Chinese version of NCCN Clinical Practice Guidelines in Oncology of fi cially authorized by NCCN
2016-03-28YanhuaZhengDingyaoXuZhaodeBu
Yanhua Zheng, Dingyao Xu, Zhaode Bu
Chinese version of NCCN Clinical Practice Guidelines in Oncology of fi cially authorized by NCCN
Yanhua Zheng, Dingyao Xu, Zhaode Bu
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Editorial Department of Chinese Journal of Cancer Research, Peking University Cancer Hospital & Institute, Beijing 100142, China
Correspondence to: Zhaode Bu. Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Editorial Department of Chinese Journal of Cancer Research, Peking University Cancer Hospital & Institute, Beijing 100142, China. Email: buzhaode@cjcrcn.org.
Submitted Jan 28, 2016. Accepted for publication Feb 19, 2016.
View this article at: http://dx.doi.org/10.3978/j.issn.1000-9604.2016.02.10 The publishing conference of the Chinese version of National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (hereinafter referred to as NCCN Guidelines) and the inaugural peer reviewer meeting of NCCN Clinical Practice Guidelines in Oncology: Digestive System Cancers (hereinafter referred to as NCCN Guidelines on Digestive System Cancers) were held in People’s Medical Publishing House in January 28th, 2016 (Figure 1). Sun Yan, the academician of Chinese Academy of Engineering, from Cancer Institute and Hospital, Chinese Academy of Medical Sciences; Guo Yinglu, the academician of Chinese Academy of Engineering, from Peking University First Hospital; Ji Jiafu, the president of Beijing Cancer Hospital as well as many leading cancer experts and representatives in China were presented at the conference (Figure 2). Hao Yang, the general manager of People’s Medical Publishing House, attended and addressed the meeting. The Chinese version of NCCN Guidelines was introduced by the People’s Medical Publishing House, and initiated by Beijing Cancer Hospital which is in charge of the review of the translation. Both sides decided to jointly push the publicity and promotion of the NCCN Guidelines in China.

Figure 1 The conventioneers pose for a group photograph.
Professor Sun Yan, one of the most leading oncologists in China and the general master translator of the NCCN Guidelines, gave guidance and made deployment on the translation and publication of the NCCN Guidelines at the meeting. He pointed out that the aim of NCCN Guidelines is to provide the latest and the best choice in diagnosis and treatment for tumor patients. With the rapid developmentof medical science, Professor Sun hopes that the publication of NCCN Guidelines can help realize indigenization of NCCN Guidelines in China as early as possible so as to improve the diagnosis and treatment of cancer and benefi t cancer patients.

Figure 2 The conventioneers are making addresses and comments in the meeting.
Professor Ji Jiafu, as the convener and the master translator of the Chinese version of NCCN Guidelines on Digestive System Cancers, first started and accomplished the translation of this section by organizing nationwide experts in digestive system cancers. The Chinese version of NCCN Guidelines on Digestive System Cancers, which covers gastric cancer, rectal cancer, anal cancer, pancreatic cancer, esophageal cancer, esophagogastric junction cancer, hepatobiliary cancer and colorectal cancer, will soon be available. Professor Ji introduced briefly the translation process in earlier stage and the review schedule in the next step at the meeting. He also gave his own opinions on the publication of the Chinese version of NCCN Guidelines, which would better suit Chinese patients.
Hao Yang, the general manager of People’s Medical Publishing House, first showed his thanks to the experts for their efforts and contributions in the translation and publication of NCCN Guidelines and emphasized that the People’s Medical Publishing House will cooperate with Beijing Cancer Hospital and all experts nationwide to popularize the publication of NCCN Guidelines by integrated promotion of both online and of fl ine.
Acknowledgements
None.
Footnote
Confl icts of Interest: The authors have no confl icts of interest to declare.
Hereby Declare: The Chinese version of the NCCN Clinical Practice Guidelines in Oncology will be formally published by People’s Medical Publishing House, and it is the only version in China of fi cially authorized by U.S. NCCN.
Cite this article as: Zheng Y, Xu D, Bu Z. Chinese version of NCCN Clinical Practice Guidelines in Oncology officially authorized by NCCN. Chin J Cancer Res 2016;28(1):144-145. doi: 10.3978/j.issn.1000-9604.2016.02.10
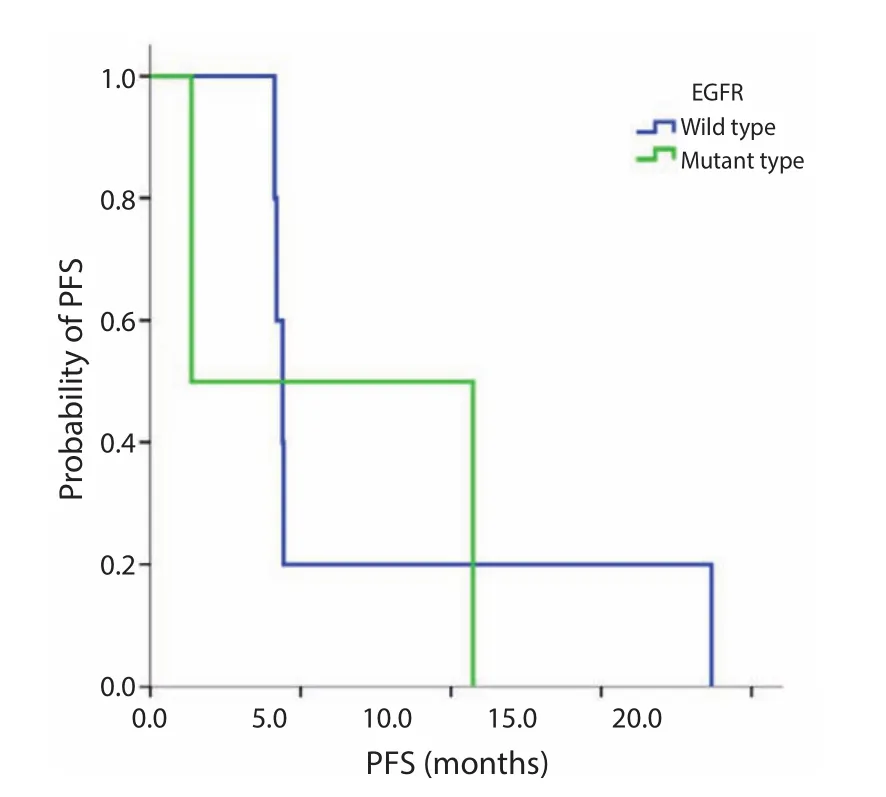
Figure S1 PFS for EGFR wild type versus mutation. Median PFS, wild type 4.4 m; mutant type, 1.3 m; P=0.806. EGFR, epidermal growth factor receptor; PFS, progression-free survival.
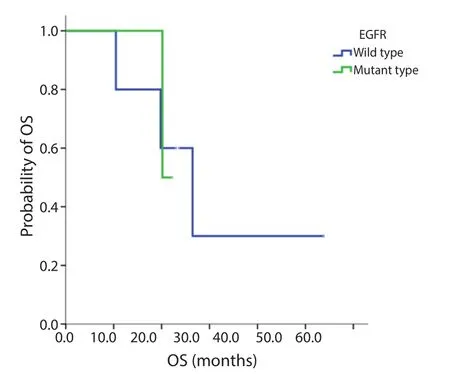
Figure S2 OS EGFR wild type versus mutation. Median OS, wild type 26.4 m; mutant type, 21.1 m; P=0.981. EGFR, epidermal growth factor receptor; OS, overall survival.

Figure S3 PFS for EGFR low or medium expression versus high expression. Median PFS, low or medium 4.4 m; high, 4.4 m; P=0.594. EGFR, epidermal growth factor receptor; PFS, progression-free survival.
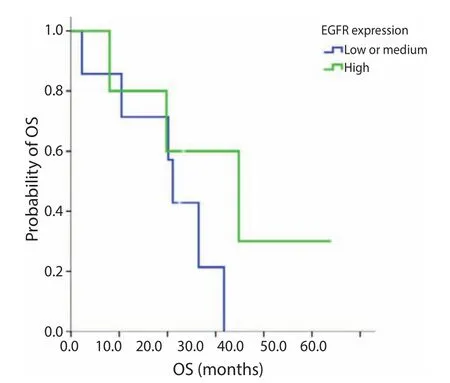
Figure S4 OS for EGFR low or medium expression versus high expression. Median OS, low or medium 21.1 m; high, 34.8 m; P=0.225. EGFR, epidermal growth factor receptor; OS, overall survival.
doi:10.3978/j.issn.1000-9604.2016.02.10
杂志排行
Chinese Journal of Cancer Research的其它文章
- The pathology of unusual subtypes of prostate cancer
- Molecular aspects of prostate cancer with neuroendocrine diff erentiation
- The pathology of urinary bladder lesions with an inverted growth pattern
- Intraductal carcinoma of prostate (IDC-P): from obscure to signifi cant
- Understanding the molecular pathogenesis and prognostics of bladder cancer: an overview
- Epigenomics of clear cell renal cell carcinoma: mechanisms and potential use in molecular pathology
