Study on the Mechanism of Zn2SiO4Formation in S Zorb Sorbents and Its Inhibition Methods
2016-03-22
(SINOPEC Research Institute of Petroleum Process, Beijing 100083)
Study on the Mechanism of Zn2SiO4Formation in S Zorb Sorbents and Its Inhibition Methods
Xu Li; Zou Kang; Xu Guangtong; Yang Xingyuan; Xu Hua; Mao Anguo
(SINOPEC Research Institute of Petroleum Process, Beijing 100083)
S Zorb adsorptive desulfurization technology is of great signi fi cance on the production of clean gasoline in China, but the formation of Zn2SiO4during the operation in sorbents would bring forth negative impacts on the desulfurization performance and the stability of the processing unit. By using the in-situ TPO, XRD, and IR techniques to study the mechanism of Zn2SiO4formation under operating conditions, it was found that the coexistence of acid and hydrothermal conditions could accelerate the formation of Zn2SiO4. Moreover, the study of Zn2SiO4inhibition method indicated that the decrease of oxygen concentration in regeneration gas would inhibit the rate of Zn2SiO4formation, and the regeneration of ZnS would take place in a mild regeneration condition.
S Zorb; zinc silicate; desulfurization; deactivation; reactivation
1 Introduction
The ultra-deep desulfurization of gasoline has become an increasingly pressing issue for refineries in China. The SINOPEC’s S Zorb adsorptive desulfurization process, a major technology for the production of clean gasoline with ultra-low sulfur content, has such outstanding characteristics as a minimum octane loss, a lower hydrogen consumption, and a highest liquid yield[1-3]. According to the S Zorb desulfurization reactions, the ZnO phase is considered to be one of the most important active phases in sorbents[3-4]. However, the ZnO phase was seriously consumed under certain conditions during the long-term running of industrial units because of the formation of inactive Zn2SiO4phase, which leads to an irreversible inactivation of sorbents[5-7]. Lin, et al.[5]studied the conditions for the formation of Zn2SiO4in the fresh FCAS-R09 sorbents, and indicated that the rate of Zn2SiO4formation increased with an increasing temperature and partial pressure of water. Zhang, et al.[6]studied the behavior of Zn2SiO4formation in fresh and spent industrial sorbents under different conditions and the influence of hydroxyl silicon on the formation of Zn2SiO4using the FT-IR spectroscopic method. Xu, et al.[7]indicated that the formation of Zn2SiO4in industrial sorbents was more likely to be detected under the combined acidic hydrothermal conditions rather than in the dry or hydrothermal atmosphere, and the actually industrial regeneration conditions agreed more properly with the former one—the combined acidic hydrothermal condition. To our knowledge, there is neither report about the mechanism of Zn2SiO4formation in the S Zorb sorbents under the combined acidic hydrothermal conditions, nor the optimization of regeneration conditions to inhibit the formation of inactive Zn2SiO4phase.
In this work, XRD and in situ IR spectrometric techniques were used to study the mechanism of Zn2SiO4formation in the S Zorb fresh sorbents under the combined conditions of acidic and hydrothermal atmosphere. The evolution of sorbent phase during regeneration was processed and monitored by TPO and XRD techniques, respectively. Efforts were then made to propose and discuss the optimal methods for regeneration conditions to inhibit the formation of the non-active phase as far as possible while maintaining a high desulfurization activity of sorbents. This study can provide the support and guidance for the design of industrial catalysts and the optimization of industrial regeneration conditions.
2 Experimental
2.1 Samples
The S Zorb fresh sorbent (S-1) was a commercial sorbent, which was produced by the Nanjing Branch of SINOPEC Catalyst Co., Ltd. The spent sorbents (D-1 and D-2) were collected from a 0.9 Mt/a industrial unit at the SINOPEC Jinan Branch Company. As shown in Table 1, the phase contents of fresh and spent sorbents were determined by using XRD analysis with the fast phase quantitative method[8-9].

Table 1 Phase contents of sorbents %
2.2 Acidic hydrothermal reaction of fresh sorbents
The acidic hydrothermal reaction was carried out in an instrument made by our lab, which was similar to the one used in the previous studies[6-7]. The reaction was conducted using nitrogen as the carrier gas at a fl ow rate of 50 L/h. The reaction mixture was preheated from the room temperature up to the required reaction temperature of 550 ℃ at a temperature increase rate of 120 ℃/min. The SOXwas produced by the combustion of ZnS with trace oxygen coming from the vapor and the carrier gas. The vapor was generated by evaporation of water injected by a high pressure fl at- fl ow pump at a liquid fl ow rate of 200 mL/h.
2.3 Simulation of spent sorbents regeneration
Simulation of spent sorbents regeneration was carried out by using the AutoChem II 2920 TPO apparatus. The volume of sorbents was about 300 mg in each experiment. The sample was fi rstly puri fi ed in Ar gas with a fl ow rate of 50 mL/min at room temperature for 30 min. Secondly, the temperature increased to reach the regeneration temperature of 500 ℃ at a rising rate of 10 ℃/min in the Ar atmosphere. Thirdly, the reaction gas was switched from Ar to air or a mixed gas of Ar containing 5% of oxygen. The WHSV value in all experiments was similar to those used in the industrial units. After a period of reaction at 500 ℃, the reaction gas was switched back to Ar gas until the temperature decreased to the room temperature. Finally, the regenerated sorbents were characterized by XRD analysis with the fast phase quantitative method[8-9].
2.4 Characterization of sorbents
The X-ray powder diffraction (XRD) patterns of the sorbents were recorded on a Rigaku TTR-III diffractometer using CuKα radiation (λ=1.540 6Å) at a tube voltage of 40 kV and a tube current of 250 mA[8-9].
The silicon hydroxyl groups of sorbents were analyzed by the Fourier transform infrared spectrophotometer (FTIR) coupled with a vacuum pretreatment system made by our lab. The FT-IR spectra were recorded using a Thermo Fisher Nicolet 560 spectrophotometer in the range of 3 830—3 160 cm-1with a 0.5 cm-1resolution in vacuum at 500 ℃.
3 Results and Discussion
3.1 Mechanism of Zn2SiO4formation
The XRD patterns of S-1 treated at 550 ℃ in the acidic hydrothermal condition at different time duration are shown in Figure 1. It can be seen that a series of sharp and obvious peaks at 2θ=31.5°, 34.3°, 36.2°, 47.6° and 56.5° are attributed to ZnO phase, and the characteristic peaks at 2θ=37.1° and 43.2° are attributed to NiO phase, which are commonly observed in S Zorb sorbents[5-9]. It is worth noting that the new characteristic peaks at 2θ=12.7°, 22.1°, 25.5°, 34.0°, 38.8° and 48.9° can be observed in XRD patterns of S-1 at the reaction time of 80 h and 136 h, which can be attributed to the formation of Zn2SiO4[5-9]. According to the enlarged view of XRD patterns (Figure 1, under), the intensity of characteristics peaks of Zn2SiO4significantly increased with an increasing reaction time beyond 80 h. This phenomenon is quite similar to our previous studies, which was attributable to the formation of silicon hydroxyl groups (OxSi-(OH)4-x) on the surface of amorphous SiO2in the sorbent[6-7]. The OxSi-(OH)4-xspecies can easily react with ZnO to form[6-7].

Figure 1 XRD patterns (upper) of S-1 treated at 550 ℃under acidic hydrothermal condition with different time and enlarged view (under)
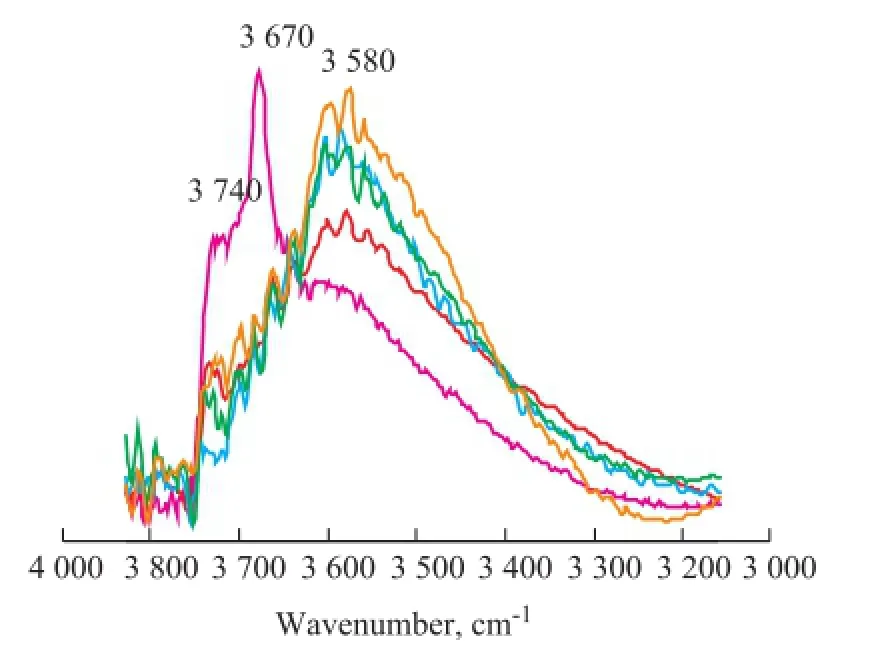
Figure 2 Vacuum FT-IR spectra of S-1 treated at 550 ℃ inacidic hydrothermal condition at different time
The vacuum FT-IR spectra of S-1 treated at 550 ℃ in the acidic hydrothermal condition with different time are shown in Figure 2. There are three types of hydroxyl silicon groups on the surface of silicon, including the‘single free’ (isolated), the ‘single geminal’, and the bridged silanol groups[6,10-11]. The fi rst two silanol groups are also known as the terminal silanol groups. The schemes of three hydroxyl silicon groups are shown in Figure 3. As regards the FT-IR spectra of original S-1 (obtained at an aging time of 0 h), the weak and broad absorption band at 3 580 cm-1is attributed to the so-called bridged silanol groups. The sharp and obvious absorption bands at 3 670 cm-1and 3 740 cm-1are attributed to the‘single geminal’ and the ‘single free’ (isolated) silanol groups[6,10-11].

Figure 3 Schemes of three hydroxyl silicon groups
Compared with Figure 1 and Figure 2, Zn2SiO4phase was not observed in the original S-1 (obtained at an aging time of 0 h). It is obvious that the silanol groups of original S-1(obtained at an aging time of 0 h) were primarily attributed to the terminal silanol groups at 3 670 cm-1and 3 740 cm-1. With the increase of aging time (at an aging time of 8 h and 24 h), the intensity of absorption band of terminal silanol groups obviously decreased, and that of the bridged silanol group at 3 580 cm-1significantly increased at the same time, indicating that under the acidic hydrothermal conditions the terminal silanol group of amorphous SiO2was massively removed and was converted to form more bridged silanol group. Through further comparative analysis of sorbents before and after the formation of Zn2SiO4(obtained at an aging time of 24 h and 80 h), the intensity of two bands at 3 580 cm-1attributed to the bridged silanol groups was basically the same, indicating that the Zn2SiO4would be generated after a certain amount of bridged silanol groups had existed in the sorbents. Comparison of sorbents with different contents of Zn2SiO4(at an aging time of 80 h and 136 h), the intensity of absorption bands at 3 580 cm-1attributed to the bridged silanol groups had signi fi cantly increased, demonstrating that the increase of Zn2SiO4content in sorbents would lead to the generation of more bridged silanol groups, which might further accelerate the formation of Zn2SiO4. Therefore, on the basis of XRD and FT-IR analysis results, the scheme of the mechanism of Zn2SiO4formation in acidic hydrothermal conditions could be proposed as follows (Figure 4).
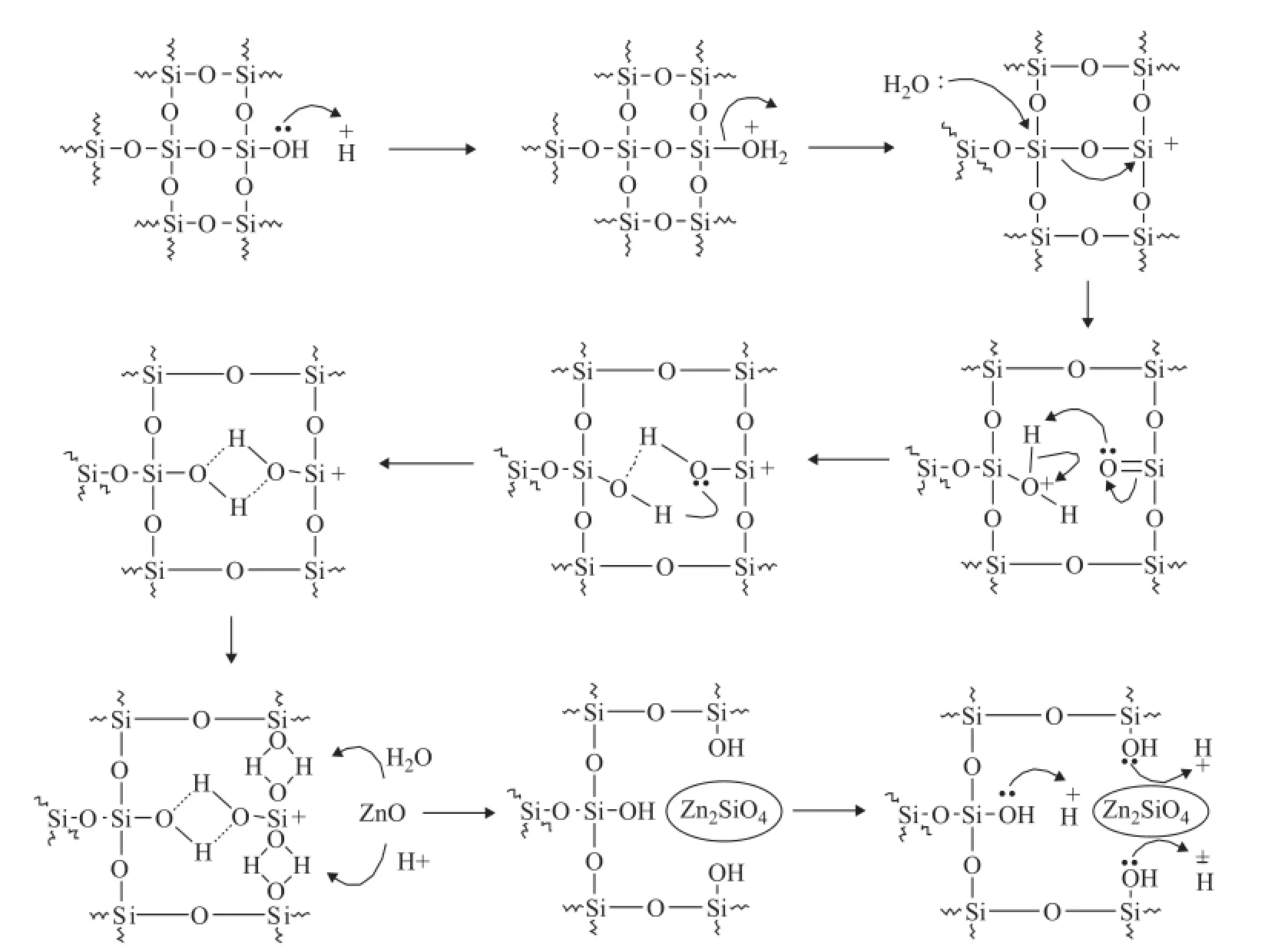
Figure 4 Mechanism of Zn2SiO4formation in sorbent under acidic hydrothermal conditions
It can be seem from Figure 4 that the formation of Zn2SiO4was closely related to the bridged silanol groups rather than the terminal silanol groups. The acid did play a key role in the reaction, leading to a dramatic decrease of terminal silanol groups and an increase of bridged silanol groups. The reaction process might be described as follows: First of all, the presence of acid could result in the loss of terminal silanol groups, producing some unstable defect silicon centers with positive charges. Secondly, the induction of these unstable defect silicon centers and the effect of vapor resulted in break and dissociation of three neighboring Si—O—Si bonds, generating a lot of bridged silanol groups. Thirdly, the bridged silanol groups reacted with ZnO to form Zn2SiO4, which could generate more exposed terminal silanol groups at once. Finally, the exposed terminal silanol groups further turned into some additional unstable defect silicon centers under the acidic hydrothermal conditions, leading to the acceleration of Zn2SiO4formation.
In order to further study the mechanism of Zn2SiO4formation in the S Zorb sorbents, the vacuum FT-IR spectra of original S-1 and S-1 that had been treated in hydrothermal and acidic hydrothermal conditions are shown in Figure 5.
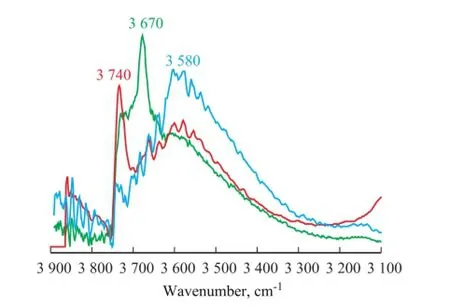
Figure 5 Vacuum FT-IR spectra of original S-1 and S-1 treated in hydrothermal and acidic hydrothermal conditions (550 ℃)
Upon comparing the FT-IR spectra between the original S-1 and S-1 that had been treated in hydrothermal condition, the sharp intensity absorption band at 3 679 cm-1in the original S-1 disappeared after the hydrothermal treatment, and the absorption band at 3 740 cm-1increased signi fi cantly in S-1 that had been treated in the hydrothermal condition. The absorption bands at 3 679 cm-1and 3 740 cm-1were both attributed to the terminal silanol groups. It should be noted that the intensity of absorption band of the bridged silanol group (at 3 580 cm-1) did not change obviously during the hydrothermal treatment, which could better explain the phenomenon that the formation of Zn2SiO4was not easy to be observed in hydrothermal condition[6-7]. After the acidic hydrothermaltreatment, the FT-IR spectrum (Figure 5) showed that the intensity of absorption bands at 3 679 cm-1and 3 740 cm-1sharply decreased, and that of the bands at 3 580 cm-1dramatically increased. The existence of acid gas signi fi cantly increased the amount of bridged silanol groups in the S Zorb sorbent.
3.2 Methods for inhibition of Zn2SiO4during regeneration process
During the regeneration process, the acidic hydrothermal environment seemed to be inevitable now. According to the mechanism of Zn2SiO4formation in the S Zorb sorbent, the reduction of vapor and acid concentration could decrease the amount of the bridged silanol groups to inhibit the formation of Zn2SiO4[12-13]. In order to reduce the concentration of vapor and acid gas, the mixed gas containing 5% of oxygen and 95% of nitrogen was adopted in the experiments in place of air, which was commonly used in the industrial units. The changing trend of ZnO, ZnS, and Zn2SiO4contents of D-1 and D-2 regenerated at 500 ℃ in the air and in the mixed gas are shown in Figures 6 and 7, respectively. According to the main regeneration reaction of S Zorb process (1)[12-13], in order to achieve the same ZnS regeneration rate, the fl ow rate of mixed gas (with 5% of O2) should be four times as much as that of air.
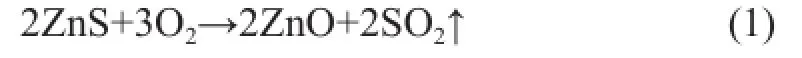
During the regeneration process of D-1 and D-2 in the presence of two different atmospheres, the content of ZnO gradually increased, while the content of ZnS gradually decreased. In addition, the content of Zn2SiO4slightly increased, which was in accordance with the results of our previous studies. It is worth noting that the contents of ZnO and ZnS were stable after regeneration in the presence of both mixed gas and air, indicating that the regeneration of active phases in the S Zorb sorbent is determined by the total amount of O2rather than the concentration of O2. Furthermore, the rate of Zn2SiO4formation was obviously decreased in the mixed gas. Thisphenomenon could be attributable to the dilution effect of mixed gas into the acid gas and vapor. Both of the vapor and acid gas were generated by the combustion of D-1 and D-2. At the same O2content, the fl ow rate of mixed gas should be four times as much as that of air, which obviously lowered the concentration of vapor and acid gas. Thus, the formation of Zn2SiO4in the sorbents was successfully inhibited.
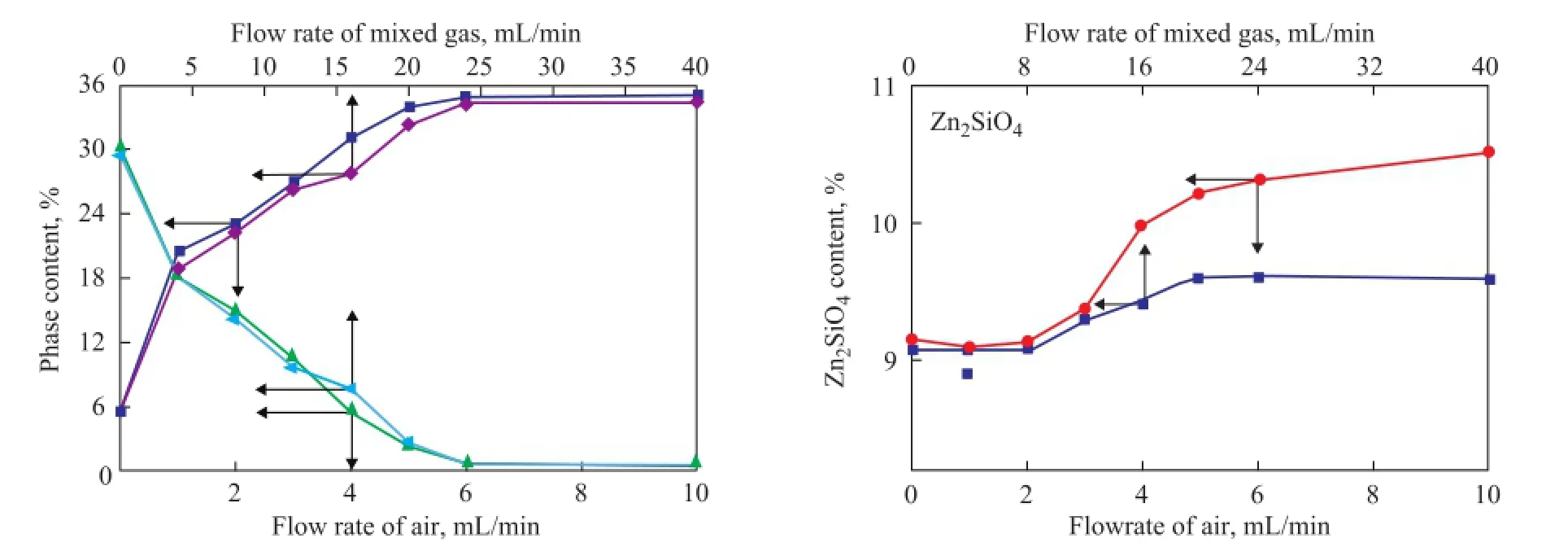
Figure 6 Relationship between phase content of D-1 and regeneration conditions

Figure 7 Relationship between phase content of D-2 and regeneration conditions
4 Conclusions
The coexistence of acidic gas and the hydrothermal environment was an important factor for the rapid formation of Zn2SiO4in the S Zorb sorbents. The presence of acid gas resulted in the loss of terminal silanol groups of S Zorb sorbents and the formation of unstable defect silicon centers, which could form the bridged silanol groups leading to the generation of Zn2SiO4. Then, the formation of Zn2SiO4resulted in more exposed terminal silanol groups of sorbents, which could be continuously turned into additional unstable defect silicon centers, leading to the acceleration of Zn2SiO4formation.
According to the mechanism for formation of Zn2SiO4in the S Zorb sorbents, the reduction of O2concentration during the regeneration process could consequently decrease the concentration of vapor and acid gas, and then inhibit the formation of Zn2SiO4in the S Zorb sorbents.
Acknowledgements: The authors thank the financial support from the funding provided by SINOPEC (CLY15053, 114010, and 114057).
[1] Zhu Yunxia, Xu Hui. Improvement and development of S Zorb process[J]. Petroleum Refinery Engineering, 2009, 39(8): 7-12 (in Chinese)
[2] Wu Defei, Zhuang Jian, Yuan Zhongxun, et al. Technology improvement and application in the localization of S Zorb technology[J]. Petroleum Processing and Petrochemicals, 2012, 43(7): 76-79 (in Chinese)
[3] Xu Guangtong, Diao Yuxia, Zou Kang, et al. Cause analysis of sorbent deactivation in S Zorb unit for gasoline desulfurization[J]. Petroleum Processing and Petrochemicals, 2011, 42(12): 1-6 (in Chinese)
[4] Long Jun, Lin Wei, Dai Zhenyu. From detailed desulfurization mechanism to successful commercial application: I. Features and advantages of S Zorb technology[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2015, 31(1): 1-6 (in Chinese)
[5] Lin Wei, Wang Lei, Tian Huiping. An analysis of the formation rate of zinc silicate in S Zorb sorbents[J]. Petroleum Processing and Petrochemicals, 2011, 42(11): 1-4 (in Chinese)
[6] Zhan Xin, Xu Guangtong, Huang Nangui. Conditions of willemite formation in S Zorb sorbent[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2013, 29(4): 619-625 (in Chinese)
[7] Xu Hua, Yang Xingyuan, Zou Kang, et al. Effect of atmosphere on zinc silicate formation in S Zorb sorbents[J]. Petroleum Processing and Petrochemicals, 2011, 45(6): 9-14 (in Chinese)
[8] Zou Kang, Huang Nangui, Xu Guangtong. Study of Rietveld quantitative phase analysis of S Zorb sorbent[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2012, 28(4): 598-604 (in Chinese)
[9] Zou Kang, Xu Guangtong, Gai Jinxiang, et al. Rapid quantitative phase analysis technology for desulphurization sorbents and its application[J]. Petroleum Processing and Petrochemicals, 2014, 45(10): 99-105 (in Chinese)
[10] Van der Voort P, D’Hamers I G, Vansant E F. Estimation of the distribution of surface hydroxyl groups on silica gel, using chemical modi fi cation with trichlorosilane[J]. J Chem Soc, Faraday Trans, 1990, 86(22): 3751-3755
[11] Van Der Voort P, D’Hamers I G, Vrancken K C, et al. Effect of porosity on the distribution and reactivity of hydroxyl groups on the surface of silica gel[J]. J Chem Soc, Faraday Trans, 1991, 87(24): 3899-3905
[12] Xu Li, Zou Kang, Xu Guangtong, et al. Investigation on structure, composition,and regeneration behavior of industrial S Zorb adsorbent[J]. Petroleum Processing and Petrochemicals, 2013, 44(6): 44-48 (in Chinese)
[13] Zou Kang, Xu Guangtong, Gai Jinxiang, et al. Study on the thermal decomposition processing of commercial S Zorb sorbent by using in situ HT-XRD and TG-MS[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2015, 31(3): 732-739 (in Chinese)
Received date: 2016-02-28; Accepted date: 2016-04-18.
Dr. Xu Li, Telephone: +86-10-82369237; E-mail: xuli.ripp@sinopec.com.
杂志排行
中国炼油与石油化工的其它文章
- Synthesis and Evaluation of Environmentally Friendly Calcium Isostearate Detergent with Excellent Oil Solubility
- Study on the Adaptability of Etheri fi cation Feedstock to Reactor Type
- Modeling of Isobutane/Butene Alkylation Using Solid Acid Catalysts in a Fixed Bed Reactor
- Analysis and Modeling of Wangqing Oil Shale Drying Characteristics in a Novel Fluidized Bed Dryer with Asynchronous Rotating Air Distributor
- Experimental and Molecular Simulations for Evaluating the Effect of Lubricity Improvers on the Property of Jet Fuel
- Preparation and Tribological Properties of Lanthanumdoped Muscovite Composite Particles as Lubricant Additives in Lithium Grease
