Effect of Hydrothermal Treatment on Suppressing Coking of ZSM-5 Zeolite during Methanol-to-Propylene Reaction
2016-03-22
(State Key Laboratory of Chemical Engineering, Department of Chemical and Biochemical Engineering, Zhejiang University, Hangzhou, 310027, China)
Effect of Hydrothermal Treatment on Suppressing Coking of ZSM-5 Zeolite during Methanol-to-Propylene Reaction
Jiang Binbo; Zhou Bingjie; Yan Lixia; Wei Lingze; Xu Caixia; Liao Zuwei; Huang Zhengliang; Wang Jingdai; Yang Yongrong
(State Key Laboratory of Chemical Engineering, Department of Chemical and Biochemical Engineering, Zhejiang University, Hangzhou, 310027, China)
Fresh ZSM-5 zeolite catalysts were pretreated at 460 ℃ and 500 ℃ with various cumulative amount of water feed (CAWF) in a fi xed bed reactor. The catalytic process was carried out under the following conditions: a temperature of 480 ℃; a methanol WHSV of 3 h-1; a methanol partial pressure of 30 kPa; and a time on stream of 12 h, 24 h and 48 h, respectively. The BET parameters of catalysts and diffusion coefficients of toluene showed that there were two types of mesopores generated under different hydrothermal conditions. Mild temperature and moderate CAWF conditions led to external open mesopores which could be entered from the external surface of the zeolite, while a high temperature or a high CAWF condition resulted in the generation of macropores or internal isolated mesopores, which were occluded in the microporous matrix. The TGA results showed that catalyst with external open mesopores had good ability to resist coke accumulation and good performance on propylene selectivity, while the internal isolated mesopores did no contribute to the increased diffusivity of reactants and products.
methanol to propylene (MTP); hydrothermal treatment; ZSM-5; coking; mesopores; diffusion
1 Introduction
The worldwide demand for propylene has been increasing because the production of propylene derivatives including polypropylene, propylene oxide and acrylonitrile keeps increasing[1-2]. On the other hand, the current propylene production sources, mainly the steam cracking and the fluid catalytic cracking (FCC) units, are not sufficient to meet future propylene demand[3]. Therefore, new production routes with high propylene selectivity are required. The methanol-to-propylene (MTP) process is a promising way to produce propylene independently and ef fi ciently[4-5].
Steam is employed in MTP process as a thinner, a coke remover and a heating medium[6-8]. Adding water to the feedstock can lower the partial pressure of reactants, reduce the accumulation of carbonaceous substances and enhance the alkene yield, while the presence of water vapor will also accelerate the dealumination of the ZSM-5 zeolite[9]. Barghi[10]found out that the activity loss due to coking was decreased and the activity loss due to sintering was increased with an increasing water content in the feed. The dual effects of water led to the research of appropriate water feeding amount and feeding time so that carbonaceous deposits would be inhibited whilst the acid sites wouldn’t be affected by water during the reaction. Hydrothermal treatment is used to produce what is termed an equilibrium catalyst with high activity and good ability to resist coking by removing the acid sites close to or on the external surface of the catalyst[11], which will decrease the hydrogen transfer from coke species[12]. Besides, the formation of new block space like mesopores after dealumination will strengthen the diffusion efficiency of light olefins and heavy hydrocarbons. Aramburo[13]found that the distribution of mesopores fully depended on the hydrothermal temperature. Mild conditions just affected the external regions, while severe conditions led to the formation of mesoporous network within the whole crystal. However, not all kinds of mesopores will contribute to improving the diffusion ef fi ciency of reactants or products[14]. The amount, shapeand distribution of mesopores in the crystal all determine whether the hierarchical pore system has better ability to resist coking than the microporous system.
In this study, we prepared several catalyst samples under different hydrothermal treatment conditions. The obtained samples differed by the amount and distribution of mesopores. Subsequently, the catalytic performance of these samples was measured based on their diffusion coefficients and coking rate. The relation between the hydrothermal treatment condition, the mesopore distribution and the catalytic performance was fi nally obtained.
2 Experimental
2.1 Catalyst Preparation
The ZSM-5 zeolite used in this study was supplied by the Sinopec Research Institute of Petroleum Processing. The Si/Al of the fresh catalyst (marked C-0) was 200 and the particle size ranged from 1.2 mm to 1.5 mm. The hydrothermal treatment conditions of the samples are listed in Table 1.
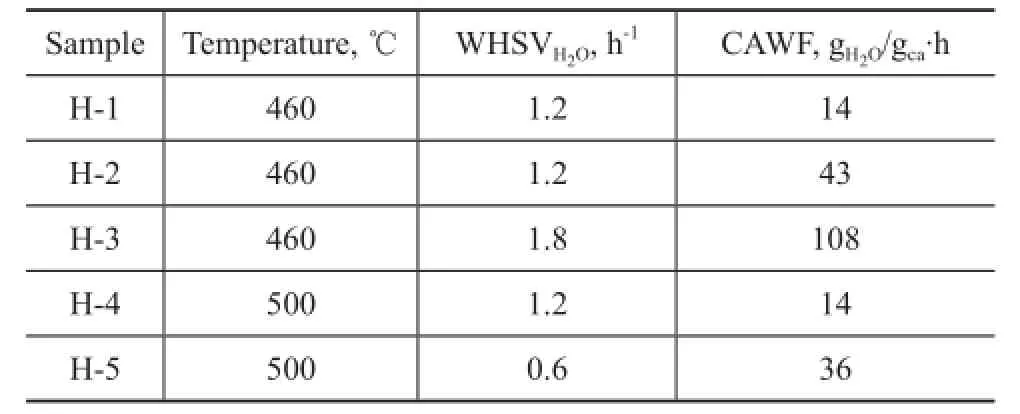
Table 1 Hydrothermal treatment conditions of samples
2.2 Characterization of catalysts
All the samples were characterized by N2adsorption/ desorption measurements at 77 K employing the BET method (using the Micromeritics ASAP 2400 instrument). Before the N2adsorption measurements, the samples were outgassed at 393 K for 5 h to remove water.
The amount of acidity and acid strength distribution of the catalysts were analyzed using the temperature programmed desorption with ammonia gas (NH3-TPD). Prior to the measurements, about 0.1 g of sample was heated at 823 K for 1 h to remove water, and after being cooled down for 30 min to 373 K the ammonia adsorption process was carried out for another 30 min. Physically adsorbed ammonia was removed by helium at 373 K for 1 h. Finally, the sample was heated from 373 K to 823 K at a temperature increase rate of 10 K/min under fl owing helium gas.
A thermo-gravimetric analyzer (TGA) was used for detecting the amount of coke deposited on the samples. Prior to the measurements, the sample was heated at 393 K for 4 h with N2, and then the nitrogen fl ow was replaced by a mixture of O2(80%) diluted with nitrogen at a total flow rate of 50 mL/min, then the reactor temperature was increased from 393 K to 1 123 K at a heating rate of 10 K/min.
The diffusion experiments were carried out with an intelligent gravimetric analyzer (IGA), model IGA-003, made by the Hiden Analytical (Warrington, United Kingdom). The samples were placed in a stainless steel cylinder with a height of 300 mm and a diameter of 34.5 mm. The temperature went up in a heating furnace on the outside of the reactor, and was then measured by a platinum resistance thermometer (Pt 100) located near the sample. The pressure was regulated with a proportionalintegral-derivative (PID) controller, and measured by a manometer. At each set pressure or temperature point, the real-time processor of IGA could record the increase or decrease in weight of samples owing to the adsorption of diffusate automatically.
2.3 Reaction equipment and product analysis
A fi xed-bed setup was used for the catalytic testing, and a stainless steel tube reactor (with an ID of 12 mm) was filled with 5 g of ZSM-5 zeolite catalyst. The reaction was carried out at 480 ℃ and 0.15 MPa, using a feed of methanol at a weight hourly space velocity (WHSV) of 3.0 h-1and a reactant partial pressure of 30 kPa through accurate mixing of the methanol with N2as a diluent gas. Then the MTP reaction was terminated after 12 h, 24 h, and 48 h on stream, respectively. The product was analyzed by a gas chromatograph equipped with a PLOT-Q column (30 m×0.32 mm×25 μm) from 393 K (maintained for 3 min) to 523 K (maintained for 15 min) at a heating rate of 20 K/min.
3 Results and Discussion
3.1 Relationship of mesoporous form and diffusion performance
As the result of BET analysis reflects the adsorption onthe “external” mesoporous surface[15], it is widely used for testing zeolites with mesoporous structure. The BET surface areas and micropore volumes of the samples are listed in Table 2. With the increase of CAWF we can also fi nd a pore size increase of the newly formed mesopores from the BJH graph of Figure 1. Higher temperature condition of hydrothermal treatment can lead to the collapse of the matrix of the catalyst[12], so it is much easier to generate mesopores (or macropores) with big size[13]. It can be obviously seen from data listed in Table 2 that the hydrothermal treatment causes a loss in surface areas of sample H-1.
As CAWF increased to 460 ℃, the micropore volume of sample H-3 decreased sharply while the mesopore volume as well as the external surface area increased notably, as compared to the sample H-1. Similar trends could be found at 500 ℃. It can be found from comparison between the treatment of samples H-1 and H-4 that higher temperature could lead to larger mesoporous volume and larger external surface area. It can be seen from Figure 1 that the mesoporous volume increased with the increase of CAWF because of the increased amount of small mesopores. The newly formed mesopores had a diameter between 2 nm and 3 nm while the diameter of macropores was around 51 nm. Besides the reduction of micropores, the collapse of macropores that led to the creation of new mesopores, 31 nm in diameter, could be attributed to the increase of mesoporous volume in the sample H-5.
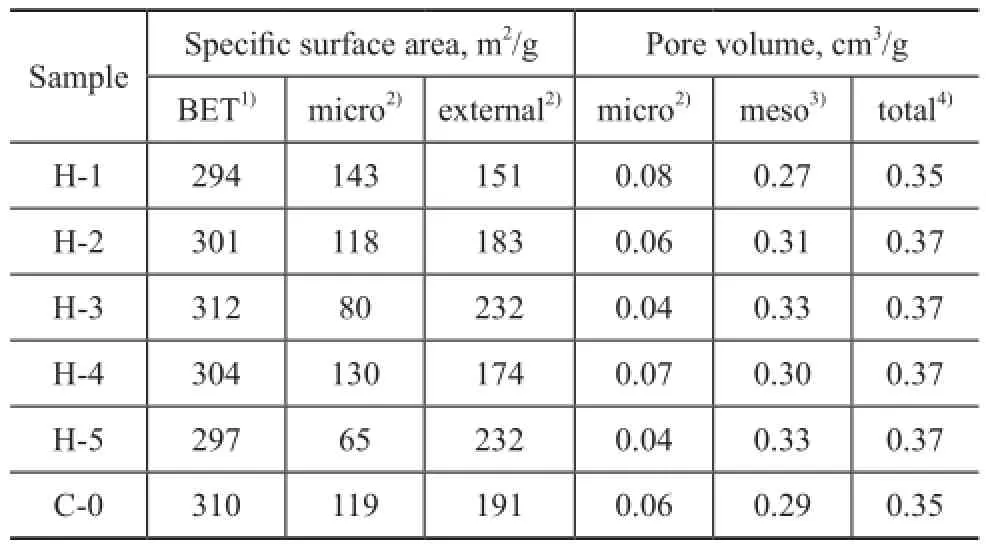
Table 2 BET surface areas and micropore/mesopore volumes
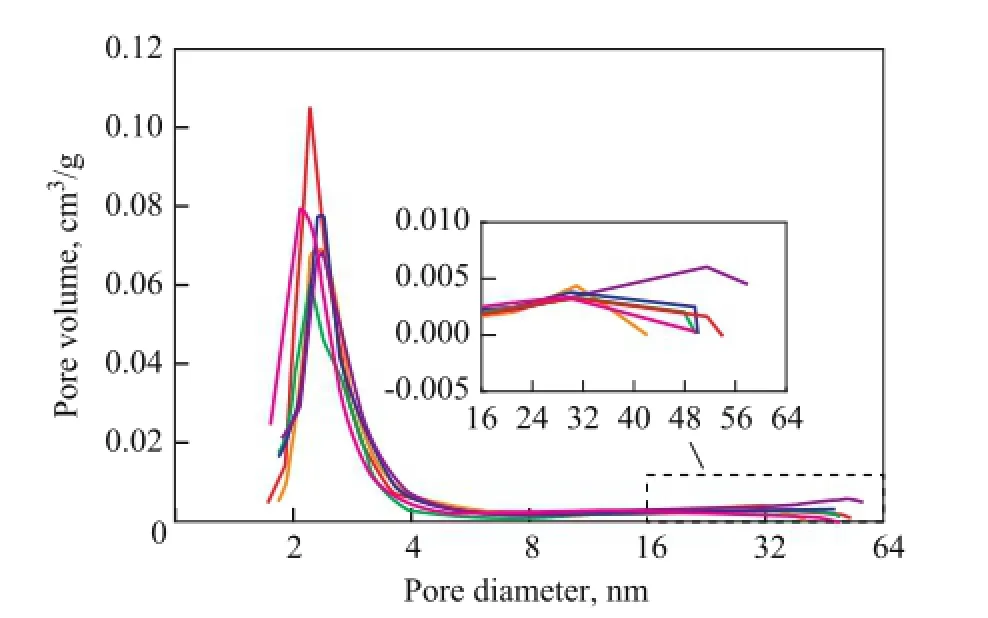
Figure 1 Pore volume derived from BJH desorption data
The NH3-TPD spectrogram was used to describe the strength and distribution of acid sites in ZSM-5 zeolite. The peak at lower temperature reflected the NH3desorption from moderate and weak acid sites, while the peak at high temperature reflected the NH3desorption from strong acid sites, which could contribute to coking reactions[16]. The NH3-TPD spectrograms of all samples are presented in Figure 2, where no clear difference is seen in the distribution and strength of acid sites among the samples except samples H-5 and H-3. As for the sample H-3, the temperature of the two peaks is lower than others. While for the sample H-5, there is no clear peak at high temperature. This result indicates that the ZSM-5 zeolite used in this study is highly resistant to the dealumination caused by the steam.
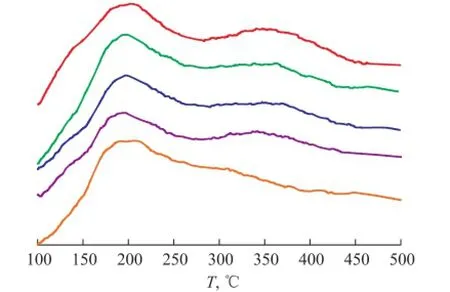
Figure 2 NH3-TPD spectrograms of hydrothermally pretreated catalysts
The observed differences in the pore structure among the samples are further con fi rmed by the IGA measurements presented in Table 3. Toluene was chosen as the diffusate in this study. The effective diffusion coef fi cients of toluene at 200 ℃ in each sample decreased in the following order: H-2>H-5>C-0>H-3>H-4>H-1. It can be seen that the diffusion coefficient increases as the micropore volumedecreases except for the sample H-3. It is denoted that the micropore and mesopore volumes are the same for samples H-3 and H-5, but the toluene diffusion ef fi ciency in H-5 is bigger than that in H-3. This can be explained that the new mesopores generated in H-3 originate from the collapse of internal micropores. Because of the higher concentration and longer time of steaming, the Brönsted acidity contained in the internal crystal is more accessible[13]. So the small mesopores formed in H-3 are mainly internal isolated mesopores (type A of Figure 3), which are occluded in the microporous matrix. As regards the sample H-2, the mild hydrothermal treatment results in generating the external open mesopores (type B of Figure 3), which can be entered from the external surface of the zeolite crystals, because the mild steaming condition induces alterations that are limited to the external regions[13].
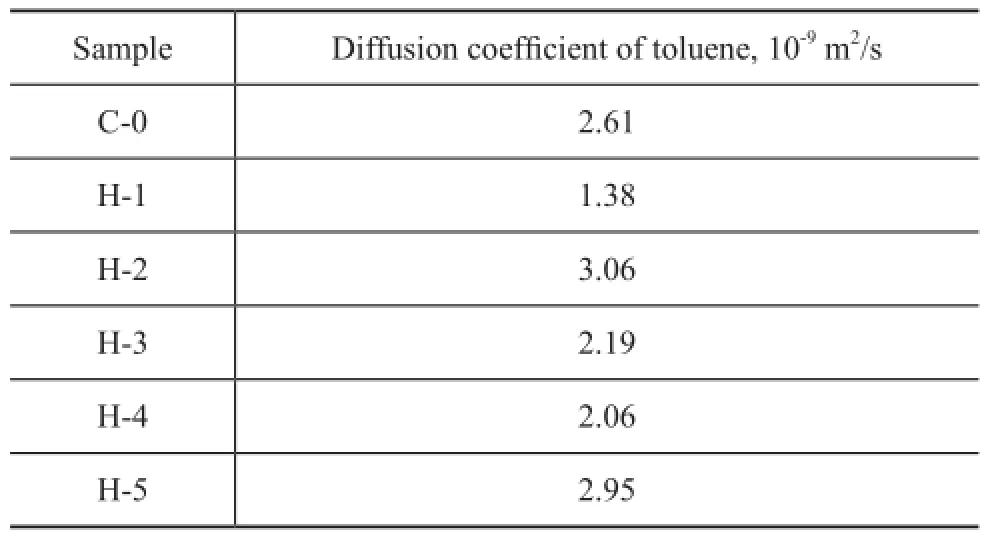
Table 3 Effective diffusion coefficients of the diffusate toluene
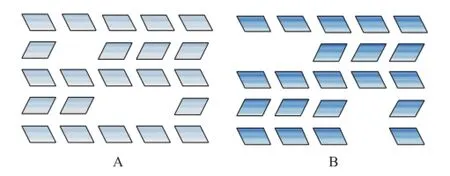
Figure 3 Two types of mesopores and their distribution: Type A: internal isolated mesopores; Type B: external open mesopores
3.2 Effect of hydrothermal treatment on the catalytic properties
The total amount of oxidizable coke was determined by TGA. The derivative thermogravimetry (DTG) curves of each sample after experiencing a TOS of 12 h, 24 h and 48 h, respectively, are shown in Figure 4. A growing trend can be seen in both the temperature and the area of the exothermic peaks as the TOS goes by, which suggests that the carbonaceous compounds expand in molecular size. Although the amount and density of acid sites are reduced after hydrothermal treatment, the temperature of the exothermic peaks in DTG curves show no clear difference among these samples. This indicates that the acidity does not affect the nature of coke species but in fl uence the rate and amount of coke compounds[17].
Figure 5 shows the coke content of each catalyst sample which varied with the TOS. It can be seen that the coking rate of samples H-3 and H-5 was lower than other samples. This occurred because of the lower density and strength of acid sites resulting in stronger resistance to the formation of coke precursor. As the time on stream went by, the coke rate of sample H-2 became the lowest, verifying the fact that the diffusivity in sample H-2 was much better than other samples and the sample H-2 had an advantageous mesoporous form and distribution. The coke content in sample H-2 became stabilized after 24 h of operation. This can be explained by the fact that the sample H-2 had an external open mesoporous structure and its diffusion ability was stronger than others.
The catalytic performance of each sample is shown in Figure 6. It can be seen that in respect of samples H-1, H-2 and H-4, the mild hydrothermal treatment conditions led to a high activity of catalyst. As regards samples H-3 and H-5,the severe conditions (higher concentration and longer time of steaming) could damage most of the micropores, which would lead to a notable loss of the acid sites. The propylene and aromatic selectivity was in fl uenced by the diffusivity of each catalyst. For samples H-1 and H-4, the lower concentration and steaming time had negligible effect on the pore structure, which could lead to nearly no difference as compared with the fresh catalyst C-0. For the sample H-3, the methanol conversion and propylene selectivity were the lowest, while the selectivity of aromatics was the highest. This result can be ascribed to the fact that the internal isolated mesopores were not conducive to the product diffusivity, but they could provide a space for the formation of products with big size. As for the sample H-5, the loss of strong acid sites could lead to the limitation on aromatization. Therefore the aromatic selectivity of the sample H-5 waslower than other samples. The sample H-2, which had an external open mesoporous system and a biggest diffusion coefficient, showed high methanol conversion, high propylene selectivity and low aromatic selectivity during the reaction.
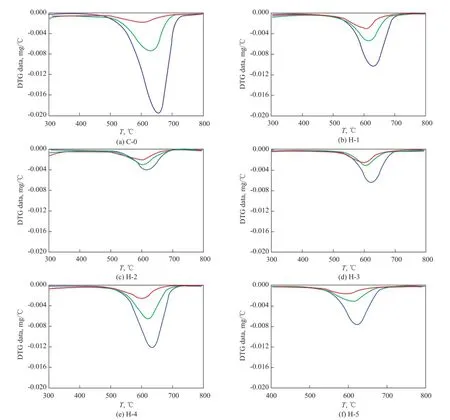
Figure 4 The DTG curve of each sample (obtained from a TOS of 12 h, 24 h and 48 h)
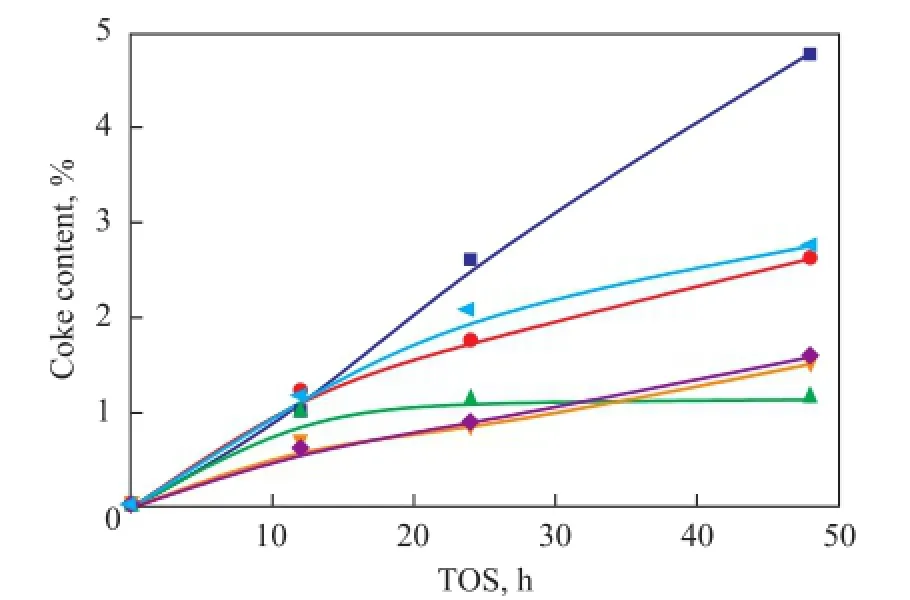
Figure 5 The coke content of each catalyst sample versus the time on stream
4 Conclusions
It can be concluded that different hydrothermal treatment conditions will lead to different types of mesopores. Lower temperature, moderate concentration and CAWF conditions can lead to slight dealumination on the external regions of the catalyst that results in the formation ofexternal open mesopores, while higher temperature condition tends to generate mesopores with bigger size due to the severe dealumination within the whole crystal of ZSM-5 zeolite.

Figure 6. The products distribution of MTP process
The performance of catalyst samples is obviously in fl uenced by the mesoporous forms in the microporous system because of their different contribution to the diffusivity of reactants and products. Catalysts with a signi fi cant number of external open mesopores apparently show higher methanol conversion and propylene selectivity (the target product) and lower selectivity of aromatics (the byproducts). During the whole reaction process, this kind of mesoporous system could restrict the accumulation of coke deposits by using its excellent diffusion ef fi ciency. As for the internal isolated mesoporous system, it could not strengthen the diffusivity, while offering a new place to accommodate secondary products with big size. So the aromatics account for a much higher ratio in the fi nal products slate.
Acknowledgments: This study is supported by the National Natural Science Foundation of China (Grant 21176208 & 61590925), the National High-Tech R & D Program of China (Grant 2012AA030304), the International S&T Cooperation Projects of China (2015DFA40660) and the Fundamental Research Funds for the Central Universities (Grant 2015QNA4033).
[1] Zhou H Q, Wang Y, Wei F. Kinetics of the reactions of the light alkenes over SAPO-34[J]. Appl Catal A, 2008, 348(1): 135-141
[2] Lee K Y, Lee H K. Influence of catalyst binders on the acidity and catalytic performance of HZSM-5 zeolites for methanol-to-propylene (MTP) process: Single and binary binder system[J]. Top Catal, 2010, 53(3): 247-253
[3] Plotkin J S. The changing dynamics of olefin supply/ demand[J]. Catal Today, 2005, 106(1): 10-14
[4] Koempel W, Liebner M, Fábio B N. Lurgi’s methanol to propylene (MTP) report on a successful commercialisation[J]. Stud Surf Sci Catal, 2007, 167: 261-267
[5] Jiang B B, Feng X, Yan L X, et al. Methanol to propylene process in a moving bed reactor with byproducts recycling: kinetic study and reactor simulation[J]. Ind Eng Chem Res, 2014, 53(12): 4623-4632
[6] Kang M, Inui T. Dynamic reaction characteristics affected by water molecules during the methanol to ole fi n conversion on NiAPSO-34 catalysts[J]. J Mol Catal A: Chem, 1999, 140(1): 55-63
[7] Xu W, Miller S J, Agrawal P K. Positive effect of water on zeolite BEA catalyzed alkylation of phenol with propylene[J]. Catal Lett, 2014, 144(3): 434-438
[8] Corma A, Marie O, Ortega F J. Interaction of water with the surface of a zeolite catalyst during catalytic cracking:A spectroscopy and kinetic study[J]. J Catal, 2004, 222(2): 338-347
[9] Ghavipour M, Behbahani R M, Moradi G R. Methanol dehydration over alkali-modified H-ZSM-5: Effect of temperature and water dilution on products distribution[J]. Fuel, 2013, 113(2): 310-317
[10] Barghi B, Fattahi M, Khorasheh F. The modeling of kinetics and catalyst deactivation in propane dehydrogenation over Pt-Sn/γ-Al2O3in presence of water as an oxygenated additive[J]. Pet Sci Technol, 2014, 32(10): 1139-1149
[11] Ivanov D P, Pirutko L V, Panov G I. Effect of steaming on the catalytic performance of ZSM-5 zeolite in the selective oxidation of phenol by nitrous oxide[J]. J Catal, 2014, 311(3): 424-432
[12] Mante O D, Agblevor F A, McClung R. The effect of hydrothermal treatment of FCC catalysts and ZSM-5 additives in catalytic conversion of biomass[J]. Appl Catal A, 2012, 445(6): 312-320
[13] Aramburo L R, Hofmann J P, Weckhuysen B M. Imaging the effect of a hydrothermal treatment on the pore accessibility and acidity of large ZSM-5 zeolite crystals by selective staining[J]. Catal Sci Technol, 2013, 3: 1208-1214
[14] Schüth F. Endo- and exotemplating to create high-surfacearea inorganic materials[J]. Angew Chem Int Ed. 2003, 42(31): 3604-3622
[15] Campbell S M, Bibby D M, Coddington J M. Dealumination of HZSM-5 zeolites. 2. Methanol to gasoline conversion[J]. J Catal, 1996, 161(1): 350-358
[16] Choudhary T V, Kinage A, Banerjee S. Influence of hydrothermal pretreatment on acidity and activity of H-GaAl MFI zeolite for the propane aromatization reaction[J]. Microporous Mesoporous Mater, 2005, 87(1): 23-32
[17] Mores D, Kornatowski J, Olsbye U. Coke formation during the methanol-to-olefin conversion: in situ microspectroscopy on individual H-ZSM-5 crystals with different Bronsted acidity[J]. Chem Eur J, 2011, 17(10): 2874-2884
Received date: 2016-01-11; Accepted date: 2016-02-23.
Dr. Liao Zuwei, E-mail: liaozw@zju. edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Synthesis and Evaluation of Environmentally Friendly Calcium Isostearate Detergent with Excellent Oil Solubility
- Study on the Adaptability of Etheri fi cation Feedstock to Reactor Type
- Modeling of Isobutane/Butene Alkylation Using Solid Acid Catalysts in a Fixed Bed Reactor
- Analysis and Modeling of Wangqing Oil Shale Drying Characteristics in a Novel Fluidized Bed Dryer with Asynchronous Rotating Air Distributor
- Experimental and Molecular Simulations for Evaluating the Effect of Lubricity Improvers on the Property of Jet Fuel
- Preparation and Tribological Properties of Lanthanumdoped Muscovite Composite Particles as Lubricant Additives in Lithium Grease
