
Andrews C W, 1906. A Descriptive Catalogue of the Tertiary Vertebrata of the Fayûm, Egypt. London: British Museum (Natural History). 1-324
Bibi F, Kraatz B, Craig N et al., 2012. Early evidence for complex social structure in Proboscidea from a Late Miocene trackway site in the United Arab Emirates. Biol Lett, 8(4): 670-673
Chen S K, Deng T, Hou S K et al., 2010. Sexual dimorphism in perissodactyl rhinocerotidChilotherium wimanifrom the Late Miocene of the Linxia Basin (Gansu, China). Acta Palaeont Pol, 55: 587-597
Darwin C, 1874. The Descent of Man, and Selection in Relation to Sex. New ed. Revised and Augmented. Philadelphia: David McKay. 1-705
Deng T, Qiu Z X, Wang X M et al., 2013. Late Cenozoic biostratigraphy of the Linxia Basin, northwestern China. In: Wang X M, Flynn L J, Fortelius M eds. Fossil Mammals of Asia: Neogene Biostratigraphy and Chronology of Asia. New York: Columbia University Press. 243-273
Fisher R A, 1930. The Genetical Theory of Natural Selection. Oxford: Clarendon Press. 1-272
Gheerbrant E, Tassy P, 2009. L'origine et l'évolution des éléphants. C R Palevol, 8: 281-294
Gingerich P D, 1981. Variation, sexual dimorphism, and social structure in the Early Eocene horseHyracotherium(Mammalia, Perissodactyla). Paleobiology, 7: 443-455
Janis C, 1982. Evolution of horns in ungulates: ecology and paleoecology. Biol Rev, 57: 261-318
Johnston R F, 1966. The adaptive basis of geographic variation in color of the purple martin. Condor, 68: 219-228
Kurt F, Hartl G B, Tiedemann R, 1995. Tuskless bulls in Asian elephantElephas maximus. History and population genetics of a man-made phenomenon. Acta Theriol, Suppl 3: 125-143
Lambert W D, 1992. The feeding habits of the shovel-tusked gomphotheres: evidence from tusk wear patterns. Paleobiology, 18: 132-147
Lee P C, Poole J H, 2011. Reproductive strategies and social relationships. In: Moss C J, Croze H, Lee P C eds. The Amboseli Elephants, a Long-term Perspective on a Long-lived Mammal. Chicago: The University of Chicago Press. 184-286
雪萤从窗台上摸出钥匙开了门,直奔卧室,趴在地上,拉出床下一双统靴,伸手一摸,没有。于是把手伸进另一只鞋子。里面果然有一个油腻腻的小本子,还有一个U盘,用一块布包着。她把证据捂在胸前,长长地出了一口气,忍不住翻开来,快速地扫了一眼,心都快跳出来了。
Maglio V J, 1972. Evolution of mastication in the Elephantidae. Evolution, 26: 638-658
Matsumoto H, 1924. A revision ofPalaeomastodondividing it into two genera, and with descriptions of two new species. Bull Am Mus Nat Hist, 50: 1-58
Nowak R M, 1999. Walker’s Mammals of the World. 6th ed. Baltimore: The Johns Hopkins University Press. 1-1936
Osborn H F, Granger W, 1932.Platybelodon grangeri, three growth stages, and a new serridentine from Mongolia. Am Mus Novit, 537: 1-13
Roth V L, Shoshani J, 1988. Dental identi fi cation and age determination inElephas maximus.J Zool Lond, 214(4): 567-588
Shoshani J, Tassy P, 1996. The Proboscidea: Evolution and Palaeoecology of Elephants and Their Relatives. Oxford: Oxford University Press. 1-472
Shoshani J, Tassy P, 2005. Advances in proboscidean taxonomy & classi fi cation, anatomy & physiology, and ecology & behavior. Quat Int, 126-128: 5-20
Stamps J A, Gon III S M, 1983. Sex-biased pattern variation in the prey of birds. Annu Rev Ecol Syst, 14: 231-253
Tassy P, 1986. Nouveaux Elephantoidea (Mammalia) dans le Miocène du Kenya: Essai de Réévaluation Systématique. Paris: E'ditions du Centre National de la Recherche Scienti fi que. 1-135
Tassy P, 1988. The classi fi cation of Proboscidea: how many cladistic classi fi cations? Cladistics, 4: 43-57
Tassy P, 1996. Growth and sexual dimorphism among Miocene elephantiformes: the example ofGomphotherium angustidens. In: Shoshani J, Tassy P eds. The Proboscidea: Evolution and Palaeoecology of Elephants and Their Relatives. Oxford: Oxford University Press. 92-100
Tassy P, 2013. L’anatomie cranio-mandibulaire deGomphotherium angustidens(Cuvier, 1817) (Proboscidea, Mammalia): données issues du gisement d'En Péjouan (Miocène moyen du Gers, France). Geodiversitas, 35(2): 377-445
Wang S Q, 2010. The variations of limb bones in the male and the femaleEquus hemionus.In: Dong W ed. Proceedings of the Twelfth Annual Meeting of the Chinese Society of Vertebrate Paleontology. Beijing: China Ocean Press. 77-84
Wang S Q, Deng T, 2010. Recovering the missing data of defective fossil samples using linear regression method. Vert PalAsiat, 48: 161-168
Wang S Q, He W, Chen S Q, 2013. Gomphotheriid mammalPlatybelodonfrom the Middle Miocene of Linxia Basin, Gansu, China. Acta Palaeont Pol, 58(2): 221-240
Wang X M, Qiu Z D, Opdyke N D, 2003. Litho-, bio-, and magneto-stratigraphy and paleoenvironment of Tunggur Formation (Middle Miocene) in central Inner Mongolia, China. Am Mus Novit, 3411: 1-31
雌性偏好促进象型类的性别异时进化
王世骐1,2,3邓 涛1,2
(1 中国科学院古脊椎动物与古人类研究所,中国科学院脊椎动物演化与人类起源重点实验室 北京 100044)
(2 中国科学院青藏高原地球科学卓越创新中心 北京 100101)
(3 中国科学院资源地层学与古地理学重点实验室(中国科学院南京地质古生物研究所) 南京 210008)
性双型的特征通常被认为产生于种内争夺交配优先权的斗争。例如,现生和化石的雄性长鼻类动物具有较大的体型和较粗壮的上门齿。本研究阐释了如下现象:化石象型类动物(Elephantiformes, 长鼻类的主要类群)一些性双型特征与其进化历史具有相关性,而与性别竞争并非直接相关。在中新世的葛氏铲齿象(Platybelodon grangeri)和狭齿嵌齿象(Gomphotherium angustidens)中,雄性比雌性倾向于具有进化中更进步的特征,如同雄性在进化中领先雌性一步。这种现象可能与雌性偏好的机制相耦合。在象型类动物进化的早期(繁荣期),性别选择压促使雄性比雌性产生更加显著的进步特征;然而,在它们进化的晚期(衰退期),性别选择压似乎减弱,性别的异时进化也减少。这种新的发现或许在大型有蹄类的演化过程中有一定的普遍意义,因为那些繁荣的类群中通常性双型显著,如鹿科和牛科;而衰落的类群中通常性双型不显著。
长鼻类,性双型,雌性偏好,进化
Q915.878
A
1000-3118(2016)01-0051-16
2015-06-30
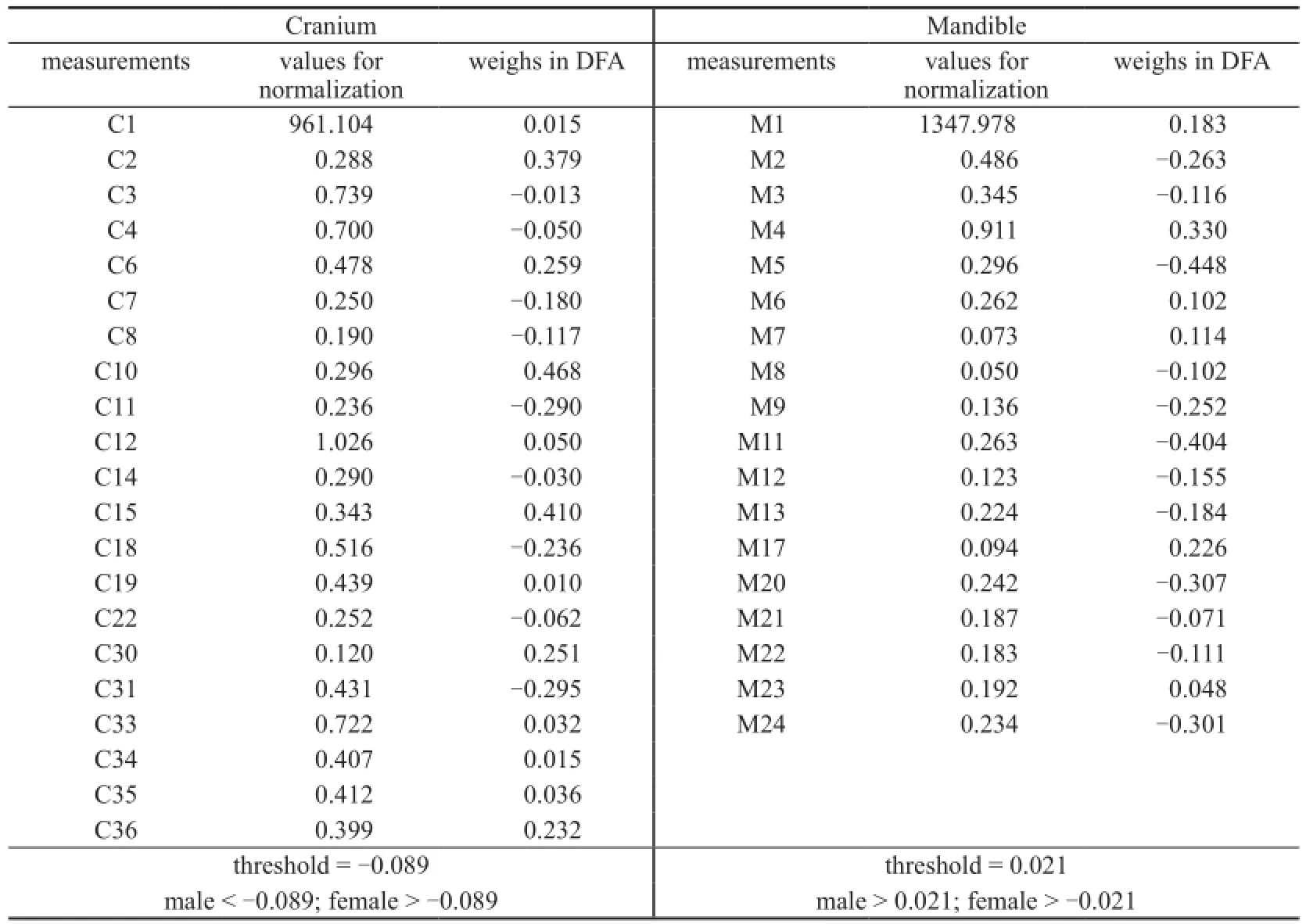
Appendix 1 The weighs of cranial and mandibular measurements in fi nal DFA
Wang S Q, Deng T, 2016. Female preference promotes asynchronous sex evolution in Elephantiformes. Vertebrata PalAsiatica, 54(1): 51-66
国家重点基础研究发展计划项目(编号:2012CB821900)、中国科学院战略性科技先导专项(编号:XDB03020104)、国家自然科学基金(批准号:41372001, 41430102, 41202017)和中国科学院资源地层学与古地理学重点实验室(中国科学院南京地质古生物研究所)资助。
猜你喜欢
杂志排行
古脊椎动物学报(中英文)的其它文章
- A new type of dinosaur eggs from Early Cretaceous of Gansu Province, China
- A new hadrosauroid dinosaur from the Late Cretaceous of Tianzhen, Shanxi Province, China
- Restudy of the Late Oligocene dormice from northern Junggar Basin
- New record of a haplocyonine amphicyonid in early Miocene of Nei Mongol fi lls a long-suspected geographic hiatus
- Morphology and taxonomy of Gazella (Bovidae, Artiodactyla) from the Late Miocene Bahe Formation, Lantian, Shaanxi Province, China
