A preparation technology to obtain 5β-hydroxyacovenosigenin from roots ofStreptocaulon juventasusing ethanol refluxing and hydrochloric acid hydrolysis method
2016-02-24ChengchengCaiHuaWangShuaiYuanNaHanZhihuiLiuJunYin
Chengcheng Cai, Hua Wang, Shuai Yuan, Na Han, Zhihui Liu, Jun Yin*
School of Traditional Chinese medicine, Shenyang Pharmaceutical University, Shenyang 110016, China
A preparation technology to obtain 5β-hydroxyacovenosigenin from roots ofStreptocaulon juventasusing ethanol refluxing and hydrochloric acid hydrolysis method
Chengcheng Cai, Hua Wang, Shuai Yuan, Na Han, Zhihui Liu, Jun Yin*
School of Traditional Chinese medicine, Shenyang Pharmaceutical University, Shenyang 110016, China
1β-hydroxyacovenosigenin (HAG) is the main sterides structure of cardiac glycosides in the family of Asclepiadaceae and Scrophulariaceae. The objective of this study is to perform a practical method to prepare HAG from roots ofStreptocaulon juventas(S. juventas) to arrange raw material to synthesize an active compound of TXA9. However, it's quite hard to get HAG from roots ofS. juventaseasily.To obtain the HAG efficiently, two orthogonal experiments were designed to optimize the parameters of extraction and hydrolysis condition ofS. juventas. A four-factor, three-level orthogonal extracting test was projected to confirm the extraction condition ofS. juventasto obtain the total cardiac glycosides (CG). The optimal parameters were determined as extraction time of 3 h, extraction number of 2 times and ethanol (EtOH, 75%) to raw material of 10-fold, respectively. The content of CG was tested with the colorimetric method. To get more HAG, the other three-factor, three-level orthogonal hydrolysis of CG experiment was studied. With the HPLC to test the peak area of HAG, the CG was hydrolyzed with hydrochloric acid (HCl, 0.1 mol/L) at 70 °C for 1.5 h and extracted with dichloromethane for three times after each reaction. The hydrolysis reaction numbers were intended to be four times. With the optimised extracting and hydrolysis method, a solution rich of HAG and other cardiac aglycones (CA) was obtained. The solution was concentrated and purified with silica gel and ODS. 4.45 g of purified HAG was acquired from 160 kg of roots ofS. juventasafter recrystallising with dichloromethane/ethyl acetate for several times. The technology has been validated; it shows good results in terms of easy operation, repeatability and high scientificity. It could be used to prepare large amount of HAG in the further research.
HAG; TXA9; extract; hydrolysis; isolation; orthogonal experiment
1 Introduction
HAG is a C21steroid compound which is the main mother nucleus of cardiac glycosides in the family of Asclepiadaceae [1-3] and Scrophulariaceae [4].TXA9, which is a product separated from the antitumor-active fraction of the roots ofS. juventas, has been certified good anti-proliferative activity in the former research [1, 2]. The structure of TXA9 is a stem nucleus of HAG and a glucose unit. TXA9 has better anticancer activity and less side effects than the same dosage of paclitaxel [2]. However, the content of TXA9 is so limited that cost too much to obtain it from the plant directly. It is a challenge to synthesize TXA9 completely in the present study. Whereas, the semi-synthesis is a good choice to solve thisproblem. We have synthesized TXA9 successfully with HAG and glucosyl donors in the preliminary study [2]. HAG is the most important raw material to synthesize TXA9. But it's not easy to get HAG from commercially available manner or other convenient ways. So, it's necessary to find a simple and convenient method to prepare sufficient HAG. It had been found HAG was most parent nucleus of cardiac glycosides in roots ofS. juventas[1, 3]. It was the reason that roots ofS. juventaswas chosen as the crude drug to extract HAG. Although it was reported that HAG and digoxigenin could be generated via biotransformation with digitoxin [5], the way to biotransform 75% of alcohol extract of roots ofS. juventaswas difficult and the product was too complex. Much of cardiac glycosides of roots ofS. juventascontain deoxy sugar that could be hydrolyzed by light concentration of mineral acid [6]. In this paper, two orthogonal experiments were designed to optimize the parameters of extraction and hydrolysis condition ofS. juventas. With the optimised extracting and hydrolysis method, a solution rich of CA was obtained. The solution was concentrated and purified with silica gel and ODS. Purified HAG could be obtained after recrystallization with dichloromethane /ethyl acetate.

Fig. 1 The structure of DIG
2 Materials and Methods
2.2 Instrumentation
2.1 Reagents
HAG (99%) was prepared in the laboratory. EtOH (95%) was purchased from Shenyang Laibo 'trade co., LTD. HCl, ammonium hydroxide (HN3. H2O), methanol (MeOH), ethyl acetate, petroleum ether and acetone were all from Damao chemical reagent factory in Tianjin. Methanol (HPLC grade) was also purchased here. Dichloromethane and sodium hydroxide (NaOH) were both purchased from Tianjin Bodi chemical co., LTD. 3,5-Dinitrobenzoic acid and 2,4,6-trinitrophenol were purchased from Tianjin kaixin chemical industry co., LTD. All solutions were prepared with deionized water and all chemicals were of analytical grade, unless stated otherwise.
Chromatographic analysis was carried out with a Liquid Chromatograph from Shimadzu (Kyoto, Japan), containing a Prominence line operating system with a quaternary pump solvent type LC-10AT, manual injector SIL-10AT, UV-Visible detector with a photodiode array SPD-M20A, column oven CTO-10VAP. The software of LC Solution N2000 (Zhejiang University Zhida IE co., LTD) was used for data acquisition and processing. A spectrometer UV/VIS Lambda 25 (Perkim Elmer) was used for the acquisition of synthetic colorants absorption spectrum. Rotary evaporator (Shanghai Yarong biochemical apparatus factory) and circulating water pump (Zhengzhou Changcheng Division industry and trade co., LTD) were used to concentrate solution and recovery solvents.
2.3 Plant material
Roots ofS. juventas(Lour.) Merr were purchased from Yunnan province in China, and was identified by professor Jun Yin, Shenyang Pharmaceutical University.
2.4 Preparation of color developing solvents
Baljet reagent was used as a color developing solvents which was 2,4,6-trinitrophenol aqueous solution (1%) and sodium hydroxide aqueous solution (5%) mixed at a volume ratio 1:1. It should be mixed before the experiment.
2.5 Preparation of reference solution
HAG (4.8 mg) was dissolved in 10 mL of measuring flask with EtOH (75%, v/v) to be a 0.48 mg/mL of reference solution that was stored at 4 °C. Took out 0.00, 0.20, 0.40, 0.60, 0.80, 1.00, 1.20 mL of reference solution into 10 mL measuring flasks and added 6 mL of Baljet reagent to each flask to make the constant volume about 10 mL, respectively. The mixed solution was placed in the dark for 30 minutes, detected with a UV-visible spectrophotometer to obtain the absorbance at 477 nm.
2.6 Extracting technology of roots of S. juventas
2.6.1 Preparation of sample solution
The crude powder sample (20.0 g, passed through a 150 μm mesh sieve) of roots ofS. juventaswas suspended with appropriate amount of EtOH (Table 1) for 30 minutes and heated reflux extraction with Soxhlet's apparatus for advisable time (Table 1). The extraction solution was cooled at room temperature and added losing EtOH to make it weighed equal to heated before. After filtering the extraction solution and merging all the filtrate, 1 mL of filtrate and 6 mL of Baljet reagent were mixed in a 10 mL measuring flask. Added 3 mL of EtOH (75%) to the flask and reacted for 30 minutes in the dark at room temperature. The solution of sample was detected with the UV-visible spectrophotometer at 477 nm.

Table 1 Factors and levels of extraction technology
2.6.2 Validation the optimal extraction technology and soaking experiment
Took out three samples and heated reflux extraction as the optimized technology above to test the yield of total cardiac glycosides. Respectively, roots ofS. juventassoaked and unsoaked were investigated in the extracting experiment. Prepared two samples of roots ofS. juventasand immersed one of the samples for 12 h before extracting, the other sample was extracted as showed above immediately. Both extracting solutions were tested absorbance at 477 nm.
2.7 Technology of hydrolysis
2.7.1 Chromatographic condition
The analysis was performed on a Shimadzu 10-ATVP HPLC system. Separation was carried out on a Diamonsil C18column (250 mm × 4.6 mm, 5 μm). The mobile phase was composed ofmethanol-water (60:40) and conducted by using an isocratic elution program. The flow rate was 1.0 mL/min and the injection volume was 20 μL. The detection wavelength was 217 nm. The column temperature was maintained at 25 °C.
2.7.2 Preparation of HAG standard solution
Standard compound of HAG was accurately weighed, then dissolved and diluted in methanol to the concentrations of 0.04 mg/mL, as mixed stock standard solution. The solution was stored at 4 °C. Measured different amount of standard working solutions (2.2, 3.0, 4.0, 5.0, 6.0 mL) into the 10 mL of measuring flasks, added appropriate MeOH to make the solution volume of 10 mL. 20 μL of solution was injected into the HPLC system.

Table 2 Factors and levels of hydrolyzation technology
2.7.3 Preparation of sample solution
50 mL of extraction solution (ρ=1.06 g/mL) of roots ofS. juventaswas put into 100 mL round flask and hydrolyzed as the designed experimental scheme (Table 2). Added moderate of NH3.H2O into reaction solution and stirred for 5 minutes to terminate the hydrolysis reaction. The dichloromethane was added to the reaction solution two times. HAG and other cardiac aglycones were extracted in the dichloromethane layers. All the solutions of dichloromethane layer were combined and moved the solvent with a rotary evaporimeter under reduced pressure. Dissolved the residue with 10 mL of MeOH and filtered the mixture with filter membrane (0.45 µm). 20 μL of filter was tested with HPLC at wave lengthen about 217 nm.
2.7.4 Investigation of hydrolysis times and extracting times
Took 500 mL of extraction solution (ρ= 1.06 g/mL) of roots ofS. juventasinto 1000 mL round flask and hydrolyzed with HCl (0.1 mol/ L) at 70 °C for 1.5 h. After the reaction, NH3.H2O was added into the liquid to stop the reaction. Then HAG and other cardiac aglycones were extracted with isometric dichloromethane for 5 times from the reaction liquid. Collected dichloromethane layers and evaporated to dryness, respectively. The aqueous layer was hydrolyzed another four times as method above. Severally, 25 samples of dry dichloromethane tier were dissolved with 10 mL of MeOH and filtered with filter membrane (0.45 µm). 20 μL of filter was tested with HPLC to record the peak area of HAG at 217 nm.
2.8 Isolation of HAG
A solution rich of CA was obtained after hydrolysis of extracting liquid and extracting with dichloromethane for three times. All the solution of dichloromethane layers were combined and concentrated to get arid extract. Extract (30 g) was subjected to a silica-gel column (300 g, 200-300 mesh, 6 × 33 cm) chromatography, followed by petrolenm-ethyl acetate (3: 1, 1-10V), petrolenmethyl acetate (3: 2, 11-25V) to obtain two subfractions. TLC displayed that HAG was resided in the subfraction (SF1, 17-21V, 2.20 g). Then SF1 was separated on a silica gel column (22 g, 200-300 mesh, 2 × 32 cm), eluted by gradient petroleumacotone 5: 1 (1-10V), 5: 2 (11-20V) to get SF2 (12-15V, 0.32 g) which was found rich of HAG.SF2 was further purified with an ODS column chromatography, eluted with MeOH-H2O 40:60 (1-5V), 50:50 (6-10V), 60:40 (11-15V) and 100:0 (16-20V). After recrystal of the subfraction (SF3, 12-13V) with dichloromethane/ethyl acetate, pure HAG was obtained.
2.9 Lab scale craft and magnification craft
The lab scale craft was validated with three powders of roots ofS. juventas(20 kg/sample) which were soaked with EtOH (200 L, 75%) for 12 h before extracting. Then the samples were extracted by reflux twice for 3 h, respectively. Concentrated the extracting solution to be 5 L and hydrolyzed with HCl (0.1 mol/L) at 70 °C for 1.5 h. Then added appropriate amount of NH3.H2O to stop the reaction. The same volume of dichloromethane was added to the solution to extract HAG for three times. The water layer was hydrolyzed another three times as above. All the solutions of dichloromethane layer were combined and concentrated to gain arid extract. HAG was isolated from the extract after acid hydrolysis as explained in 2.8. 100 kg of roots power ofS. juventaswas extracted to investigate the magnification craft, the process was showed as Fig. 2.

Fig. 2 Flow chart of preparation technology of HAG
3 Results and discussion
3.1 The total CG with the colorimetric method
Baljet solution and cardiac glycosides may react to produce new substance which has maximum wavelength in the area of UV-Visible light. The solution of the roots ofS. juventaswas of the same maximum wavelength as HAG standard. It could measure total cardiac glycosides ofS. juventasat 477 nm.
For assessment of linearity, the calibration range was extended down to 80% of the lowest and up to 120% of the highest calibrator. The coefficient of regression R was desired to be higher than 0.999 for sufficient linearity. The result of curve line was showed as Fig. 3. The method was linear in the range of 7.2-31.2 µg /mL of cardiac glycoside (R2= 0.9992).

Fig. 3 Standard curve of HAG
3.2 Optimization of the extraction parameters of S. juventas
It was considered four factors in the heating reflux extraction process to select the optimal process. The range of extraction times (C) was the largest that was the main factors influencing the content, followed by solvent inventory (B), extracting time (D) and ratio of EtOH (A). Thus, the best combination scheme was A2B2C3D3 (10-fold amount of EtOH (75%), extracting 3 times, 3 h each time). However, it was no difference between extracting 2 times and 3 times in the mean value of CG, the best extracting technology was adjusted to A2B2C2D3 when considered the comprehensive cost of technology. The optimized extract technology ofS. juventaswas ten times the amount of EtOH (75%), extracting 3 h × 2 times.
Three samples were prepared to validate the optimization extracting technology as 2.6.1. The contents of CG in roots ofS. juventaswere 2.633, 2.612 and 2.560 mg/g (Table 4). It was proved that the technology was scientific and reasonable. Usually, the yield of extracting root crude drug was affected by dipping and other extraction methods [6]. The result of roots ofS. juventassoaked and unsoaked was showed in Table 5. The content of total cardiac in roots ofS. juventassoaked (2.813 mg/g) before the extracting was higher than the technology that was not to soak (2.582 mg/g). Therefore, the roots ofS. juventaswere soaked for 12 h before extracting as the optimization method above.

Table 3 Orthogonal tests result of extraction technology

Table 4 Extraction process validation test results

Table 5 Investigation of dousing the roots ofS. juventas
3.3 The content of HAG tested with HPLC
More sensitive HPLC method was adopted for determination the content of HAG in the experiment. HAG standard solution was proceeded full wavelength scanning with a 720N-UV-visible spectrophotometer. The maximum absorption wavelength of HAG was at 216.6 nm (Fig. 4) so that 217 nm was selected as the detection wavelength of HAG.

Fig. 4 UV wavelength scanning gram of HAG standard
Took different concentrations of standard solution with HPLC to detect and record peak area, the concentration of the reference substance as the abscissa, peak area of integral value of the vertical to draw standard curve as shown in Fig. 5. The linearity range was 8-24 μg/mL. Calculated coefficient of regression R was equal to 0.999 for investigated DIG, demonstrating good linearity in the investigated calibration ranges.

Fig. 5 HPLC standard curve of HAG
3.4 Optimization of hydrolysis parameters of CG
CG was similar with other nucleoside that was easily hydrolyzed by acid and enzyme. Molecules with ester bond can also be alkaline hydrolysis [6]. However, the surge of cardenolide was different structure and place so that the hydrolysis product was largely different. Usually, the enzyme hydrolysis was selective to obtain specific product. However, it cost much higher than acid hydrolysis method in the industrial production. In this paper, acid hydrolysis method was chosen to get the CA. To obtain HAG more efficiently, the three-factor, threelevel orthogonal hydrolyzing test was designed to optimize the hydrolysis condition based on the results of single-factor experiments. The content of HAG was affected by different concertration of HCl (0.05, 0.10 and 0.20 mol/L), when the other two factors were fixed at hydrolysis time (0.5, 1.5 and 2.5 h) and temperature (60, 70 and 80 °C). As seen in Table 6, the peak area of HAG increased with the hydrolysis temperature, reaching a peak at 70 °C with the maximum peak area as 104357.4. The effect of the hydrolysis time and concentration of HCl on the content of HAG was less than temperature (R1>R2>R3). Considering the value of K, A2B2C2 (70 °C, 1.5 h, 0.1 mol /L of HCl) was found to be the best condition.

Table 6 Orthogonal tests result of hydrolyzation technology

Continued Table 6
With the optimal hydrolysis method, 25 samples of dry dichloromethane layer were tested with HPLC. HPLC chromatogram of HAG was shown in Fig.6 A. The peak area of HAG in the first hydrolyzation and the first extraction (Fig. 6 B) was higher than the sample of the first hydrolyzation and the forth extraction (Fig. 6 C). The results showed the content of HAG was the lowest after extracting reaction solution four times. It was three times as the best extracting strategy to minimum the cost and improve the efficiency of experiment. The peak of HAG was almost invisible in Fig. 6 D. That means HAG was inexistent after hydrolysis four times of solution ofS. juventas. Therefore, the optimization of hydrolysis technology ofS. juventaswas hydrolyzed (0.1 mol/L HCl, 70 °C and 1.5 h) three times and extracted four times each hydrolysis solution.


Fig. 6 HPLC chromatograms of periplocenin and the samples after hydrolyzation. (A) HPLC chromatogram of HAG; (B) HPLC chromatogram of the sample of the first hydrolyzation and the first extraction; (C) HPLC chromatogram of the sample of the first hydrolyzation and the forth extraction; (D) HPLC chromatogram of the sample of the fifth hydrolyzation and the first extraction.
3.5 Isolation of HAG
With the optimal technology of extracting and hydrolysis, an extract that rich of HAG and other cardiac aglycones was obtained. The chemical polarity of cardiac aglycone was so small that was easy to separate caidiac aglycone with silica-gel by petrolenm-ethyl acetate and petroleum-acetone [6]. Traced with TLC, fraction that rich of HAG was found and further purified with an ODS column chromatography. The crude product of HAG was recrystallized with dichloromethane/ethyl acetate for several times, crystallization of HAG could be acquired.
3.6 Lab scale craft and magnification craft
In the preliminary study, the technology was mainly lab scale research. Two orthogonal experimenst were designed to investigate the optimal technology of extraction and hydrolysis. The best craft was validated with three samples and the result was showed as Table 7. The content of HAG of three samples was no significant difference that the preparation technology of HAG was demonstrated reasonable and scientific. In this paper, a magnification craft was also validated as Fig. 2. Ultimately, 2.918 g of HAG was acquired from 100 kg of roots ofS. juventas, and the yield was 0.02918‰. It was also proved the preparation technology of HAG was feasibility and stability.
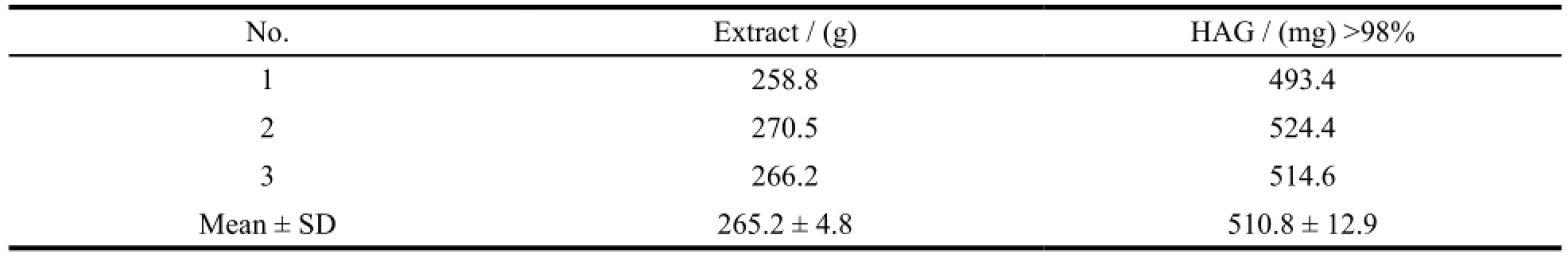
Table 7 The confirmatory experiment of the preparation technology for HAG
4 Conclusion
It was difficult to obtained HAG in commercial manner or other easy ways to prepare raw material to synthesize the active compound of TXA9. We have studied the preparation technology of HAG from roots ofS. juventas.In the present study, a quick technology to prepare HAG was successfully finished in the laboratory research. More than 4.45 g of HAG was gained from roots ofS. juventas(160 kg) with extracting, hydrolysis and isolation process. The method was fully validated; it showed good results in terms of easy operation, repeatability and scientificity. This technology would be used to prepare large amount of HAG in the next study.
Reference
[1] Han N, Yang JY, Li LW, et al. Inhibitory activity of a phytochemically characterized fraction fromStreptocaulon juventason lung cancer in nude mice. Planta Med, 2010,76: 561-565.
[2] Xue R, Han N, Xia MY, et al. TXA9, a cardiac glycoside fromStreptocaulon juventas, exerts a potent anti-tumor activity against human non-small cell lung cancer cells in vitro and in vivo. Steroids, 2015, 94: 51-59.
[3] Ye C, Wang H, Xue R, et al. Minor cytotoxic cardenolide glycosides from the root ofStreptocaulon juventas. Steroids. 2015, 93: 39-46.
[4] Cui XB. The application of digitoxin. World Health Digest Medical Periodieal. 2010, 7(5): 34-35.
[5] Luo JM, Song T, Zhu FZ, et al. Optimization on biotransformation technology of digitoxin by aspergillus ochraceus and the products activity analysis. Natural Product Research and Develop. 2015, 27: 398-403.
[6] Wu LJ. Natural Pharmaceutical Chemistry. 2003, 4: 323-324.
* Author to whom correspondence should be addressed. Address: School of Traditional Chinese Materia Medica 48#, Shenyang Pharmaceutical University, 103 Wenhua Road, Shenhe District, Shenyang 110016, China; Tel./fax: +86-24-23986491; E-mail: yinjun826@sina.com
Received: 2016-05-18 Accepted: 2016-07-19
