盐霉素诱导的细胞自噬机制在抗癌治疗中的应用进展
2016-03-13陈思远胡济安
陈思远,胡济安
(浙江大学医学院附属口腔医院,病理科,杭州310006)
盐霉素诱导的细胞自噬机制在抗癌治疗中的应用进展
陈思远,胡济安*
(浙江大学医学院附属口腔医院,病理科,杭州310006)
摘要自噬在肿瘤的发生、发展、治疗过程中扮演着重要的角色.盐霉素作为一种聚醚类离子载体型抗生素,一直被用于防治鸡球虫病和促进反刍动物生长;它也能有效杀伤多种肿瘤干细胞及多药耐药肿瘤细胞系,且在此过程中伴随自噬的发生.盐霉素诱导的自噬是复杂的,可由细胞内活性氧升高而激活自噬;可通过损伤线粒体引发线粒体自噬;可引起内质网应激,通过AKT1-m TOR信号通路激活自噬.此外,盐霉素可抑制自噬潮的晚期阶段,有助于肿瘤微环境的破坏和自身抗肿瘤作用的提高.本文综述了盐霉素在禽畜疾病中的应用及机制、盐霉素抗肿瘤的作用机制及盐霉素诱导自噬的机制.
关键词盐霉素;肿瘤;自噬;凋亡;球虫病
目前,肿瘤的化学治疗药物主要包括4大类:烷化剂、抗代谢药、抗生素类抗肿瘤药、抗肿瘤植物药.盐霉素作为一种离子载体型抗生素,一直用于家禽球虫病防治及禽畜的生长促进.自2009年以来,研究发现盐霉素可抑制小鼠原发性胶质瘤、乳腺癌细胞转移[12],并可杀伤具有耐药性和抗凋亡的肿瘤细胞系、肿瘤干细胞[39]等多种肿瘤细胞.盐霉素在杀伤肿瘤细胞过程中还伴随自噬现象发生,且大多为肿瘤细胞的自我保护作用.这为肿瘤治疗提供了新思路,使联合自噬抑制剂作为肿瘤治疗药物成为可能.
1 盐霉素在家禽中的应用及机制
1.1盐霉素的研究历史及理化性质
盐霉素(C42H70O11),于上世纪70年代初由MIYAZAKI与其同事从白色链球菌发酵培养中获得,为一元羧酸聚醚类离子载体型抗生素类,具有抑制革兰氏阳性菌和防治禽类球虫病的作用,并可促进牛、猪等禽畜的生长.KINASHI等通过X射线晶体分析,鉴定了盐霉素的化学结构式[10],其相对分子质量为750,如图1所示;其紫外光吸收波长为285 nm,红外光吸收波长为5.85μm,熔点为113℃[11].
1.2盐霉素抗球虫、抗菌作用及其机制
家禽类在饲养过程中易出现球虫感染,导致生长缓慢、发育不良,甚至死亡,造成严重的经济损失.目前,抗球虫药按其化学结构和生产过程大致可分为2类:一类是聚醚类离子载体抗生素;另一类是化学合成的抗球虫药.其中,莫能菌素、盐霉素和拉沙里菌素3种离子载体型抗球虫药应用广泛,而盐霉素因其较低的药物毒性[12]、低廉的用药成本,其年使用量占所有离子载体型抗球虫药的50%以上[1314].
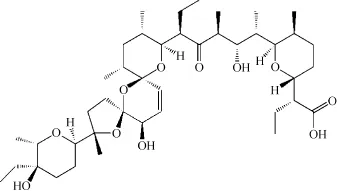
图1 盐霉素的分子结构式Fig.1 Molecular structure of salinomycin
研究发现,低质量浓度的盐霉素(0.5~16 mg/ L)即可有效杀伤多种革兰氏阳性菌,如肠道内的粪肠球菌、屎肠球菌、葡萄球菌、产气荚膜杆菌等,且不易产生耐药性[1516].MITANI等[17]最早报道了盐霉素抗菌及抗球虫的机制:盐霉素主要与胞外K+和Na+形成络合物,利用膜转运蛋白将阳离子送入细胞内,通过破坏细胞膜和线粒体膜内外的离子平衡,从而损伤线粒体功能,使细胞失活,达到抗菌、抗球虫的目的.近年来,LAVINE等[18]发现,盐霉素还可通过激活线粒体DNA修复酶Tg MSH-1导致弓形球虫细胞周期S期阻滞,发挥抗球虫作用.
1.3盐霉素促进鸡、猪、牛等禽畜生长及其机制
盐霉素还广泛用于鸡、猪、牛、羊等日常饲养,可有效降低料肉比,促进禽畜体质量增加[1920].早期研究表明,盐霉素主要通过增加瘤胃中丙酸含量,降低乙酸、丁酸含量,提高瘤胃的消化吸收功能而促进反刍动物(牛、羊)生长[21].JÓZEFIAK等[22]发现,盐霉素(60 mg/kg)添加到肉鸡饲料后,肉鸡回肠中拟杆菌属和肠杆菌科细胞减少,特异的细菌酶α-葡萄糖苷酶和β-半乳糖苷酶活性增高最为明显,有利于营养物质的吸收,促进肉鸡生长.TSINAS等[23]报道了盐霉素通过抑制猪肠腺瘤的发生而促进断奶仔猪生长.
直至2009年,GUPTA等[24]从1.6万种化合物中筛选出盐霉素可选择性杀伤乳腺癌干细胞,且效果为紫杉醇的100倍.从此开启了盐霉素在肿瘤研究中的新篇章.
2 盐霉素抗肿瘤作用的主要机制
2.1盐霉素通过抑制Wnt/β-链蛋白信号通路促进肿瘤细胞凋亡
Wnt/β-链蛋白(β-catenin)信号通路作为Wnt信号转导的经典途径,与细胞自我更新、分化、增殖、凋亡等密切相关.在成熟细胞内,Wnt/β-链蛋白信号通路处于沉默状态;当细胞质内β-链蛋白浓度升高至一定水平,它可转移至细胞核内,与转录因子家族TCF/LEF结合,进而激活MMP-7、Myc、细胞周期素D1、CD44等多种原癌基因[25],导致细胞增殖、分化、成熟,促进肿瘤的发生发展.近年来,已报道Wnt/β-链蛋白在前列腺癌[26]、乳腺癌[27]、肝癌[28]等多种肿瘤中异常表达,并与肿瘤的侵袭性和耐药性相关.MAO等[29]发现:过表达Wnt1的AGS细胞株在裸鼠体内形成肿瘤的能力更强,体积更大;盐霉素能有效减小肿瘤体积,主要通过抑制Wnt/β-链蛋白信号通路.
低密度脂蛋白受体相关蛋白(low-density lipoprotein receptor related protein,LRP)是Wnt信号通路中的受体蛋白之一,磷酸化的LRP可使β-链蛋白在细胞内积累,调控肿瘤的发生.LU等[30]报道了盐霉素可有效抑制LRP6的表达进而抑制β-链蛋白浓度的上升,阻止Wnt/β-链蛋白信号通路的激活,促进乳腺癌及前列腺癌细胞系凋亡.WU等[31]发现盐霉素作用于鼻咽癌细胞系后,LRP6和β-链蛋白表达下调,细胞凋亡增加及侵袭性降低:提示盐霉素可通过抑制Wnt/β-链蛋白信号通路来抑制肿瘤的发展.
2.2盐霉素通过抑制肿瘤细胞周期进程而诱导凋亡
肿瘤细胞的快速增殖,往往与细胞周期调控失调有关.通过抑制细胞周期的进程从而抑制肿瘤细胞分裂,也为肿瘤治疗提供了思路.已知细胞周期素D1可促进细胞周期G1向S期转变,并在多种肿瘤中过表达[32].P27kip1蛋白通过抑制细胞周期转变起到抑制肿瘤发生的作用,Skp2表达上升后,可降解P27kip1,从而诱导肿瘤的发生[33].在卵巢癌中,盐霉素可抑制Skp2及细胞周期素D1的表达,上调P27kip1的表达,从而使细胞周期停滞在G1期[34]及细胞凋亡.AL DHAHERI等[35]将不同浓度盐霉素作用于乳腺癌细胞系后发现:低浓度盐霉素主要通过下调细胞周期素D1从而诱导细胞周期G1阻滞;高浓度盐霉素则是通过下调细胞周期素B1,诱导细胞周期G2阻滞,进而诱导细胞凋亡.
2.3盐霉素逆转上皮-间质转化
上皮-间质转化,是指上皮细胞通过特定程序转化为具有间质表型细胞的生物学过程,其主要特征有细胞黏附分子(如E-钙黏蛋白)表达的减少,细胞角蛋白骨架转化为波形蛋白(vimentin);上皮-间质转化往往与肿瘤的侵袭和转移有关.GUPTA等[24]发现盐霉素可选择性杀伤乳腺癌干细胞,且效果为紫杉醇的100倍,随后在小鼠移植瘤模型中,也验证了盐霉素的抑癌作用,并且增加了肿瘤细胞向上皮分化.KOPP等[36]发现,低浓度盐霉素即可抑制小鼠乳腺癌细胞系4T1和小鼠Lewis肺癌细胞系转移,随后将4T1细胞通过尾静脉注射到Balb/c小鼠中,形成肺部原发肿瘤,再给予5 mg/kg盐霉素处理数日后,肺部原发肿瘤的大小未见明显改变,但有效抑制了其向脑、肾、脾的转移.KOPP等[37]在随后的研究中进一步证明,盐霉素作用于4T1和Lewis细胞系后,通过促进miR-200c表达而增强E-钙黏蛋白的表达,抑制波形蛋白的表达和上皮-间质转化的发生.在小鼠乳腺癌移植瘤模型和小鼠Lewis肺癌模型中,盐霉素作用后促进上皮细胞标记蛋白表达,对肿瘤生长无明显抑制作用,但有效抑制肿瘤转移.CD44+的前列腺癌细胞可通过TGFβ1-CD44信号通路促进上皮-间质转化的发生,使肿瘤细胞具有更强的侵袭性.在移植瘤模型中,盐霉素有效抑制CD44及波形蛋白的表达,促进E-钙黏蛋白的表达,逆转上皮-间质转化[38].阿霉素为肝细胞癌治疗的常用药物,但体外实验表明,阿霉素作用于肝细胞癌后,可通过β-链蛋白/TCF复合体引起的上皮-间质转化使肝癌细胞系产生耐药及转移.而体内外实验均验证了盐霉素可通过激活FOXO3a抑制β-链蛋白/TCF复合体的表达,促进E-钙黏蛋白的表达及波形蛋白的表达,逆转了由阿霉素引起的上皮-间质转化[39].
3 盐霉素诱导肿瘤细胞产生自噬的机制
3.1自噬与肿瘤的关系
3.1.1自噬概述
细胞自噬是细胞通过溶酶体自我降解的过程,主要通过溶酶体将错误折叠的蛋白质及受损的细胞器降解.在应激、代谢、维持内环境稳定及保持基因组完整性方面发挥重要作用.当细胞受到外界刺激时,包括紫外线、饥饿、氧化应激、DNA损伤、缺氧等,可通过一系列协同的应答通路发生自噬,包括AMPK信号通路[40]、PI3K/AKT信号通路[41]、ROS信号通路[42]等.
3.1.2自噬抑制肿瘤形成
肿瘤的发生是基因突变逐渐累积的结果,自噬可通过将细胞内损伤的细胞器、侵入的微生物、错误折叠的蛋白质清除以维持细胞内稳态及基因组的稳定性.因此,在肿瘤的初始阶段,自噬发挥抑制作用. Beclin-1是自噬核心复合物的重要组成部分,调控自噬的发生.小鼠体内自噬相关基因Atg5[43]缺失后可引起多发性肝肿瘤,Beclin-1杂合缺失后可引起自发性肺癌、肝癌和淋巴瘤[44],Bif-1纯合缺失后可导致自发性淋巴瘤、肝细胞癌及食管癌[45],进一步证明了自噬可抑制肿瘤形成.MORTENSEN等[46]发现,小鼠造血干细胞Atg7表达沉默后,造成骨髓发育不良,出现类似人类急性髓性白血病的表型:表明自噬在维持造血干细胞的正常功能中不可或缺.因此,自噬缺陷有利于肿瘤的形成.
3.1.3自噬促进肿瘤生存和转移
肿瘤在发展过程中会经历低氧、营养物质匮乏等不良生长环境.肿瘤细胞可通过自噬水解受损的蛋白质及细胞器等,产生氨基酸和脂类,满足自身的营养需求,促进自身的存活.在恶性胶质瘤中,低氧可通过HIF-1α/AMPK信号通路激活自噬,促进肿瘤细胞生存[47].肿瘤血管生成在肿瘤生长和扩散中起重要作用.在裸鼠移植瘤中,自噬主要存在于肿瘤的低氧、血供尚未建立区域[48],抑制自噬后,血管内皮生长因子VEGF表达降低,瘤体内血管数量减少[49]:表明自噬有助于肿瘤血管的生成,促进肿瘤发展.肿瘤休眠是临床普遍存在的现象,与肿瘤复发和远处转移密切相关[50].肿瘤细胞内PI3K-AKT信号通路表达下调后,可出现类休眠样形态及自噬激活.ras同源基因家族成员ARHⅠ可抑制PI3K-m TOR信号通路,激活自噬并促进肿瘤休眠.应用自噬抑制剂氯喹作用于由ARHⅠ诱导的已进入休眠的移植瘤,可见肿瘤重新开始生长:表明自噬可促进肿瘤休眠[51],促进肿瘤复发及转移.
3.2盐霉素与自噬的关系
3.2.1盐霉素诱导肿瘤细胞自噬过程中伴随坏死
在肿瘤治疗过程中,常伴有肿瘤细胞的自噬、凋亡和坏死.盐霉素作用于肿瘤细胞后,也可引起细胞发生这3种改变.QIN等[52]发现,盐霉素作用于神经胶质瘤细胞系U87MG、U251MG后,可通过ROS-P53-CyPD信号通路调控坏死.XIPELL等[53]发现,盐霉素作用于神经胶质瘤细胞系后,细胞内活性氧增加,ATP减少,溶酶体膜破裂,释放组织蛋白酶B及溶酶体膜蛋白Lamp1,细胞质肿胀:提示肿瘤细胞发生程序性坏死.高迁移率族蛋白HMGB1作为经典的损伤相关模式分子,与细胞程序性坏死密切相关,可促进炎症反应[54],并可通过与自噬相关蛋白Beclin-1结合,使之从Beclin-1/Bcl-2复合体解离,诱导自噬的发生[55].JANGAMREDDY等[56]发现,盐霉素作用于乳腺癌细胞系SKBR3后,细胞内HMGB1表达明显升高,并出现明显的程序性坏死.此外,KANG等[57]报道了,在胰腺癌细胞系Panc02中,HMGB1与晚期糖基化终末产物受体RAGE相互作用后,抑制哺乳动物雷帕霉素靶蛋白m TOR的活性,调控自噬的发生.将HMGB1敲除后,细胞凋亡增加,自噬明显受到抑制,且对化疗药物敏感性增加.这表明HMGB1与肿瘤细胞生存和耐药密切相关.提示盐霉素联合HMGB1抑制剂,可有效增强药物的敏感性,避免坏死及体内的炎症反应.
3.2.2盐霉素诱导肿瘤细胞发生自噬
放疗、化疗是除手术外最主要的治疗方法,在治疗过程中,肿瘤细胞往往可以通过自噬来保护自己,逃避死亡.WANG等[58]通过将微粒体装载的羟氯喹和微粒体装载的阿霉素联合应用于小鼠移植瘤模型中,增强了阿霉素的抗肿瘤作用:表明自噬具有促生存的作用.YOU等[59]将克唑替尼作用于肺癌细胞系后,细胞内出现大量自噬泡结构,联合自噬抑制剂羟氯喹或RNA干扰Beclin-1后,可明显增强克唑替尼的抗肿瘤作用,也体现了自噬的促生存作用.然而,APO866[60]和氯碘羟喹[61]诱导的自噬,都有促死亡的作用.盐霉素作用于不同细胞系后,大部分细胞内出现的自噬都有保护作用[62-63];然而,在VERDOODT等[64]的研究中,自噬具有促进SW620细胞系死亡的作用.同一药物应用于不同细胞系,联合自噬抑制剂,出现截然相反的效果可能与药物浓度、细胞内机制调控密切相关,为未来的联合用药提供了实验基础,也给予了警示.
3.2.3盐霉素抑制自噬潮
自噬潮为一个动态过程,包括诱导、自噬前体的形成及延伸、吞噬物选择及包裹、自噬体形成及运输、与溶酶体融合、内容物降解及再利用.KLOSE等[65]联合盐霉素和氯化铵或氯喹作用于肝癌细胞系后发现,相比于单独用药,LC3B-Ⅱ的表达量并未出现明显升高:表明盐霉素可抑制自噬潮.YUE等[66]发现,在乳腺癌肿瘤干细胞样细胞中,盐霉素作用后,可见自噬相关蛋白P62表达增高,而在正常情况下,自噬激活后,自噬体最终在溶酶体内降解,P62作为LC3与泛素化底物之间的枢纽,能整合到成熟自噬体中,并在溶酶体内降解,即当LC3表达上升,P62表达下降,表明自噬潮过程被抑制;进一步研究表明,在此过程中,自噬体与溶酶体仍可以结合,但溶酶体降解功能异常,具体机制仍需进一步研究.XIPELL等[53]发现,盐霉素作用于恶性胶质瘤细胞系后,产生的活性氧可使脂质氧化,溶酶体膜通透性改变,组织蛋白酶B表达量降低:提示溶酶体酸性环境被破坏,致使溶酶体降解功能异常,从而抑制自噬潮.JANGAMREDDY等[67]分别将低浓度和高浓度盐霉素作用于小鼠胚胎成纤维细胞后,发现低浓度盐霉素可激活自噬,高浓度(30μmol/ L)可抑制自噬,使细胞内LC3B聚集.因此,我们推测:低浓度盐霉素作用于肿瘤细胞后,溶酶体仍有降解功能;当高浓度盐霉素作用于肿瘤细胞后,溶酶体降解功能破坏,从而抑制自噬潮.
3.2.4盐霉素破坏肿瘤微环境
肿瘤微环境由肿瘤细胞和基质细胞、细胞外基质、免疫炎性细胞等构成,具有低氧、营养匮乏、酸性、间质流体压力高等特点,与肿瘤干细胞的形成[68]及肿瘤的发展、转移、耐药密切相关[6970]. LARZABAL等[71]发现,将小鼠Lewis肺癌细胞系接种到C57/BL6小鼠皮下,盐霉素有效抑制移植瘤的肺部转移.免疫组化分析移植瘤及肺转移灶表明,盐霉素有效抑制移植瘤中趋化受体因子CXCR4、肿瘤相关成纤维细胞分泌的SDF-1的表达(SDF-1可有效促进表达CXCR4的肿瘤细胞增殖及肿瘤血管形成[72])及减少CD11B+、F4/80+的巨噬细胞浸润(与淋巴管新生密切相关,促进炎症反应及肿瘤的形成[73]):表明盐霉素主要通过破坏肿瘤微环境而抑制转移.
低氧作为肿瘤微环境中研究最多的一个特征,与肿瘤血管的形成、免疫监视、DNA过度复制及耐药都存在密切关系[74].在肿瘤的低氧区域往往伴随自噬的激活,主要信号通路如下:HIF-1α可通过激活BNIP3,使得Bcl-2和Beclin-1复合物分离而激活自噬[75];低氧可激活腺苷酸活化蛋白激酶,抑制m TOR活性,从而激活自噬;低氧还可通过内质网应激及其相应通路激活自噬[76].进一步研究表明,低氧诱导的自噬具有促进肿瘤转移及保护肿瘤细胞的作用.酸性条件也为肿瘤微环境的特征之一,可通过影响药物代谢而使肿瘤细胞具有耐药性,且此处的肿瘤细胞往往通过增强自噬来保护自身[77].氯喹——自噬潮的晚期阶段抑制剂,联合其他抗肿瘤药物,取得了一定的抗癌效果[78];然而, PELLEGRINI等[79]发现,将肿瘤细胞株HCT116和Me30966置于p H为6.8的酸性环境中培养,氯喹失去了抑制自噬潮的功能,小鼠体内实验进一步证明,氯喹在酸性环境中无法抑制自噬潮:暗示了在酸性肿瘤微环境中,氯喹无法很好地发挥其作用. PELLEGRINI等[80]的近期成果表明,盐霉素在酸性环境下能更明显地抑制自噬潮,可作为自噬抑制剂;通过微囊化肿瘤细胞培养模型(模拟体内的低氧及酸性环境)培养大肠癌细胞系后,盐霉素可同时抑制囊表面及核心区的细胞自噬,然而,氯喹及Lys-01只能抑制囊表面细胞的自噬,且酸性条件增强了盐霉素对乳腺癌干细胞的杀伤性.
综上所述,肿瘤微环境往往呈低氧、酸性且含有大量的肿瘤干细胞,肿瘤细胞在这些区域往往通过增强自噬以保护自己免于凋亡及促进肿瘤发展.如前所述,盐霉素可杀伤多种肿瘤干细胞和多药耐药肿瘤细胞,抑制自噬潮,酸性环境可增强其对肿瘤细胞的杀伤作用.因此,我们认为盐霉素通过抑制自噬潮,进一步破坏了肿瘤微环境.
3.2.5盐霉素诱导肿瘤细胞产生自噬的具体机制
自2012年首次报道盐霉素可诱导肿瘤细胞产生自噬至今,学者们已用不同肿瘤细胞系研究盐霉素诱导自噬的相关机制.盐霉素作用后,大部分肿瘤细胞内都产生了活性氧,其在自噬激活过程中发挥了重要而复杂的作用.KIM等[81]将盐霉素作用于骨肉瘤细胞系后,活性氧表达上升,自噬相关蛋白LC3B表达上升,通过NAC去除细胞内活性氧后,盐霉素诱导细胞产生的自噬被明显抑制:表明活性氧在调控自噬过程中发挥着重要作用. VERDOODT等[64]将盐霉素作用于结肠癌和乳腺癌细胞系后,产生的活性氧可部分通过JNK信号通路激活自噬.ZHU等[62]发现,盐霉素诱导破骨细胞瘤细胞系U2OS和MG-63产生自噬主要通过ROSAMPK-m TOR途径.然而,LI等[63]发现,盐霉素诱导非小细胞性肺癌细胞株产生自噬是通过内质网应激,经ATF4-DDIT3/CHOP-TRIB3-AKT1-m TOR信号通路激活自噬.JANGAMREDDY等[56]发现,盐霉素作用于前列腺癌细胞系PC3后,导致细胞内线粒体功能异常,包括线粒体膜去极化、ATP减少,进而发生线粒体自噬.
综上所述,盐霉素诱导不同肿瘤细胞系发生自噬的机制是复杂的,可通过损伤线粒体而发生线粒体自噬,通过内质网应激而激活AKT1-m TOR信号通路发生自噬,诱导活性氧的产生进而通过多信号通路介导自噬的发生.
4 结语
自噬作为细胞内的“清道夫”,在细胞正常功能的维持上发挥着至关重要的作用.近年来的研究揭示了自噬与肿瘤、发育、神经退行性疾病、免疫和衰老等存在密切联系,因此,已成为研究热点.当细胞受到不同的外界刺激时,产生的自噬大不相同.如本文中抗肿瘤药物盐霉素诱导的自噬,既可以促进肿瘤细胞生存,又可以加速肿瘤细胞死亡.因而,在研究盐霉素的抗肿瘤作用时,要明确它诱导的自噬在此过程中的作用,以便于联合自噬激活剂或者抑制剂达到更好的抗肿瘤效果.此外,已有少量文献报道了盐霉素作用于肿瘤细胞后,HMGB1表达上升,细胞出现程序性坏死,因此,可进一步探讨自噬与程序性坏死的关系.盐霉素诱导肿瘤细胞自噬的机制尚未完全阐明,仍需通过深入研究,探索盐霉素激活肿瘤细胞的具体机制,也有助于发现自噬调控的新机制.近年来研究表明,miRNA与肿瘤细胞自噬、凋亡、耐药、转移、侵袭等存在密切关系.而盐霉素可通过促进miR-200c 表达而抑制上皮-间质转化的发生.但目前对于miRNA是否参与调控盐霉素诱导肿瘤细胞自噬的过程还未见报道,仍具有研究意义.
参考文献(References):
[1] CHEN T,YI L,LI F,et al.Salinomycin inhibits the tumor growth of glioma stem cells by selectively suppressing gliomainitiating cells.Molecular Medicine Reports,2015,11(4): 2407-2412.
[2] ZHAO P,XIA G Q,DONG S Y,et al.An iTEP-salinomycin nanoparticle that specifically and effectively inhibits metastases of 4T1 orthotopic breast tumors. Biomaterials,2016,93:1-9.
[3] SCHENK M,AYKUT B,TESKE C,et al.Salinomycin inhibits growth of pancreatic cancer and cancer cell migration by disruption of actin stress fiber integrity.Cancer Letters, 2015,358(2):161-169.
[4] ZHOU J,LI P,XUE X F,et al.Salinomycin induces apoptosis in cisplatin-resistant colorectal cancer cells by accumulation of reactive oxygen species.Toxicology Letters, 2013,222(2):139-145.
[5] WANG F,HE L,DAI W Q,et al.Salinomycin inhibits proliferation and induces apoptosis of human hepatocellularcarcinoma cells in vitro and in vivo.PLoSOne,2012,7(12): e50638.
[6] KUO S Z,BLAIR K J,RAHIMY E,et al.Salinomycin induces cell death and differentiation in head and neck squamous cell carcinoma stem cells despite activation of epithelial-mesenchymal transition and Akt.BMC Cancer, 2012,12:556.
[7] LI T,SU L,ZHONG N,et al.Salinomycin induces cell death with autophagy through activation of endoplasmic reticulum stress in human cancer cells.Autophagy,2013,9 (7):1057-1068.
[8] YUE W,HAMAI A,TONELLI G,et al.Inhibition of the autophagic flux by salinomycin in breast cancer stem-like/ progenitor cells interferes with their maintenance. Autophagy,2013,9(5):714-729.
[9] ROWAN K.High-throughput screening finds potential killer of cancer stem cells.Journal of the National Cancer Institute,2009,101(21):1438-1439.
[10] KINASHI H,ŌTAKE N.An interpretation of mass spectra of salinomycin and its derivatives.Agricultural and Biological Chemistry,1976,40(8):1625-1632.
[11] MIYAZAKI Y,SHIBUYA M,SUGAWARA H,et al. Salinomycin,a new polyether antibiotic.The Journal of Antibiotics,1974,27(11):814-821.
[12] OEHME F W,PICKRELL J A.An analysis of the chronic oral toxicity of polyether ionophore antibiotics in animals. Veterinary and Human Toxicology,1999,41(4):251-257.
[13] HANSEN M,BJÖRKLUND E,KROGH K A,et al. Analytical strategies for assessing ionophores in the environment.Trends in Analytical Chemistry,2009,28(5): 521-533.
[14] BAK S A,HANSEN M,PEDERSEN K M,et al. Quantification of four ionophores in soil,sediment and manure using pressurized liquid extraction.Journal of Chromatography A,2013,1307:27-33.
[15] DORNE J L C M,FERNÁNDEZ-CRUZ M L,BERTELSEN U,et al.Risk assessment of coccidostatics during feed crosscontamination:Animal and human health aspects.Toxicology and Applied Pharmacology,2013,270(3):196-208.
[16] FURTULA V,FARRELL E G,DIARRASSOUBA F,et al.Veterinary pharmaceuticals and antibiotic resistance of Escherichia coli isolates in poultry litter from commercial farms and controlled feeding trials.Poultry Science,2010,89 (1):180-188.
[17] MITANI M,YAMANISHI T,MIYAZAKI Y,et al. Salinomycin effects on mitochondrial ion translocation and respiration.Antimicrobial Agents and Chemotherapy,1976, 9(4):655-660.
[18] LAVINE M D,ARRIZABALAGA G.The antibiotic monensin causes cell cycle disruption of Toxoplasma gondii mediated through the DNA repair enzyme Tg MSH-1. Antimicrobial Agents and Chemotherapy,2011,55(2): 745-755.
[19] CORPET D E.Mechanism of antimicrobial growth promoters used in animal feed.Revue De Médecine Vétérinaire,2000, 151(2):99-104.
[20] PEEBLES E D,BAFUNDO K W,WOMACK S K,et al. Effects of nicarbazin on the blood glucose and liver glycogen statuses of male broilers.Poultry Science,2012,91(9): 2183-2188.
[21] MERCHEN N R,BERGER L L.Effect of salinomycin level on nutrient digestibility and ruminal characteristics of sheep and feedlot performance of cattle.Journal of Animal Science,1985,60(5):1338-1346.
[22] JÓZEFIAK D,KIERON'CZYK B,JUS'KIEWICZ J J,et al. Dietary nisin modulates the gastrointestinal microbial ecology and enhances growth performance of the broiler chickens. PLoS One,2013,8(12):e85347.
[23] TSINAS A C,KYRIAKIS S C,LEKKAS S,et al.Control of proliferative enteropathy in growing/fattening pigs using growth promoters.Zoonoses and Public Health,1998,45 (2):115-127.
[24] GUPTA P B,ONDER T T,JIANG G,et al.Identification of selective inhibitors of cancer stem cells by high-throughput screening.Cell,2009,138(4):645-659.
[25] YOSHIDA G J,SAYA H.Inversed relationship between CD44 variant and c-Myc due to oxidative stress-induced canonical Wnt activation.Biochemical and Biophysical Research Communications,2014,443(2):622-627.
[26] CRISTÓBAL I,ROJO F,MADOZ-GU'RPIDE J,et al.Cross talk between Wnt/β-catenin and CIP2A/Plk1 signaling in prostate cancer:Promising therapeutic implications. Molecular and Cellular Biology,2016,36(12):1734-1739.
[27] JAMDADE V S,SETHI N,MUNDHE N A,et al. Therapeutic targets of triple-negative breast cancer:A review.British Journal of Pharmacology,2015,172(17): 4228-4237.
[28] VILCHEZ V,TURCIOS L,MARTI F,et al.Targeting Wnt/β-catenin pathway in hepatocellular carcinoma treatment.World Journal of Gastroenterology,2016,22 (2):823-832.
[29] MAO J,FAN S,MA W,et al.Roles of Wnt/β-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment.Cell Death&Disease,2014, 5:e1039.
[30] LU W,LI Y.Salinomycin suppresses LRP6 expression and inhibits both Wnt/β-catenin and m TORC1 signaling in breast and prostate cancer cells.Journal of Cellular Biochemistry, 2014,115(10):1799-1807.
[31] WU D X,ZHANG Y,HUANG J,et al.Salinomycin inhibits proliferation and induces apoptosis of human nasopharyngeal carcinoma cell in vitro and suppresses tumor growth in vivo.Biochemical and Biophysical Research Communications,2014,443(2):712-717.
[32] MUSGROVE E A,CALDON C E,BARRACLOUGH J,et al.Cyclin D as a therapeutic target in cancer.Nature Reviews Cancer,2011,11(8):558-572.
[33] MICEL L N,TENTLER J J,SMITH P G,et al.Role of ubiquitin ligases and the proteasome in oncogenesis:Novel targets for anticancer therapies.Journal of Clinical Oncology,2013,31(9):1231-1238.
[34] KOO K H,KIM H,BAE Y K,et al.Salinomycin induces cell death via inactivation of Stat3 and downregulation of Skp2.Cell Death&Disease,2013,4:e693.
[35] AL DHAHERI Y,ATTOUB S,ARAFAT K,et al. Salinomycin induces apoptosis and senescence in breast cancer:Upregulation of p21,downregulation of survivin and histone H3 and H4 hyperacetylation.Biochimica et Biophysica Acta:General Subjects,2013,1830(4): 3121-3135.
[36] KOPP F,HERMAWAN A,OAK P S,et al.Salinomycin treatment reduces metastatic tumor burden by hampering cancer cell migration.Molecular Cancer,2014,13:16.
[37] KOPP F,HERMAWAN A,OAK P S,et al.Sequential salinomycin treatment results in resistance formation through clonal selection of epithelial-like tumor cells.Translational Oncology,2014,7(6):702-711.
[38] SHANG Z Q,CAI Q L,ZHANG M H,et al.A switch from CD44(+)cell to EMT cell drives the metastasis of prostate cancer.Oncotarget,2015,6(2):1202-1216.
[39] ZHOU Y,LIANG C,XUE F,et al.Salinomycin decreases doxorubicin resistance in hepatocellular carcinoma cells by inhibiting the beta-catenin/TCF complex association via FOXO3a activation.Oncotarget,2015,6(12):10350-10365.
[40] REHMAN G,SHEHZAD A,KHAN A L,et al.Role of AMP-activated protein kinase in cancer therapy.Archives of Pharmacal Research,2014,347(7):457-468.
[41] ZHAO G X,PAN H,OUYANG D Y,et al.The critical molecular interconnections in regulating apoptosis and autophagy.Annals of Medicine,2015,47(4):305-315.
[42] LI L L,TAN J,MIAO Y Y,et al.ROS and autophagy: Interactions and molecular regulatory mechanisms.Cellular and Molecular Neurobiology,2015,35(5):615-621.
[43] TAKAMURA A,KOMATSU M,HARA T,et al. Autophagy-deficient mice develop multiple liver tumors. Genes&Development,2011,25(8):795-800.
[44] QU X P,YU J,BHAGAT G,et al.Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene.Journal of Clinical Investigation,2003, 112(12):1809-1820.
[45] TAKAHASHI Y,COPPOLA D,MATSUSHITA N,et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis.Nature Cell Biology,2007,9 (10):1142-1151.
[46] MORTENSEN M,WATSON A S,SIMON A K.Lack of autophagy in the hematopoietic system leads to loss of hematopoietic stem cell function and dysregulated myeloid proliferation.Autophagy,2011,7(9):1069-1070.
[47] HU Y L,DELAY M,JAHANGIRI A,et al.Hypoxiainduced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Research,2012,72(7):1773-1783.
[48] DEGENHARDT K,MATHEW R,BEAUDOIN B,et al. Autophagy promotes tumor cell survival and restricts necrosis,inflammation,and tumorigenesis.Cancer Cell, 2006,10(1):51-64.
[49] CHEN Y S,LI X H,GUO L M,et al.Combining radiation with autophagy inhibition enhances suppression of tumor growth and angiogenesis in esophageal cancer.Molecular Medicine Reports,2015,12(2):1645-1652.
[50] GOSSP E,CHAMBERS A F.Does tumour dormancy offer a therapeutic target?Nature Reviews Cancer,2010,10(12): 871-877.
[51] SOSA M S,BRAGADO P,AGUIRRE-GHISO J A. Mechanisms of disseminated cancer cell dormancy:An awakening field.Nature Reviews Cancer,2014,14(9): 611-622.
[52] QIN L S,JIA P F,ZHANG Z Q,et al.ROS-p53-cyclophilin-D signaling mediates salinomycin-induced glioma cell necrosis.Journal of Experimental&Clinical Cancer Research,2015,34(1):57.
[53] XIPELL E,GONZALEZ-HUARRIZ M,DE IRUJO J J,et al.Salinomycin induced ROS results in abortive autophagy and leads to regulated necrosis in glioblastoma.Oncotarget, 2016,7(21):30626-30641.
[54] BIANCHI M E,MANFREDI A A.High-mobility group boxⅠ(HMGBⅠ)protein at the crossroads between innate and adaptive immunity.Immunological Reviews,2007,220: 35-46.
[55] KANG R,LIVESEY K M,ZEH H J,et al.HMGBⅠ:A novel Beclin 1-binding protein active in autophagy. Autophagy,2010,6(8):1209-1211.
[56] JANGAMREDDY J R,GHAVAMI S,GRABAREK J,et al.Salinomycin induces activation of autophagy,mitophagy and affects mitochondrial polarity:Differences between primary and cancer cells.Biochimica et Biophysica Acta: Molecular Cell Research,2013,1833(9):2057-2069.
[57] KANG R,TANG D,SCHAPIRO N E,et al.The receptor for advanced glycation end products(RAGE)sustains autophagy and limits apoptosis,promoting pancreatic tumor cell survival.Cell Death and Differentiation,2009,17(4): 666-676.
[58] WANG Y,SHI K R,ZHANG L,et al.Significantly enhanced tumor cellular and lysosomalhydroxychloroquine delivery by smart liposomes for optimal autophagy inhibition and improved antitumor efficiency with liposomal doxorubicin.Autophagy,2016,12(6):949-962.
[59] YOU L K,SHOU J W,DENG D C,et al.Crizotinib inducesautophagy through inhibition of the STAT3 pathway in multiple lung cancer cell lines.Oncotarget,2015,6(37): 40268-40282.
[60] YANG P,ZHANG L,SHI Q J,et al.Nicotinamide phosphoribosyltransferase inhibitor APO866 induces C6 glioblastoma cell death via autophagy.Die Pharmazie:An International Journal of Pharmaceutical Sciences,2015,70 (10):650-655.
[61] CAO B Y,LI J,ZHOU X M,et al.Clioquinol induces prodeath autophagy in leukemia and myeloma cells by disrupting the m TOR signaling pathway.Scientific Reports,2014, 4:5749.
[62] ZHU L Q,ZHEN Y F,ZHANG Y,et al.Salinomycin activates AMP-activated protein kinase-dependent autophagy in cultured osteoblastoma cells:A negative regulator against cell apoptosis.PLoSOne,2013,8(12):e84175.
[63] LI T L,SU L,ZHONG N,et al.Salinomycin induces cell death with autophagy through activation of endoplasmic reticulum stress in human cancer cells.Autophagy,2013,9 (7):1057-1068.
[64] VERDOODT B,VOGT M,SCHMITZ I,et al.Salinomycin induces autophagy in colon and breast cancer cells with concomitant generation of reactive oxygen species.PLoS One,2012,7(9):e44132.
[65] KLOSE J,STANKOV M V,KLEINE M,et al.Inhibition of autophagic flux by salinomycin results in anti-cancer effect in hepatocellular carcinoma cells.PLoS One,2014,9 (5):e95970.
[66] YUE W,HAMAI A,TONELLI G,et al.Inhibition of the autophagic flux by salinomycin in breast cancer stem-like/ progenitor cells interferes with their maintenance. Autophagy,2013,9(5):714-729.
[67] JANGAMREDDY J R,PANIGRAHI S,LOS M J. Monitoring of autophagy is complicated-salinomycin as an example.Biochimica et Biophysica Acta:Molecular Cell Research,2015,1853(3):604-610.
[68] CHAFFER C L,BRUECKMANN I,SCHEEL C,et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state.Proceedings of the National Academy of Sciences of the USA,2011,108(19): 7950-7955.
[69] PATEL A,SANT S.Hypoxic tumor microenvironment: Opportunities to develop targeted therapies.Biotechnology Advances,2016,34(5):803-812.
[70] OMIDI Y,BARAR J.Targeting tumor microenvironment: Crossing tumor interstitial fluid by multifunctional nanomedicines.Bioimpacts,2014,4(2):55-67.
[71] LARZABAL L,EL-NIKHELY N,REDRADO M,et al. Differential effects of drugs targeting cancer stem cell(CSC) and non-CSC populations on lung primary tumors and metastasis.PLoS One,2013,8(11):e79798.
[72] ORIMO A,GUPTA P B,SGROI D C,et al.Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion.Cell,2005,121(3):335-348.
[73] JI R C.Macrophages are important mediators of either tumor-or inflammation-induced lymphangiogenesis.Cellular and Molecular Life Sciences,2012,69(6):897-914.
[74] ROHWER N,CRAMER T.Hypoxia-mediated drug resistance:Novel insights on the functional interaction of HIFs and cell death pathways.Drug Resistance Updates, 2011,14(3):191-201.
[75] BELLOT G,GARCIA-MEDINA R,GOUNON P,et al. Hypoxia-induced autophagy is mediated through hypoxiainducible factor induction of BNIP3 and BNIP3L via their BH3 domains.Molecular and Cellular Biology,2009,29 (10):2570-2581.
[76] WU H W,HUANG S Y,ZHANG D S.Autophagic responses to hypoxia and anticancer therapy in head and neck cancer.Pathology:Research and Practice,2015,211(2): 101-108.
[77] WOJTKOWIAK J W,ROTHBERG J M,KUMAR V,et al. Chronic autophagy is a cellular adaptation to tumor acidic p H microenvironments.Cancer Research,2012,72(16): 3938-3947.
[78] HU Y L,JAHANGIRI A,DELAY M,et al.Tumor cell autophagy as an adaptive response mediating resistance to treatments such as antiangiogenic therapy.Cancer Research, 2012,72(17):4294-4299.
[79] PELLEGRINI P,STRAMBI A,ZIPOLI C,et al.Acidic extracellular p H neutralizes the autophagy-inhibiting activity of chloroquine:Implications for cancer therapies. Autophagy,2014,10(4):562-571.
[80] PELLEGRINI P,DYCZYNSKI M,SBRANA F V,et al. Tumor acidosis enhances cytotoxic effects and autophagy inhibition by salinomycin on cancer cell lines and cancer stem cells.Oncotarget,2016,7(24):35703-35723.
[81] KIM S H,CHOI Y J,KIM K Y,et al.Salinomycin simultaneously induces apoptosis and autophagy through generation of reactive oxygen species in osteosarcoma U2OS cells.Biochemical and Biophysical Research Communications, 2016,473(2):607-613.
CHEN Siyuan,HU Ji'an*(Department of Pathology,Affiliated Hospital of Stomatology,School of Medicine,Zhejiang University,Hangzhou 310006,China)
Summary Autophagy is a cell self-eating process that allows the orderly degradation and recycling of misfolded proteins and damaged organelles through lysosome.It plays a vital role in cells'adaptation to metabolic stress and homeostasis as well as the formation,development and treatment of tumors.Autophagy is a double-edged sword, both suppressing and facilitating tumorigenesis.For example,multiple benign tumors formed in the liver when autophagy gene Atg5 was mosaically deleted in mice.But at the same time,cancer cells can upregulate autophagy to survive under microenvironmental stress,promote tumor dormancy and increase their growth and aggressiveness.
Salinomycin is a polyether ionophore antibiotic isolated from Streptomyces albus.Compared with lasalocid and monensin,salinomycin is less toxic,lower priced and less susceptible to drug resistance,which enables worldwide use to prevent chicken coccidiosis and promote the growth of ruminants.As an antimicrobial drug,salinomycin acts as an ionophore for K+and Na+ions and is able to alter cytoplasmic and mitochondrial membrane potentials, thereby inhibiting oxidative phosphorylation.Besides,salinomycin can activate a MutS homolog 1-dependent cell cycle checkpoint,leading to the ultimate death of the coccidial parasites.
In 2009,salinomycin was reported to exert a powerful effect on breast cancer stem cells(CSCs),and itreduced the proportion of CSCs by more than 100 folds relative to paclitaxel.Up to now,it has been shown that salinomycin could effectively kill a variety of CSCs and multi-drug resistant cell lines by inhibiting Wnt/β-catenin signaling pathways,curbing cell cycle progression and reversing epithelial-mesenchymal transformation. Anticarcinogen can directly trigger apoptosis,autophagy and necrosis simultaneously.Most of cancer cell lines utilize autophagy to protect themselves from death after treatment with salinomycin.However,inhibiting autophagy through siRNA or PI3K inhibitor partially prevents cell death upon the addition of salinomycin in SW620 cells,indicating that autophagy induced by salinomycin can provoke cell death.The process of autophagy induced by salinomycin is complicated.Salinomycin can activate autophagy through different ways:1)increasing intracellular reactive oxygen species(ROS),partly via ROS-JNK/AMPK signaling pathways;2)triggering mitophagy by damaging mitochondria;3)inducing endoplasmic reticulum stress to activate autophagy via AKT1-m TOR signaling pathway.
In addition,salinomycin does not interfere with the autophagosome-lysosome fusion except suppressing autophagic flux at the late stage by diminishing the lysosome activity.Poor microenvironmental conditions,such as hypoxia,elevated interstitial fluid pressure,glycolysis,low p H and inflammatory cell infiltration,are common features of solid tumors,which are closely related to tumor progression,metastasis and therapy.Salinomycin significantly reduces F4/80+and CD11B+inflammatory cells of the tumor microenvironment in mice with pulmonary metastasis.Hypoxia,the focus of most studies,is involved in tumor angiogenesis and the generation and maintenance of CSCs.Low p H has a great influence on drug metabolism,rendering multi-drug resistant of the cancer cells.Autophagy is always upregulated in hypoxic and low p H regions of tumors for the metabolic adaptation of cancer cells.The efficiency of hydroxychloroquine,a late-stage autophagy inhibitor by disrupting lysosome acidification under clinical investigation,is strongly impaired in acidic tumor environments due to drug resistance.However,according to a new study,salinomycin could effectively inhibit autophagy flux under conditions of transient and chronic acidosis.This contributed to the elimination of several cancer cell lines and enhanced cytotoxic effects of salinomycin on CSCs.In short,the inhibition of autophagy flux by salinomycin leads to a considerable change in the tumor microenvironment,which makes salinomycin more potent in terms of the antitumor ability and provides new insights into drug combination.
In sum,this paper reviews the antitumor mechanism and application of salinomycin in livestock,the relationship between autophagy and tumor,and the mechanism of autophagy induced by salinomycin.
Key wordssalinomycin;tumor;autophagy;apoptosis;coccidiosis
Progress and application of salinomycin-induced cell autophagy in anti-cancer treatment.Journal of Zhejiang University(Agric.&Life Sci.),2016,42(6):694- 702
DOI:10.3785/j.issn.1008-9209.2016.07.123
中图分类号R 979.14
文献标志码A
基金项目:国家自然科学基金(81172560,81400511);浙江省自然科学基金(Y14H140014).
*通信作者(Corresponding author):胡济安(http://orcid.org/0000-0002-1150-0399),Tel:+86- 571- 87217221,E-mail:hja@zju.edu.cn
收稿日期(Received):2016 07 12;接受日期(Accepted):2016 10 19;网络出版日期(Published online):2016 11 19
第一作者联系方式:陈思远(http://orcid.org/0000-0003-3284-4489),E-mail:chensiyuan081023@163.com
URL:http://www.zjujournals.com/agr/CN/article/download ArticleFile.do?attach Type=PDF&id=10430
