Preparation and Tribological Behavior of Hydrophobic Lanthanum Borate Nanosheets in Rapeseed Oil
2016-03-22GuKechengChenBoshuiXueMingWangJiuFangJianhuaWuJiang
Gu Kecheng; Chen Boshui; Xue Ming; Wang Jiu; Fang Jianhua; Wu Jiang
(1. Department of Oil Application and Management Engineering, Logistical Engineering University, Chongqing 401311; 2. Department of National Defense Architectural Planning and Environment Engineering, Logistical Engineering University, Chongqing 401311)
Preparation and Tribological Behavior of Hydrophobic Lanthanum Borate Nanosheets in Rapeseed Oil
Gu Kecheng1; Chen Boshui1; Xue Ming2; Wang Jiu1; Fang Jianhua1; Wu Jiang1
(1. Department of Oil Application and Management Engineering, Logistical Engineering University, Chongqing 401311; 2. Department of National Defense Architectural Planning and Environment Engineering, Logistical Engineering University, Chongqing 401311)
Oleic acid-capped lanthanum borate (abbreviated as OA/LaBO3·H2O) nanosheets were prepared by the hydrothermal method. The microstructures of as-prepared OA/LaBO3·H2O were characterized by means of SEM, TEM, EDS, FTIR and XRD, respectively. Moreover, the friction and wear properties of OA/LaBO3·H2O as a lubricant additive in rapeseed oil were evaluated on a four-ball tribotester. The tribochemical characteristics of worn surfaces were investigated by SEM and XPS. The results showed that the hydrophobic OA/LaBO3·H2O nanosheets exhibited their morphology with a diameter in the range of 100 nm to 300 nm and a thickness of about 25 nm, and displayed excellent dispersing stability in rapeseed oil. In the meantime, the rapeseed oil doped with OA/LaBO3·H2O nanosheets markedly decreased the friction and wear of steel balls, and the optimal friction-reducing and antiwear ability of rapeseed oil was obtained at an OA/LaBO3·H2O content of 1.0%. The outstanding tribological performance of OA/LaBO3·H2O in rapeseed oil was attributed to the formation of a composite boundary lubrication fi lm mainly composed of lubricious tribochemical species of B2O3, La2O3and Fe2O3, and deposits of OA/LaBO3·H2O nanosheets as well as the adsorbates of rapeseed oil on rubbed surfaces.
lanthanum borate; nanosheet; hydrophobicity; friction; wear; rapeseed oil.
1 Introduction
Over the past decades, nanoscale materials have been widely investigated and applied in many scientific fields thanks to their fascinating physical and chemical characteristics[1-7]. In tribological applications, much effort has also been paid to the development of efficient nanometric lubricant additives to reduce the friction and wear of machines. Indeed, many inorganic nanomaterials such as metals[8-9], sul fi des[10-11], oxides[12-14]and borates[15-16], have been proven to be promising as lubricant additives since they exhibit excellent friction-reducing and antiwear capability when they are formulated into lubricants, although the practical application of inorganic nanomaterials used as lubricant additives is usually impeded because of their poor dispersing stability in lubricants, and surface modi fi cation has to be adopted to resolve this defect[17-18]. Among various inorganic nanomaterials used as lubricant additives, nanometric lanthanum borate has been reported to possess excellent ability in the reduction of friction and wear owing to the special electronic structure of elements La and B[19-20]. Unfortunately, the previous studies on the tribological performance of nanometric lanthanum borate mainly focused on that of one-/ three-dimensional nanoparticles[20-21], few reports are currently available about the tribological performance of two-dimensional (2D) lanthanum borate nanospecies. As we know, nanometric thickness endows twodimensional nanomaterials with strikingly peculiar and fascinating properties, which differ greatly from those of their bulk parent counterparts[3,22]. Consequently, further investigation on the tribological behavior of 2D lanthanum borate nanospecies in lubricants is still needed. During the last decade, contamination of eco-system caused by conventional petroleum-based lubricants has attracted much attention because of their inherenttoxicity and unreadily biodegradable nature[23-26], and environmentally friendly lubricant has become a topic of interested research with an increasing awareness of health, safety and conservation of the environment. The key issue in formulating environmentally friendly lubricant oils is to develop reliable base oils and additives with proper capability. Nowadays, many base fl uids such as vegetable oils and synthetic esters have found practical applications in the formulation of biodegradable lubricants thanks to their good lubricity, high biodegradability and nontoxicity[27-28]. The present paper aims at exploring the possibility of lanthanum borate nanosheets used as lubricant additives and comprehending their friction and wear behavior in biodegradable rapeseed oil. Herein, lanthanum borate nanosheets were prepared with their surfaces chemically modi fi ed with oleic acid to enhance their dispersing stability in rapeseed oil. The tribological performance and mechanisms of lanthanum borate nanosheets in rapeseed oil were also investigated.
2 Experimental
2.1 Materials
Oleic acid (OA) was of chemically pure grade, and other reagents used in the experiments were of analytically pure grade. All reagents were employed without further purification. Distilled water was used in all preparation and treatment processes.
2.2 Preparation of hydrophobic lanthanum borate nanosheets
2.15 g of La(NO3)3·6H2O and 1.0% of oleic acid were dissolved in 50 mL of anhydrous ethanol. The resultant mixture was marked as A. In addition, 2.86 g of Na2B4O7·10H2O was dissolved in 50 mL of distilled water under vigorous stirring. The resultant solution was marked as B. Then the solution A was added dropwise into solution B under magnetic stirring at ambient temperature. The pH value of the mixture was carefully adjusted to about 3 with diluted nitric acid solution. After addition of solution A, the obtained mixture was subjected to stirring for 1 h prior to being transferred into a 200-mL Te fl on-lined stainless-steel autoclave which was filled with anhydrous ethanol up to 80% of the total volume, sealed and treated hydrothermally at 180 ℃ for 4 h. Thereafter, the treated mixture was cooled to room temperature and filtered to obtain precipitates, which were then washed repeatedly with distilled water and anhydrous ethanol to remove the un-reacted reagents, OA and by-products, followed by drying at 80 ℃ for 4 h in air to obtain the fi nal white, oleic acidcapped lanthanum borate nanopowder (abbreviated as OA/LaBO3·H2O). The un-capped lanthanum borate nanopowder (abbreviated as LaBO3·H2O) was prepared without addition of OA in solution A while keeping all other process conditions unchanged.
2.3 Friction and wear tests
Different mass percentages of LaBO3·H2O and OA/ LaBO3·H2O were separately dispersed ultrasonically into rapeseed oil (RP). The friction coef fi cients and wear scar diameter (WSD), which could well characterize the friction and wear properties of a lubricant, were measured with a MMW-1P universal four-ball tribotester operating at 1200 rpm and 392 N for 30 min. The frictional pairs used in the present tests were the GCr15 bearing steel balls (consisting of: 0.95%—1.05% of C; 0.15%—0.35% of Si; 0.25%—0.45% of Mn; <0.025% of P; <0.025% of S; Cr, 1.40%—1.65% of Cr; <0.30% of Ni; and <0.025% of Cu) with a diameter of 12.7 mm and a hardness of 59—61 HRC. Each test was repeated three times and the average value was reported. Prior to tests, the balls were cleaned in an ultrasonic bath with petroleum ether for 10 min and then dried with a hair dryer. Upon completion of the tests, the tested balls were rinsed ultrasonically in petroleum ether for 10 min for surface analysis purpose.
2.4 Characterization of additives and analysis of worn surfaces
The crystalline and chemical structures of as-obtained LaBO3·H2O and OA/LaBO3·H2O were analyzed with a Rigaku Dmax-2500 X-ray diffractometer (XRD; using Cu Kα irradiation) and a Perkin-Elmer 400 Fourier transform infrared spectrometer (FTIR), respectively. The morphology as well as crystalline size of LaBO3·H2O and OA/LaBO3·H2O were measured by a JEOL S-4800 fi eld emission scanning electron microscope (SEM) and an FEI Tecnai F20 transmission electron microscope (TEM), while the elemental composition of samples was determined with an energy dispersive X-ray spectrometer (EDS) attached to the SEM. Furthermore,the hydrophobic characteristics of LaBO3·H2O and OA/ LaBO3·H2O were detected via measuring the water contact angles on an Easydrop DSA14 contact angle goniometer, after pressing the samples into a wafer with an IR powder-pressing machine under 15 MPa for 2 min. Finally, the morphology of worn steel surfaces was observed by SEM, and the chemical species on the worn steel surfaces were analyzed by a Thermo ESCALab-250 X-ray photoelectron spectroscope (XPS), where the binding energy of the tested elements was measured at a pass energy of 29.4 eV and a resolution of ±0.3 eV, with the Al Kαradiation used as the excitation source and the binding energy of contaminated carbon (C1s: 284.80 eV) serving as the reference.
3 Results and Discussion
3.1 Characterization of LaBO3·H2O and OA/LaBO3·H2O nanosheets
Figure 1 shows the SEM images of LaBO3·H2O and OA/LaBO3·H2O. It can be seen from Figure 1(a) that LaBO3·H2O are mainly composed of agglomerated nanoparticles and nanolumps. It can be seen from Figure 1(b) that OA/LaBO3·H2O has a sheet-like morphology with a diameter in the range of 100 nm to 300 nm and an average thickness of around 25 nm, and the OA/ LaBO3·H2O nanosheets are much less agglomerated as compared to LaBO3·H2O (in Figure 1(a)), indicating that the agglomeration of OA/LaBO3·H2O nanosheets is obviously weakened by addition of oleic acid. Figure 2 shows the TEM image of OA/LaBO3·H2O nanosheets. It is evident that the OA/LaBO3·H2O sample exhibits a sheet-like topography with its diameter in the range of 100 nm to 300 nm, and the corresponding electron diffraction pattern of OA/LaBO3·H2O shows several diffused rings, indicating that the OA/LaBO3·H2O nanosheets are amorphous.
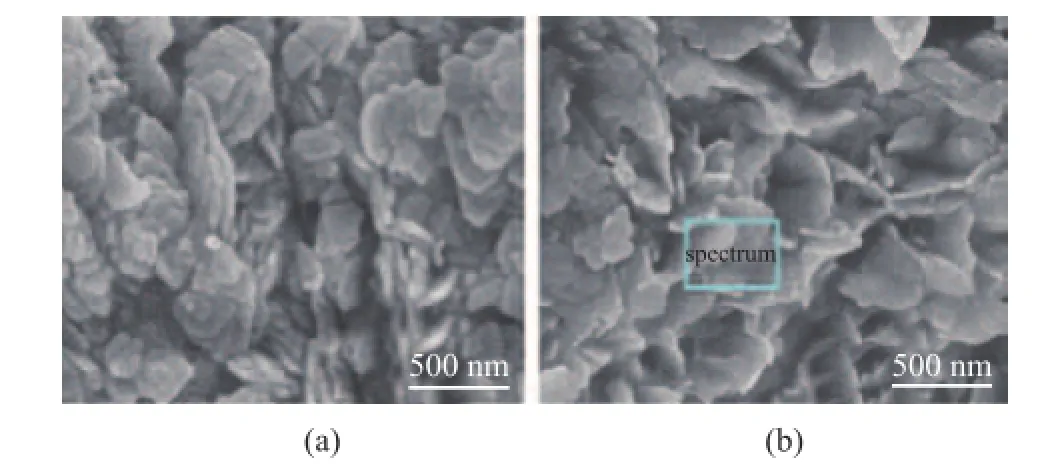
Figure 1 SEM images of (a) LaBO3·H2O and (b) OA/LaBO3·H2O

Figure 2 TEM image of OA/LaBO3·H2O (inset in higher right corner is the related electron diffraction pattern)
Figure 3 shows the EDS spectrum of OA/LaBO3·H2O nanosheets. It can be observed that the elements B, C, O and La existed in EDS profile. Quantitative calculation from EDS profile indicates that the atomic ratio of B, O, and La is 0.90: 3.92:1.04 (H cannot be identified), which is very close to the stoichiometry of LaBO3·H2O. Moreover, the peak of elemental C arises from the conductive tape.
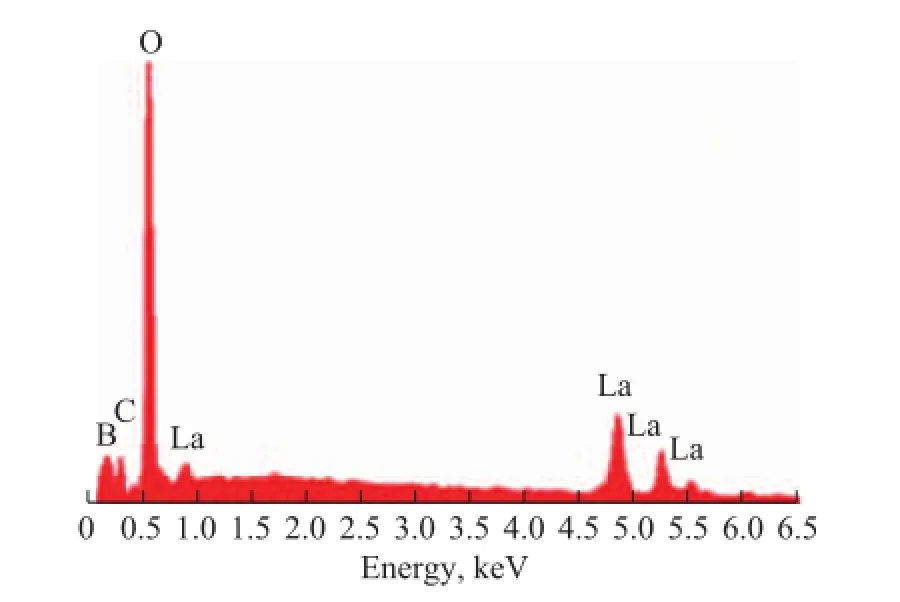
Figure 3 EDS pro fi le of OA/LaBO3·H2O
Figure 4 shows XRD patterns of LaBO3·H2O and OA/ LaBO3·H2O nanosheets. Two broad peaks indicate that the samples are almost amorphous[20], which is in agreement with the relevant TEM analytical result. Extremely similar patterns of LaBO3·H2O and OA/LaBO3·H2O nanosheets demonstrate that oleic acid has negligible influence on the crystallization of lanthanum borate nanosheets. Moreover, no characteristic peaks of impurities, such as La(NO3)3, other borates and other unreacted compounds, are detected, indicating that the as-prepared LaBO3·H2Oand OA/LaBO3·H2O nanosheets are of high purity.

Figure 4 XRD patterns of (a) LaBO3·H2O and (b) OA/ LaBO3·H2O
Figure 5 shows the IR spectra of oleic acid, LaBO3·H2O and OA/LaBO3·H2O nanosheets. In Figure 5(b) and 5(c), the band at 3 442 cm-1is attributed to the stretch vibration of O—H[29], and the band at 1 650 cm-1corresponds to the bending vibration of H—O—H, respectively, which indicates that lanthanum borate nanosheets contain crystalline water. The band at 1 388 cm-1is assigned to the asymmetric stretch vibration of B(3)—O, while the band at 1048 cm-1is ascribed to the asymmetric strecth vibration of B(4)—O, respectively. In Figure 5(a) and 5(c), the bands at 2 927 cm-1and 2 856 cm-1correspond to the symmetric and asymmetric stretch vibration of—CH2— species, respectively. The intensive peak at 1 712 cm-1in Figure 5(a) is ascribed to the carbonyl stretch vibration of oleic acid. This peak “disappears”in the IR spectrum of OA/LaBO3·H2O in Figure 5(c). However, as regards OA/LaBO3·H2O in Figure 5(c), the band at 1 540 cm-1is attributed to the carbonyl stretch vibration of carbonylate[30], suitably corresponding to the formation of oleates[12]and demonstrating that the surface of OA/LaBO3·H2O has been chemically grafted upon oleic acid[31].

Figure 5 IR spectra of (a) OA, (b) LaBO3·H2O and (c) OA/LaBO3·H2O
Figure 6 shows the water contact angles of LaBO3·H2O and OA/LaBO3·H2O. In Figure 6(a), the water contact angle of LaBO3·H2O is 46°, implying that the surface of LaBO3·H2O is hydrophilic. While in Figure 6(b), the water contact angle of OA/LaBO3·H2O increases to 139°, demonstrating that the surface of OA/LaBO3·H2O becomes hydrophobic.This indicates that the surfaces of LaBO3·H2O have been chemically well capped by oleic acid, which is in good agreement with the results of IR analysis. In fact, the dispersing stability of LaBO3·H2O and OA/LaBO3·H2O in rapeseed oil has also been tested and compared by calculating the mass percentages of precipitates of OA/LaBO3·H2O and LaBO3·H2O after centrifugally separating the precipitates of OA/ LaBO3·H2O and LaBO3·H2O, a certain amount of which is previously ultrasonicly dispersed in rapeseed oil, and then centrifuged at a rotation speed of 10 000 r/min for 5 min, respectively. Figure 7 shows the optical images of centrifuged lubricating oils. It can be seen that the addition of OA/LaBO3·H2O in rapeseed oil has not affected the transparency of rapeseed oil, and in contrast, the rapeseed oil becomes very turbid due to the addition of LaBO3·H2O. Moreover, the results show that the masspercentage of precipitate of OA/LaBO3·H2O (0.8%) is much less than that of LaBO3·H2O (98.4%), indicating that OA/LaBO3·H2O provides excellent dispersing stability in rapeseed oil, and the dispersing stability of OA/LaBO3·H2O is much better as compared to that of LaBO3·H2O in rapeseed oil.
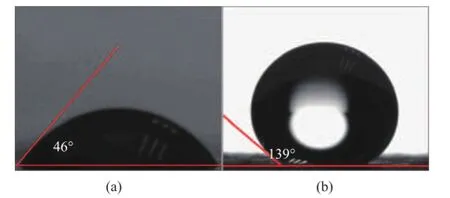
Figure 6 Water contact angles of (a) LaBO3·H2O and (b) OA/LaBO3·H2O
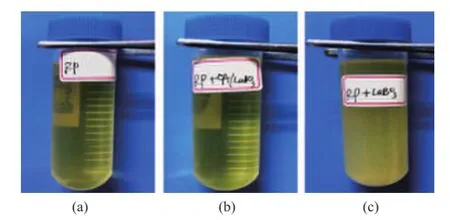
Figure 7 Optical images of centrifuged lubricating oils: (a) RP, (b) RP + OA/LaBO3·H2O and (c) RP + LaBO3·H2O
3.2 Tribological performance of OA/LaBO3·H2O nanosheets in rapeseed oil
Figure 8 shows the friction coefficients of GCr15 steel balls lubricated by rapeseed oil (RP) containing 1.0% of different additives. It can be seen from Figure 8 that RP+OA/LaBO3·H2O exhibits the lowest friction coefficient as compared to other lubricating oils, indicating that OA/LaBO3·H2O nanosheets has obviously improved the friction-reducing ability of RP. The excellent ability of OA/LaBO3·H2O in reducing friction might be attributed to its high lipophilicity and thus good dispersancy in rapeseed oil[15]. However, RP+LaBO3·H2O shows a relatively higher friction coefficient than neat RP, indicating that the un-capped LaBO3·H2O impairs the friction-reducing ability of RP, possibly partly because of its poor dispersing stability in RP. It also can be seen from Figure 8 that the friction-reducing capacity of RP can be enhanced to some extent by addition of oleic acid, which might be attributed to the adsorption of RP on rubbed surface through the organic chain segments of oleic acid[32].
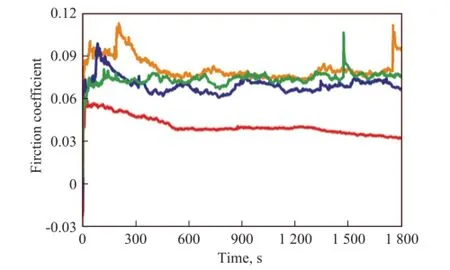
Figure 8 Friction coef fi cients of GCr15 steel balls lubricated with different lubricating oils
Figure 9 shows the friction coef fi cients and WSD versus the content of OA/LaBO3·H2O for RP doped with OA/ LaBO3·H2O nanosheets. It can be seen from Figure 9 that both the friction coef fi cients and WSD markedly decrease with an increasing content of OA/LaBO3·H2O up to 1.0%, indicating that OA/LaBO3·H2O acts as an ef fi cient friction and wear reducer in RP at a relatively low content. However, the friction coefficient and WSD obviously increase with an increasing content of OA/LaBO3·H2O in the range of between 1.0% and 3.0%. This demonstrates that the friction-reducing and antiwear ability of RP is decreased at a high content of OA/LaBO3·H2O, which could be attributed to the conglomeration of OA/ LaBO3·H2O nanosheets under frictional heat[33].
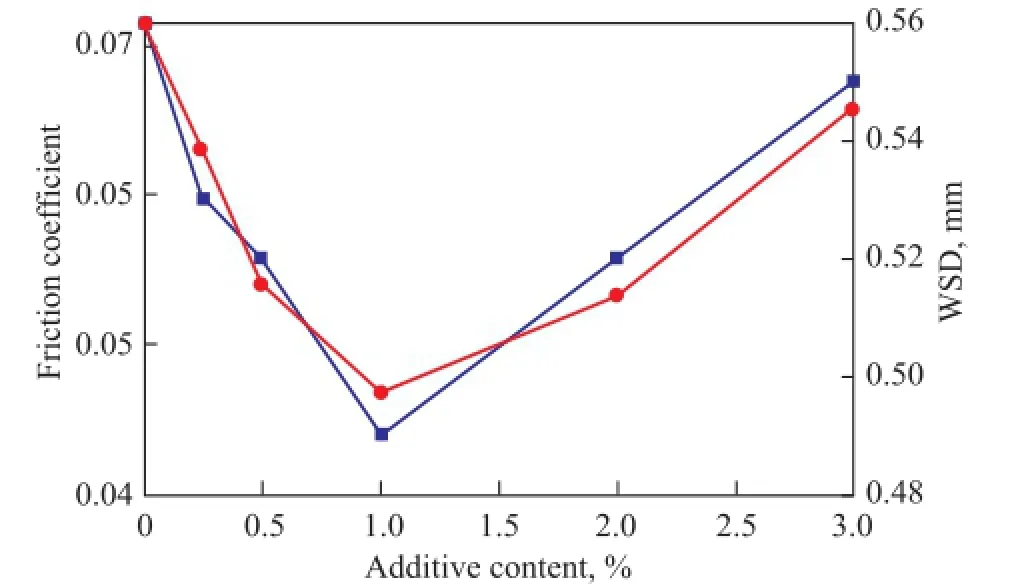
Figure 9 Friction coef fi cients and WSD vs contents of OA/LaBO3·H2O
3.3 SEM and XPS analysis of worn surfaces
Figure 10 shows the SEM images of worn steel surfaces lubricated by RP and RP+OA/LaBO3·H2O. It can be seen that the steel ball lubricated by RP+OA/LaBO3·H2O shows a much smoother worn surface with shallower, fewer and subtler furrows than it is lubricated by RP alone, which identifies that OA/LaBO3·H2O nanosheets can act as an ef fi cient wear reducer in RP.

Figure 10 SEM images of worn surfaces of GCr15 steel balls lubricated by: (a) RP, and (b) RP+OA/LaBO3·H2O
Figure 11 presents the XPS spectra of carbon, iron,lanthanum, boron and oxygen on the worn steel surfaces lubricated by RP+OA/LaBO3·H2O. In Figure 11a, The C1speak at a binding energy of 288.5 eV implies the existence of carbonyl species, which should be attributed to the adsorption of OA/LaBO3·H2O and triglycerides of rapeseed oil on rubbed surfaces. In Figure 11b, the Fe2ppeak at a binding energy of 710.8 eV indicates that iron is oxidized to Fe2O3. The La3dpeak (Figure 11c) at a binding energy of 835.1eV reveals that lanthanum exists on rubbed surfaces in the chemical state of La2O3, while in Figure 11d, the B1speak at a binding energy of 192.0 eV demonstrates that B2O3is formed on rubbed surfaces. In Figure 11e, the O1speaks at a binding energy of 530.2 eV, 533.0 eV, 528.6 eV, and 531.4 eV, respectively, correspond to the chemical state of oxygen in Fe2O3, B2O3, La2O3and carbonyl groups, respectively. These oxides play an important role in improving the friction-reducing and antiwear ability of rapeseed oil[14,34-35].
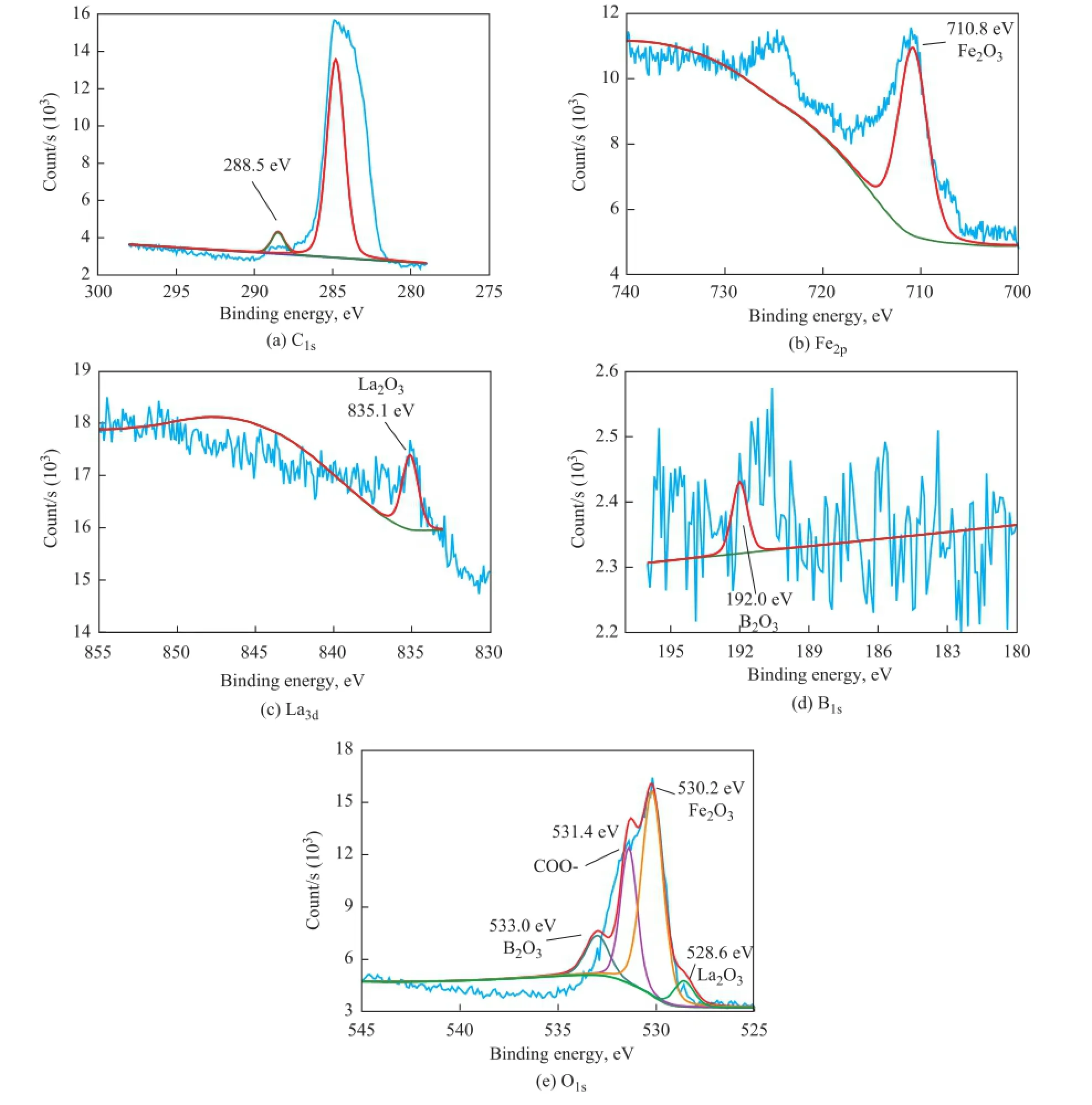
Figure 11 XPS spectra of: (a) C1s, (b) Fe2p, (c) La3d, (d) B1s, and (e) O1son worn steel surfaces lubricated by RP+OA/aBO3·H2O
In view of the above-mentioned surface analyses, it can be reasonably inferred that the excellent capability of OA/LaBO3·H2O used as rapeseed oil dopants in reducingfriction and wear is mainly ascribed to the formation of densely-packed composite boundary lubrication films. At the beginning of friction process, OA/LaBO3·H2O and triglycerides of rapeseed oil are physically and chemically adsorbed on sliding steel surfaces to form an adsorption fi lm composed of carbonyl compounds thereby reducing friction and wear of the steel sliding pairs. With the exception of an adsorption film, OA/LaBO3·H2O nanosheets are also squeezed into the furrows and then spread out on the contact surfaces to form a deposition film under the shear force, and this deposited film acts as a friction and wear reducer via separating the contact surfaces of steel balls. Finally, the heat released by the frictional force accelerates the complicated tribo-chemical reaction to form a complex oxide fi lm mainly consisting of Fe2O3, B2O3and La2O3on rubbed surfaces, and this oxide fi lm is also responsible for reducing the friction and wear of the steel sliding pairs[13-14,34-36].
4 Conclusions
(1) Lanthanum borate nanosheets were prepared by the hydrothermal method and their surfaces were chemically modified in-situ by oleic acid. Surface modification effectively alleviated the agglomeration of lanthanum borate nanosheets. The hydrophobic lanthanum borate nanosheets, namely OA/LaBO3·H2O, exhibited the nanosheet morphology with a diameter in the range of 100 nm to 300 nm and an average thickness of about 25 nm, and possessed an excellent dispersing stability in rapeseed oil.
3)风塔资料显示海风锋到达时,风向风速有显著改变,而海风锋相遇内陆强对流系统时,将激发和加强对流系统,在风场上显示突然地风向转变和迅速地风速增大。同时对流有效位能CAPE显示,CAPE自内陆向沿岸增加,与海风锋移动路径相向。配合水汽通量辐合中心,构成有利于海风锋对强对流系统激发的环境条件,并形成强风与强降水过程。
(2) OA/LaBO3·H2O nanosheets worked as excellent friction and wear reducers in rapeseed oil. The optimal friction-reducing and anti-wear ability of rapeseed oil was achieved at an OA/LaBO3·H2O content of 1.0%.
(3) During the friction process, an adsorption film of carbonyl compounds as well as a deposition fi lm of OA/ LaBO3·H2O plus an oxide film mainly composed of Fe2O3, B2O3and La2O3were formed on rubbed surfaces, which contributed to an ef fi cient reduction of the friction and wear of the steel surfaces.
Acknowledgement: We gratefully acknowledge the financial support of the National Natural Science Foundation of China (Project 50975282).
[1] Wong A B, Brittman S, Yu Y, et al. Core-shell CdS-Cu2S nanorod array solar cells[J]. Nano Lett, 2015, 15: 4096-4101
[2] Fu A, Yang P. A lower threshold for nanowire lasers[J]. Nature Matter. 2015, 14(6): 557-558
[3] Xia Y, Yang P, Sun Y, et al. One-dimensional nanostructures: synthesis, characterization, and applications[J]. Adv Mater, 2003, 15(5): 353-389
[5] Niu Z Q, Peng Q, Gong M, et al. Oleylamine-mediated shape evolution of palladium nanocrystals[J]. Angewandte Chemie, 2011, 123(28): 6439-6443
[6] Rycenga M, Cobley C M, Zeng J, et al. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications[J]. Chem Rev, 2011, 111(6): 3669-3712
[7] Xia Y N, Xiong Y J, Lim B, et al. Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics?[J]. Angew Chem Int Ed, 2009, 48(1): 60-103
[8] Pardo A, Buijnsters J G, Endrino J L, et al. Effect of the metal concentration on the structural, mechanical and tribological properties of self-organized a-C: Cu hard nanocomposite coatings[J]. Appl Surf Sci, 2013, 280: 791-798
[9] Yu H L, Xu Y, Shi P J, et al. Tribological properties and lubricating mechanisms of Cu nanoparticles in lubricant[J]. Trans Nonferrous Met Soc China, 2008, 18(3): 636-641
[10] Wu J F, Zhai W S, Jie G F. Preparation and tribological properties of tungsten disulfide hollow spheres assisted by methyltrioctylammonium chloride[J]. Tribol Int, 2010, 43(9): 1650-1658
[11] Hu K H, Liu M, Wang Q J, et al. Tribological properties of molybdenum disul fi de nanosheets by monolayer restacking process as additive in liquid paraf fi n[J]. Tribol Int, 2009, 42(1): 33-39
[12] Zhang L, Chen L, Wan H Q, et al. Synthesis and tribological properties of stearic acid-modified anatase (TiO2) nanoparticles[J]. Tribol Lett, 2011, 41(2): 409-416
[13] Peng D X, Chen C H, Kang Y, et al. Size effects of SiO2nanoparticles as oil additives on tribology of lubricant[J].Ind Lubr Tribol, 2010, 62(2): 111-120
[14] Ye W Y, Chen T F, Ye Q, et al. Preparation and tribological properties of tetrafluorobenzoic acid-modified TiO2nanoparticles as lubricant additives[J]. Mater Sci Eng A, 2003, 359(1): 82-85
[15] Chen B S, Gu K C, Fang J H, et al. Tribological characteristics of monodispersed cerium borate nanospheres in biodegradable rapeseed oil lubricant[J]. Appl Surf Sci, 2015, 353: 326-332
[16] Kong L T, Hu H, Wang T Y, et al. Synthesis and surface modification of the nanoscale cerium borate as lubricant additive[J]. J Rare Earths, 2011, 29(11): 1095-1099
[17] Mosleh M, Atnafu N D, Belk J H, et al. Modi fi cation of sheet metal forming fl uids with dispersed nanoparticles for improved lubrication[J]. Wear, 2009, 267(5): 1220-1225
[18] Liu N, Tian Y M, Yu L X, et al. Synthesis and surface modification of uniform barium borate nanorods for lubrication[J]. J Alloy Compd, 2008, 466(1): L11-L14
[19] Jia Z F, Xia Y Q. Hydrothermal synthesis, characterization, and tribological behavior of oleic acid-capped lanthanum borate with different morphologies[J]. Tribol Lett, 2011, 41(2): 425-434
[20] Hu Z S, Dong J X, Chen G X, et al. Preparation and tribological properties of nanoparticles lanthanum borate[J]. Wear, 2000, 243(1): 43-47
[21] Gu K C, Chen B S, Wang X M, et al. Preparation and tribological properties of hydrophobic lanthanum borate nanorods in rapeseed oil[J]. Trans Nonferrous Met Soc China, 2014, 24(11): 3578-3584
[22] Song X F, Hu J L, Zeng H B. Two-dimensional semiconductors: recent progress and future perspectives[J]. J Mater Chem C, 2013, 1: 2952-2969
[23] Erhan S Z, Asadauskas S. Lubricant basestocks from vegetable oils[J]. Ind Crops Prod, 2000, 11(2): 277-282
[24] Boyde S. Green lubricants: Environmental benefits and impacts of lubrication[J]. Green Chem, 2002, 4(4): 293-307
[25] Willing A. Lubricants based on renewable resources- an environmentally compatible alternative to mineral oil products[J]. Chemosphere, 2001, 43(1): 89-98
[26] Goyan R L, Melley R E, Wissmer A W, et al. Biodegradable lubricants[J]. Lubr Eng, 1998, 54(7): 10-17
[27] Fox N J, Stachowiak G W. Vegetable oil-based lubricantsa review of oxidation[J]. Tribol Int, 2007, 40(7): 1035-1046
[28] Cheenkachorn K, Fungtammasan B. Development of engine oil using palm oil as a base stock for four-stroke engines[J]. Energy, 2010, 35(6): 2552-2556
[29] Li J, Xia S P, Gao S P. FT-IR and Raman spectroscopic study of hydrated borates[J]. Spectrochim Acta A, 1995, 51(4): 519-532
[30] Gu K C, Chen B S, Chen Y. Preparation and tribological properties of lanthanum-doped TiO2nanoparticles in rapeseed oil[J]. J Rare Earths, 2013, 31(6): 589-594
[31] Tian Y M, Guo Y P, Jiang M, et al. Synthesis of hydrophobic zinc borate nanodiscs for lubrication[J]. Mater Lett, 2006, 60(20): 2511-2515
[32] Gu K C, Chen B S, Wang X M, et al. Preparation, friction, and wear behaviors of cerium-doped anatase nanophases in rapeseed oil[J]. Ind Eng Chem Res, 2014, 53(15): 6249-6254
[33] Qian J H, Yin X Y, Wang N, et al. Preparation and tribological properties of stearic acid-modi fi ed hierarchical anatase TiO2microcrystals[J]. Appl Surf Sci, 2012, 258(7): 2778-2882
[34] Song H J, Jia X H, Li N, et al. Synthesis of α-Fe2O3nanorod/graphene oxide composites and their tribological properties[J]. J Mater Chem, 2012, 22(3): 895-902
[35] Xue Y J, Jia X Z, Zhou Y W, et al. Tribological performance of Ni-CeO2composite coatings by electrodeposition[J]. Surf Coat Technol, 2006, 200(20): 5677-5681
[36] Chen B S, Fang J H, Wang J, et al. Friction and wear performances of borates and lanthanum chloride in water[J]. J Rare Earths, 2008, 26(4): 590-593
Received date: 2016-01-11; Accepted date: 2016-03-23.
Dr. Prof. Chen Boshui, E-mail: boshuichen@163.com; Phone: +86-23- 86730832.
猜你喜欢
杂志排行
中国炼油与石油化工的其它文章
- Experimental and Molecular Simulations for Evaluating the Effect of Lubricity Improvers on the Property of Jet Fuel
- Electrospinning Preparation and Mechanical Properties of Polymethyl Methacrylate (PMMA)/Halloysite Nanotubes (HNTs) Composite Nano fi bers
- Study on the Adaptability of Etheri fi cation Feedstock to Reactor Type
- Modeling of Isobutane/Butene Alkylation Using Solid Acid Catalysts in a Fixed Bed Reactor
- Analysis and Modeling of Wangqing Oil Shale Drying Characteristics in a Novel Fluidized Bed Dryer with Asynchronous Rotating Air Distributor
- Preparation and Tribological Properties of Lanthanumdoped Muscovite Composite Particles as Lubricant Additives in Lithium Grease
