Advanced Treatment Planning in Cancer Thermal Therapies
2016-02-07TheodorosSAMARASEsraNEUFELDNielsKUSTER
Theodoros SAMARAS,Esra NEUFELD,Niels KUSTER
1.Aristotle University of Thessaloniki,Greece;2.IT'IS Foundation,Zeughausstrasse 43,8004 Zurich,Switzerland;3.ZMT,Zurich MedTech AG,Switzerland;4.Swiss Federal Institute of Technology (ETH),Rämistrasse 101,8092 Zurich,Switzerland
Advanced Treatment Planning in Cancer Thermal Therapies
Theodoros SAMARAS1,Esra NEUFELD2,3,Niels KUSTER2,4
1.Aristotle University of Thessaloniki,Greece;2.IT'IS Foundation,Zeughausstrasse 43,8004 Zurich,Switzerland;3.ZMT,Zurich MedTech AG,Switzerland;4.Swiss Federal Institute of Technology (ETH),Rämistrasse 101,8092 Zurich,Switzerland
CEM43 thermal dose is a very common concept in thermal oncology.Thermal dose is the maximum amount of energy that can be transmitted during hyperthermia therapy conducted on temperature-sensitive tissue.Thermal dose is also the maximum value of local energy accumulation in human bodies,which can lead to tissue injury and pain.Thermal dose can also decrease the fnishing temperature and reduce the energy to the tolerable range.There are two functions of the individualized hyperthermia treatment plan: it determines the setting and location that can realize the best tumor hyperthermia therapy;at the same time,it can decrease the effect of hyperthermia therapy on healthy tissues.There are four steps in the treatment plan of hyperthermia therapy for tumors: the frst step is to establish a three dimensional human body model and its corresponding an atomical structure that can be used in numerical algorithmviamedical imaging resources;the second step is to determine the volume of the electromagnetic energy accumulation.Based on the peculiarity of frequency and materials,even full-wave electromagnetic wave or quasi-static technique can be used to determine the tissue distribution.Evaluation of the therapy can be conducted based on thermal dose and the corresponding tissue damage model;the third step is to use Arrhenius model to provide direct evaluation of tissues in the thermal ablation zone,solidifcation zone,as well as the necrotic area;the last step is the optimization of the treatment plan.
cancer;thermal therapy/hyperthermia therapy;treatment plan;radiofrequency
0 INTRODUCTION
Thermal therapies in cancer treatment are used either alone or in conjunction with radiotherapy and chemotherapy,in order to enhance their effcacy.In the former case,the change in tissue temperature is large enough to directly kill the cells (target temperatures of <-40℃ for cryoablationand >50℃for thermal ablation).In the latter case,temperature rise is mild (between 3 ℃ and 8 ℃) and this treatment modality is known as hyperthermia.Hyperthermia enhances the cytotoxic effects of chemo- and radiotherapy through several biological mechanisms[1-2].Depending on the heated body area,hyperthermia can be local,regional or whole-body.However,here we only discuss locoregional hyperthermia applied to deep or superfcial tissues.
There are many physical mechanisms that allow deposition of energy at the site of the tumor,leading to local temperature increase.Such mechanisms include warm or hot water flow in tubes,infrared (IR) radiation,the implantation of magnetic microspheres of nanoparticles,the conduction of radiofrequency (RF) currents and the absorption of light,microwaves (MW) or ultrasound radiation.The emphasis in this article will be on electrothermal applications.
1 THE NEED FOR TREATMENT PLANNING
1.1 Deep and superfcial hyperthermia
It has been shown in many studies that treatment outcome is correlated positively to treatment quality characterized by the achieved temperature rise for the desired time within the entire target volume[3-8].Moreover,it is known that in clinical practice "hot-spots" in sensitive tissues are the limiting factor for the maximum power that can be delivered in a treatment session.These "hot-spots" are local maxima in the deposited energy insidethe patient’s body,which result in undesirable tissue damage or pain and compromise either the final temperature that can be reached or the duration that a specifc power level can be tolerated.Therefore,individualized treatment planning in hyperthermia has a dual role: it determines the applicator settings and positioning to accomplish optimal heating of the tumor,while minimizing effects on healthy tissue,following the "tissue sparing" paradigm in radiotherapy[9].At the same time,it can provide to the clinician or technician alternative treatmentplans during the therapeutic session,readily available to be delivered by the applicators,so that patient discomfort does not put treatment objectives at risk.The benefits of personalized treatment planning include alsobetter coverage of the tumor either with very simple methods[10]or by more complicated power steering techniques available for phased arrays[11-12].
1.2 RF and MW ablation
The aims of treatment planning in thermal ablation are different from hyperthermia.The volume affected by the electromagnetic field in this case is limited.However,side-effects from healthy tissue heating remain a problem,perhaps more important than for hyperthermia,due to the high temperatures reached during ablation.Blood vessel destruction,resulting in internal bleeding,and organ perforation are the most serious complications for this treatment[13-14],which limit the final temperature rise.In thermal ablation,blood vessel proximity to the treated tumor does not only carry the risk of bleeding or blood clotting;it also reduces the efficacy of treatment with the "heat-sink" effect,especially in RF ablation,since in MW ablation blood vessels act more as heat conduits to neighboring healthy tissue.The "heat-sink" effect describes the ability of fowing blood to remove from the treatment area large quantities of the deposited heat,thus inhibiting the development of therapeutic temperatures throughout the tumor volume and resulting in its non-uniform heating.Therefore,it is important to determine temperature rise distribution in the target area prior to treatment and optimize the positioning and excitation settings of the interstitial applicator.
2 TREATMENT PLANNING WORKFLOW
2.1 Outline
Treatment planning in cancer thermal therapies is a fourstep process (Figure 1).The first step is the creation from medical imaging data of the personalized computational 3D model with the realistic anatomy that will be used in the numerical solvers.The calculation of the temperature in the model requires another two steps.Initially,the electromagnetic problem is solved to calculate the distribution of energy dissipated in the tissues.In the next step the energy deposited is considered as a heat source (thermal load) and the temperature rise is evaluated using,e.g.,a bioheat transfer equation,so that thermal effects in the tissue can be assessed using the appropriate physiological model.Finally,if the applicator employed allows for energy steering inside the body,the treatment plan can be optimized,by choosing the distribution of either deposited electromagnetic energy or temperature,which is expected to maximize the therapeutic outcome.
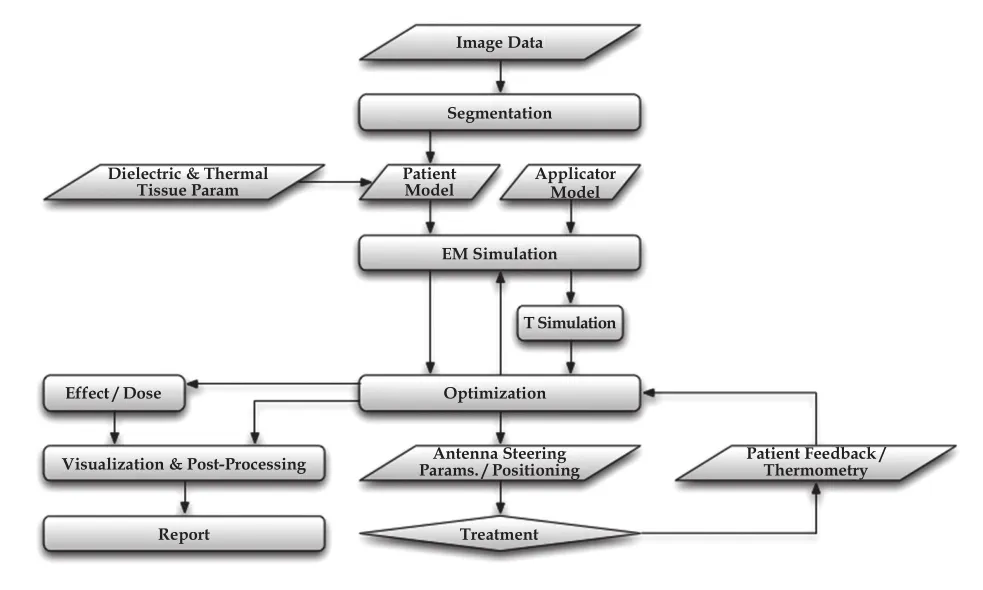
Figure 1 The process of treatment planning in thermal therapy of cancer.
Treatment planning in thermal therapies for cancer is much more demanding and complex compared with radiotherapy treatment planning.It entails state-of-the-art software implementing the latest numerical techniques,and may even require high performance computing platforms compared to RT.Due to the strongly inhomogeneous nature of the human body and the impact of physiological factors,such as blood perfusion and thermoregulation,high-quality treatment planning is a demanding task,nevertheless,attainable with present computational means.
One positive side effect of the effort to introduce treatment planning in the clinics has been the generation of tools that can assist in the development of novel applicators[15-16]and the production of guidelines[17]to promote treatment quality.
2.2 Model segmentation
Model segmentation is the process of identifying three dimensional regions of tissues from medical imaging data (e.g.,MRI,CT).By assigning physical properties to these regions,it is possible to generate computable models that can be used by physics solvers to calculate electromagnetic absorption and temperature distributions.The model detailed nessnecessary for thermal therapy treatment planning depends on the application,although it is normally higher than for radiotherapy.Deep hyperthermia treatment planning is performed for larger body volumes than superficial hyperthermia and thermal ablation.It also requires a better description of organs and their surfaces,since material interfaces have an impact in the resulting electromagnetic field distributions.Consequently,it is required that more tissues are distinguished in the model,than for radiotherapy.On the other hand,it should always be checked beforehand how big a model should be in the case ofsuperfcial hyperthermia or thermal ablation,so that evaluated physical quantities are not contaminated by boundary effects.In particular,for thermal ablation,it may be necessary to employ specialized algorithms for obtaining vasculature in the proximity of the treatment area,as it may affect treatment effcacy.
Segmentation is a time-consuming task for hyperthermia compared to radiotherapy,as explained above.Therefore,the software used must facilitate the rapid generation of anatomical models by offering a wide range of both automatic and interactive segmentation algorithms.Manual interaction is necessary in situations of low-quality images,as well as disease,which differentiates the picture in the image from anatomical atlases.However,the variation in hyperthermia treatment planning quality might be small between the two approaches[18].Moreover,it is necessary that segmented images can beconverted into highquality surface-based body models[19]without self-intersections and holes,suitable for mesh creation and simulation.
Tissue properties are assigned to organs in a homogeneous fashion,although in reality they can vary spatially.There is certainly a big variation of dielectric properties among the population (depending on age,sex,or pathology) and their values have been obtained usuallyex vivo,but,fortunately,the infuence of this variation on the calculation of the electromagnetically deposited power is less than 10% for high frequencies (hyperthermia and microwave ablation).In RF ablation the contrast electrical conductivity contrast between tumor and healthy tissue may be more critical[10-22].Currently,there are online databases[23]that include electrical,as well as mechanical and physiological tissues properties,although in some case the literature is scarce and the values are based on studies with small samples.
When the generation of individual patient models is not necessary,e.g.,for mechanism investigations,applicator development,or when it is considered suffcient to personalize tumor location and shape but not anatomy,highly-detailed computational body models[24]are available and can be used instead.
2.3 Power absorption calculation
In the next step of treatment planning electromagnetic energy deposition is determined.Depending on the frequency and the material properties,either full-wave electromagneticor quasistatic[25-27]techniques can be used to determine the field distribution in the tissues.In hyperthermic oncology and MW ablation it is most often the former,while RF ablation modeling typically benefits from the latter.Nonlinear solvers may be necessary for thermal ablation,where temperature rise is large and changes the dielectric properties of tissues,e.g.,due to coagulation or tissue water evaporation.The accurate implementation of the partial differential equation (PDE) solvers has to be verifed against reference solutions,before the software can be used for treatment planning.
Since in clinical practice it may be necessary to use more than one applicator elements to increase effectiveness,it is important that treatment planning software supports simultaneous setup of multi-antenna simulations and flexible coherent and incoherent field combining[28].It is quite often that antenna coupling and loading effects are overseen in treatment plans,carrying over miscalculations of optimal excitation parameters to the clinical setting.Applicator models are equally signifcant with patient models for accurate treatment planning.They have to be validated in terms of the predicted electric fields or the induced temperature rise,e.g.,in fat or cylindrical experimental phantoms orex vivoand the corresponding uncertainties have to be assessed,especially for quantitative predictions.Validation measurements should use phantoms containing well-characterised tissue-simulating materials and calibrated equipment,such as fibre-optic temperature sensors,or electric field sensors[29-31].Parameters that need consideration during validation include amplitude and phase changes of a single or multiple applicators.
2.4 Temperature rise evaluation and thermal dosimetry
Computational simulation of the interactions between electromagnetic fields and human tissues has reached a high level of accuracy in recent years,mainly due to the progress made in assessing the safe use of wireless telecommunication devices.On the other hand,temperature prediction still includes large uncertainties,because of large variations in the values of tissue thermal properties between patients,within the patient,or the tissue itself.Furthermore,important thermal parameters of tissues are strongly affected bytemperature (mainly through thermoregulation) leading to a highly nonlinear problem.
A review of the models of heat transfer in blood perfused tissues is given in Arkinet al[32].For RF ablation techniques,it may be necessary to include the discrete vasculature[33],although the respective information is not always available from medical imaging.An alternative model to simulate directional heat flow is the use of tensorial effective conductivity[34].Although the directional effect of blood fow for vessels with diameters exceeding 0.2 mm can be signifcant,most treatment planning software,especially for hyperthermia,uses the Pennes Bioheat Transfer Equation (PBHE)[35]which models perfused tissues as a media continuum with an isotropic heat sink.
The use of the PBHE results in reasonable estimates of temperature rise even for large computational models.Nevertheless,the final temperature distribution is sensitive to thermal parameters for which there is scarce data in the literature,in particular about their change under heat stress.Therefore,it is always necessary to perform an uncertainty analysis.The Monte-Carlo technique is a typical approach for such an analysis,due to the strong correlation between thermal parameters.The IT’IS database[23]can be used to derive the reference values of thermal properties for the sensitivity study.
Recently,the PBHE equation has been extended to accommodate the effect of systemic thermoregulation on local blood perfusion and,consequently,local temperature rise[36].Moreover,other physical mechanisms which are important in the heat balance in thermal ablation,like phase transition,have been included in the PBHE[37].
The distribution of temperature rise does not directly reveal tissue damage.This can be assessed through thermal dose,which can be evaluated with the appropriate tissue damage model.The Arrhenius model directly assesses tissue damage in coagulation and necrosis zones in ablation therapies.In hyperthermic oncology,use of the CEM43 thermal dose concept is common.The CEM43 thermal dose expresses the thermal history of a tissue location in terms of minutes of heating at 43 ℃ that would result in an equivalent thermal effect.It offers the advantage of not depending on tissue specific material parameters,and that CEM43 thresholds for thermal damage have been experimentally determined for a wide range of tissues and biological effects.Correlations between CEM43 and treatment outcome have been established clinically[38].
2.5 Treatment optimization
Treatment optimization in current clinical applications of thermal therapies is often performed by optimizing the energy deposition distribution,although the actual temperature or thermal dose level achieved in the tumor volume determine treatment quality.For phased array applicators,such as in deep hyperthermia treatments or ablation treatment with multiple catheters,the phases and amplitudes of the individual antennas canbe optimizedto achieve optimal tumor coverage,while avoiding exposure of sensitive tissues.Optimization is currently moving towards the utilization of either pre-computed information to enable real-time changes during the treatment[39]or multi-goal optimization to determine a large number of Pareto-optimal excitation settings[40].
Modeling can also be used to optimize the impact of treatment parameters other than applicator amplitude and phase.In superfcial hyperthermia,for example,the temperature of the water-bolus can be adjusted for each individual to optimally heat or cool the surface.
3 ADVANTAGES AND SHORTCOMING OF SIM4LIFE FOR TREATMENT PLANING
The computational life sciences platform Sim4Life (Zurich Med Tech,Zurich,Switzerland)[40-41]offers a range of features that make it uniquely suited for thermal therapy treatment planning:
(1) The software platform offers strong support for medical image data,which can be co-visualized with the model and simulation results to offer intuitive interpretation and localization of the results for the medical staff.This is facilitated by the integration of the powerful Visualization Toolkit (VTK)[42]that permits for example volume rendering in combination with a unique OpenGL visualization framework[43].The medical image data can be segmented and processed to generate personalized patient models.For that purpose a wide range of automatic and interactive segmentation methods is available (Figure 2).The discretization methods (gridding,voxeling,meshing) are capable of dealing with the resulting large and noisy models.Sim4Life also allows to load previously segmented data from radiotherapy planning as a basis for hyperthermia treatment planning.
(2) The numerical solvers that have been extensively verified and documented are optimized for modeling human anatomy and living tissue.By using high performance computing approaches,such as multi-GPU acceleration,simulations at high resolution and detail can be performed.As mentioned above,this is a crucial issue for the reliable detection of hot-spots.A large range of electromagnetic (full-wave and quasi-static)and thermal solvers are available.The thermal solvers offer a range of features to account for perfusion effects and thermal regulation,including the possibility of accounting for local vasodilation and body-core-temperature increase.
(3) The numerical solvers are supplemented by tissue models,such as the Arrhenius tissue damage and CEM43 thermal dose models,for the quantification of induced biological and therapeutic effects (Figure 3).

Figure 3 Assessment of the thermal treatment dose in treatment optimization with Sim4Life.
(4) Sim4Life has the functionalized,computable Virtual Population 3.0 anatomical phantoms[24]at its core,which is a range of high fidelity and resolution models generated from medical image data of volunteers selected to represent a broad patient population.These (poseable) models can be used for applicator development,basic research,guideline development,etc.In addition to the anatomical models a synchronized literature-based tissue properties database is available that facilitates assignment of dielectric and thermal properties[23].
(5) Sim4Life already includes tools for focus steering,antenna array parameter optimization,and feld control that can account for tissue sensitivity and heating priorities (Figure 4).
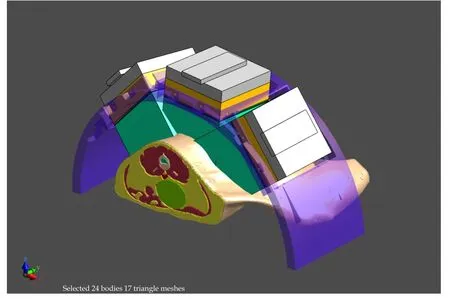
Figure 4 Model of an exposure set-up with a body model including the cancer,and a phased array applicator for electromagnetic treatment.The treatment settings are optimized with Sim4Life.
(6) The Sim4Life environment offers a Python scripting framework that has been used to automate treatment planning,reporting,and other tasks in a clinical environment,thus,reducing effort,time,and human error.
In addition,a range of features have been developed in Sim4Life for research purposes that are not yet available for the general public: advanced thermal models (accounting for example for the impact of discrete vasculature and anisotropic tissue),novel treatment optimization paradigms (multiobjective optimization combined with means to achieve realtime reoptimization based on patient or measurement feedback;PDE-constrained optimization for simultaneous treatment optimization and thermal modeling),cell water evaporation and rehydration models for high temperature treatment modeling,and support for image-derived inhomogeneity information and tissue property personalization (e.g.,using MRI perfusion maps).
4 CONCLUSIONS
There exist currently software packages[40,44]that can be used in clinical practice for thermal therapy treatment planning.Even with the current limitations in the calculation of temperature distribution,treatment planning can help improve treatment quality,design new applicators,investigate the role of multiple treatment parameters,and develop updated treatment protocols and clinical guidelines.The progress of non-invasive thermometry and real-time optimization are expected to reinforce the use of individualized treatment planning in the near future.
[1]Hurwitz MD.Today’s Thermal Therapy:Not Your Father’s Hyperthermia[J].Am J Clin Oncol,2010,33(1):96-100.
[2]Toraya-Brown S,Fiering S.Local tumour hyperthermia as immunotherapy for metastatic cancer[J].Int J Hyperthermia,2014,30(8):531-539.
[3]Cox RS,Kapp DS.Correlation of thermal parameters with outcome in combined radiation therapy-hyperthermia trials[J].Int J Hyperthermia,1992,8(6):719-732.
[4]Wust P,Stahl H,Dieckmann K,et al.Local hyperthermia of N2/N3 cervical lymph node metastases:Correlation of technical/thermal parameters and response[J].Int J Radiat Oncol Biol Phys,1996,34(3):635-646.
[5]Sherar M,Liu FF,Pintilie M,et al.Relationship between thermal dose and outcome in thermoradiotherapy treatments for superfcial recurrences of breast cancer:Data from a phase III trial[J].Int J Radiat Oncol Biol Phys,1997,39(2):371-380.
[6]Maguire PD,Samulski TV,Prosnitz LR,et al.A phase II trial testing the thermal dose parameter CEM43T90 as a predictor of response in soft tissue sarcomas treated with pre-operativethermoradiotherapy[J].Int J Hyperthermia,2001,17(4):283-290.
[7]Gellermann J,Hildebrandt B,Issels R,et al.Noninvasive magnetic resonance thermography of soft tissue sarcomas during regional hyperthermia:Correlation with response and direct thermometry[J].Cancer,2006,107(6):1373-1382.
[8]Franckena M,Fatehi D,de Bruijne M,et al.Hyperthermia dose-effect relationship in 420 patients withcervical cancer treated with combined radiotherapy andhyperthermia[J].Eur J Cancer,2009,45(11):1969-1978.
[9]Bruggmoser G,Bauchowitz S,Canters R,et al.Quality assurance for clinical studies in regional deep hyperthermia[J].Strahlenther Onkol,2011,187(10):605-610.
[10]De Bruijne M,Wielheesen DHM,van der Zee J,et al.Benefts of superficial hyperthermia treatment planning:Five case studies[J].Int J Hyperthermia,2007,23(5):417-429.
[11]Canters RAM,Paulides MM,Franckena MF,et al.Implementation of treatment planning in the routine clinical procedure of regional hyperthermia treatment of cervical cancer:An overview and the Rotterdam experience[J].Int J Hyperthermia,28(6):570-581.
[12]Karampatzakis A,Kühn S,Tsanidis G,et al.Heating characteristics of antenna arrays used in microwave ablation:A theoretical parametric study[J].Comput Biol Med,2013,43(10):1321-1327.
[13]Livraghi T,Meloni F,Di Stasi M,et al.Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis:Is resection still the treatment of choice[J]?Hepatology,2008,47(1):82-89.
[14]Livraghi T,Meloni L,Solbiati L,et al.Complications of Microwave Ablation for Liver Tumors:Results of a Multicenter Study[J].Cardiovasc Intervent Radiol,2012,35(4):868-874.
[15]Togni P,Rijnen Z,Numan WCM,et al.Electromagnetic redesign of the HYPERcollar applicator:toward improved deep local head-and-neck hyperthermia[J].Phys Med Biol,58(17):5997-6009.
[16]Capstick M,Neufeld E,Gosselin MC,et al.A new approach to high-quality patient-specific hyperthermia treatment[A].9thEuropean Conference on Antennas and Propagation(EuCAP) [C].2015:13-17.
[17]Van derGaag ML,de Bruijne M,Samaras T,et al.Development of a guideline for the water bolus temperature in superficial hyperthermia[J].Int J Hyperthermia,2006,22(8):637-656.
[18]Verhaart RF,Fortunati V,Verduijn GM,et al.CT-based patient modeling for head and neck hyperthermia treatment planning:Manual versus automatic normal-tissue-segmentation[J].Radiother Oncol,2014,111(1):158-163.
[19]Wust P,Nadobny J,Seebass M,et al.Influence of patient models and numerical methods on predicted power deposition patterns[J].Int J Hyperthermia,1999,15(6):519-540.
[20]Solazzo SA,Liu Z,Lobo SM,et al.Radiofrequency ablation: Importance of background tissue electrical conductivity-An agar phantom and computer modeling study[J].Radiology,2005,236(2):495-502.
[21]Liu Z,Ahmed M,Weinstein Y,et al.Characterization of the RF ablation-induced ‘oven effect’:Theimportance of background tissue thermal conductivity on tissue heating[J].Int J Hyperthermia,2006,22(4):327-342.
[22]Zorbas G,Samaras T.Parametric study of radiofrequency ablation in the clinical practice with the use of two-compartment numerical models[J].Electromagn Biol Med,2013,32(2):236-243.
[23]Hasgall PA,Di Gennaro F,Baumgartner C,et al.IT’IS Database for thermal and electromagnetic parameters of biological tissues[EB/OL].Available at www.itis.ethz.ch/database.
[24]Gosselin MC,Neufeld E,Moser H,et al.Development of a new generation of high-resolution anatomical models for medical device evaluation:the Virtual Population 3.0[J].Phys Med Biol,59(18):5287-5303.
[25]Lagendijk JJW.Hyperthermia treatment planning[J].Phys Med Biol,2000,45(5):61-76.
[26]Hand JW.Modelling the interaction of electromagnetic fields(10 MHz–10 GHz) with the human body:methods and applications[J].Phys Med Biol,2008,53(16):243-286.
[27]Berjano EJ.Theoretical modeling for radiofrequency ablation:state-of-the-art and challenges for the future[J].Biomed Eng Online,2006,5:24.
[28]Neufeld E.High Resolution hyperthermia treatment planning.PhD Thesis,ETH Zurich,Nr.17947[EB/OL].Available at http://e-collection.library.ethz.ch/eserv/eth:31164/eth-31164-02.pdf.
[29]Paulides MM,Bakker JF,van Rhoon GC.Electromagnetic head-and-neck hyperthermia applicator:Experimental phantom verification and FDTD model[J].Int J Radiat Oncol Biol Phys,2008,68(2):612-620.
[30]De Bruijne M,Samaras T,Chavannes T,et al.Quantitative validation of the 3D SAR profle of hyperthermia applicators using the gamma method[J].Phys Med Biol,2007,52(11): 3075-3088.
[31]Wang Z,Aarya I,Gueorguieva M,et al.Image-based 3D modeling and validation of radiofrequency interstitial tumor ablation using a tissue-mimicking breast phantom[J].Int J Comput Assist Radiol Surg,2012,7(6):941-948.
[32]Arkin H,Xu LX,Holmes KR.Recent developments in modeling heat transfer in blood perfused tissues[J].IEEE Trans Biomed Eng,1994,41(2):97-107.
[33]Kotte AN,van Leeuwen GM,Lagendijk JJ.Modelling the thermal impact of a discrete vessel tree[J].Phys Med Biol,1999,44(1):57-74.
[34]Crezee J,Lagendijk JJW.Temperature uniformity during hyperthermia:The impact of large vessels[J].Phys Med Biol,1992,37(6):1321-1337
[35]Pennes HH.Analysis of tissue and arterial blood temperatures in the resting human forearm[J].J Appl Physiol,1948,1(2):93- 122.
[36]Hirata A,Asano T,Fujiwara O.FDTD analysis of body-core temperature elevation in children and adults for whole-body exposure[J].Phys Med Biol,2008,53(18):5223-5238.
[37]Yang D,Converse MC,Mahvi DM,et al.Expanding the bioheat equation to include tissue internal water evaporation during heating[J].IEEE Trans Biomed Eng,2007,54(8):1382-1388.
[38]Jones E,Thrall D,Dewhirst MW,et al.Prospective thermal dosimetry:The key to hyperthermia's future[J].Int J Hyperthermia,2007,22(3):247-253
[39]Neufeld E,Chavannes N,Paulides MM,et al.Fast(re-) optimization for hyperthermia:Bringing treatment planning into the treatment room[A].10thInternational Congress on Hyperthermic Oncology[C].9-12 April 2008,Munich.
[40]Neufeld E,Szczerba D,Chavannes N,et al.A novel medical image data-based multi-physics simulation platform for computational life sciences[J].Interface Focus,2013,3:2012 0058.
[41]Sim4Life v2.0[EB/OL].http://www.zurichmedtech.com/sim4life.Last accessed 16 November 2015.
[42]The Visualization Toolkit(VTK),v6.3.0[EB/OL].http://www.vtk.org.Last accessed 16 November 2015.
[43]OpenGL[EB/OL].https://www.opengl.org.Last accessed 16 November 2015.
[44]Gellermann J,Wust P,Stalling D,et al.Clinical evaluation and verification of the hyperthermia treatment planning system hyperplan[J].Int J Radiat Oncol Biol Phys,2000,47(4):1145-1156.
R318.08 [Document code]A
10.3969/j.issn.1674-1633.2016.04.005
1674-1633(2016)04-0023-07
Received: 2015-11-30
Niels Kuster,Founder &Chairman,IT'IS Foundation.
E-mail: kuster@itis.ethz.ch
