The decision-making process for senior cancer patients: treatment allocation of older women with operable breast cancer in the UK
2015-12-29JennaMorganPaulRichardsOsamaZamanSueWardKarenCollinsThompsonRobinsonKwokLeungCheungRiccardoAudisioMalcolmReedLyndaWyldonbehalfoftheBridgingtheAgeGapinBreastCancerTrialManagementTeam
Jenna L. Morgan, Paul Richards, Osama Zaman, Sue Ward, Karen Collins, Thompson Robinson, Kwok-Leung Cheung, Riccardo A. Audisio, Malcolm W. Reed, Lynda Wyld; on behalf of the Bridging the Age Gap in Breast Cancer Trial Management Team
1Academic Unit of Surgical Oncology, University of Sheffeld Medical School, Sheffeld S10 2RX, UK;2Health Economics and Decision Science, School for Health and Related Research, University of Sheffeld, Sheffeld S1 4DA, UK;3Center for Health and Social Care Research, Sheffeld Hallam University, Collegiate Crescent, Sheffeld S10 2BP, UK;4Department of Cardiovascular Sciences, University of Leicester, Leicester LE2 7LX, UK;5School of Medicine, University of Nottingham, Royal Derby Hospital Center, Derby DE22 3DT, UK;6Department of Surgery, University of Liverpool, St Helens Teaching Hospital, St Helens WA9 3DA, UK;7Brighton and Sussex Medical School, University of Sussex, Brighton BN1 9PX, UK
ORIGINAL ARTICLE
The decision-making process for senior cancer patients: treatment allocation of older women with operable breast cancer in the UK
Jenna L. Morgan1, Paul Richards2, Osama Zaman1, Sue Ward2, Karen Collins3, Thompson Robinson4, Kwok-Leung Cheung5, Riccardo A. Audisio6, Malcolm W. Reed7, Lynda Wyld1; on behalf of the Bridging the Age Gap in Breast Cancer Trial Management Team
1Academic Unit of Surgical Oncology, University of Sheffeld Medical School, Sheffeld S10 2RX, UK;2Health Economics and Decision Science, School for Health and Related Research, University of Sheffeld, Sheffeld S1 4DA, UK;3Center for Health and Social Care Research, Sheffeld Hallam University, Collegiate Crescent, Sheffeld S10 2BP, UK;4Department of Cardiovascular Sciences, University of Leicester, Leicester LE2 7LX, UK;5School of Medicine, University of Nottingham, Royal Derby Hospital Center, Derby DE22 3DT, UK;6Department of Surgery, University of Liverpool, St Helens Teaching Hospital, St Helens WA9 3DA, UK;7Brighton and Sussex Medical School, University of Sussex, Brighton BN1 9PX, UK
Objective: Up to 40% of women over 70 years with primary operable breast cancer in the UK are treated with primary endocrine therapy (PET) as an alternative to surgery. A variety of factors are important in determining treatment for older breast cancer patients. Tis study aimed to identify the patient and tumor factors associated with treatment allocation in this population.
Methods: Prospectively collected data on treatment received (surgery vs. PET) were analysed with multivariable logistic regression using the variables age, modified Charlson Comorbidity Index (CCI), activities of daily living (ADL) score, Mini-Mental State Examination (MMSE) score, HER2 status, tumour size, grade and nodal status.
Results: Data were available for 1,122 cancers in 1,098 patients recruited between February 2013 and June 2015 from 51 UK hospitals. About 78% of the population were treated surgically, with the remainder being treated with PET. Increasing patient age at diagnosis, increasing CCI score, large tumor size (5 cm or more) and dependence in one or more ADL categories were all strongly associated with non-surgical treatment (P<0.05).
Conclusion: Increasing comorbidity, large tumor size and reduced functional ability are associated with reduced likelihood of surgical treatment of breast cancer in older patients. However, age itself remains a significant factor for non-surgical treatment; reinforcing the need for evidence-based guidelines.
Frail elderly; breast neoplasms; decision-making
Introduction
A third of new breast cancer diagnoses occur in women aged over 70 years in the UK1. Te number of women afected will increase over time due to the aging population2. The prevalence of comorbidity and frailty increases with age, resulting in deaths from other causes exceeding breast cancer mortality in older women with breast cancer3,4. Additionally, tolerance of some breast cancer therapies is reduced in this age group5,6. The majority (about 90%) of older women are diagnosed with estrogen receptor (ER) positive disease3. Older patients with operable breast cancer may be offered alternative treatment modalities, such as primary endocrine therapy (PET)7,8, where ER positive disease is treated with endocrine therapy alone without surgery.Non-operative management of older breast cancer patients accounts for up to 40% of treatment in the UK9.
PET gained popularity in the 1980s for the management of older women after tamoxifen was shown to be effective in this setting10and a succession of randomised controlled trials comparing its efficacy with surgery followed. A subsequent Cochrane review comparing PET with surgery in the over 70s demonstrated superior rates of local control with surgery but no difference in survival rates11. However the studies included in the review were small and flawed by modern standards because tumor ER status was not always tested and the age range of the trials included relatively young older women with no documentation of health, ftness or frailty, in fact all patients were deemed fit for surgery under general anaesthesia and therefore frail patients were excluded. A recent review of case series indicated that older frailer women tend to be treated with PET in routine practice and have inferior survival rates as would be expected due to higher other-cause mortality12. Since the trials included in the Cochrane review were conducted there have been signifcant increases in population life expectancy, so it is not clear that whether results of these trials are applicable in contemporary clinical practice.
Recent reports have advocated the use of PET only in the very old or frail13. Current national guidelines state that patients with operable breast cancer should be treated with surgery, and not PET, “irrespective of age” unless this is precluded by comorbidities14; whilst the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA) recommend that PET should only be ofered to patients with a “short estimated life expectancy (less than 2 to 3 years), who are considered unft for surgery… or who refuse surgery”15. However, as a large number of older women are treated with PET in UK and other countries, it is not clear that this guidance is being followed consistently.
Population cohort studies have been carried out to investigate the factors associated with non-surgical treatment of breast cancer in older women and found that increasing age, comorbidities and worsening scores on activities of daily living (ADL) were all associated with lower rates of surgery16. An analysis of English cancer registry data showed that after adjusting for disease characteristics, age and comorbidity, there remained significant variation in the rate of PET use at the hospital level17. This may reflect genuine variation in practice, which would contradict current recommendations. However, this analysis could not account for other potential confounding factors such as frailty and cognitive function, which would be expected to infuence treatment decision making. Tere are also limitations concerning data quality such as missing data due to the retrospective and routine nature of data collection. A further confounding factor is the infuence of the patient’s preference for one or other option if they are given a choice.
The study presented here aims to identify patient and tumor factors associated with treatment allocation in the older UK breast cancer population, including data on functional status, cognitive function and comorbidities, as well as disease characteristics. A planned interim analysis was undertaken using prospectively collected patient data from a large multicenter UK cohort study.
Materials and methods
Study design
This study is part of a prospective observational cohort study of women aged over 70 years diagnosed with operable primary breast cancer in 51 UK breast cancer units between February 2013 and June 2015. Recruitment for this trial is ongoing, and results presented here represent an interim analysis of the first 1,447 patients. Data were collected on patient and tumor characteristics, and treatment type. Data collection time points for the study are at baseline (prior to any treatment being administered), 6 weeks post-treatment, 6 months post treatment, 12 months post-treatment, 18 months post-treatment, and 24 months post-treatment. Analyses are restricted to data collected up to 6 months post-treatment as the outcome of interest was primary treatment allocation.
Analyses are also restricted to the subgroup of patients with operable ER positive disease at diagnosis. Variables used within the analysis are shown in Table 1. Patients with missing treatment data or with known ER negative disease were excluded from the analysis. Patients with missing ER status who did not receive endocrine therapy were assumed to be ER negative and also excluded from the analysis. Bilateral cancers are counted individually. Figure 1 shows the baseline features of the study population.
Statistical analysis
Primary treatment was dichotomised as “surgery” or “PET”according to whether or not the patient received surgery to the primary tumor. Simple logistic regression was used to investigate associations between complete covariates and treatment. The joint efect of patient level factors on the probability of surgical treatment was assessed using multivariable logistic regression.
Variables with missing data cannot be included in standard regression models, but excluding cases with missing values(complete case analysis) can produce biased results where data is not missing completely at random, as well as reducing statistical precision. Missing data on disease characteristics and comorbidity was handled using the method of multiple imputation by chained equations (MICE)18. Missing covariate values were imputed using predictive mean matching19, and all variables included in the main analysis were incorporated into the imputation models. Twenty-five completed datasets were used for subsequent analysis. Results of the regression models for each dataset were combined using the standard methods described by Rubin20. Covariates with over 50% missing data were not included in the regression models.
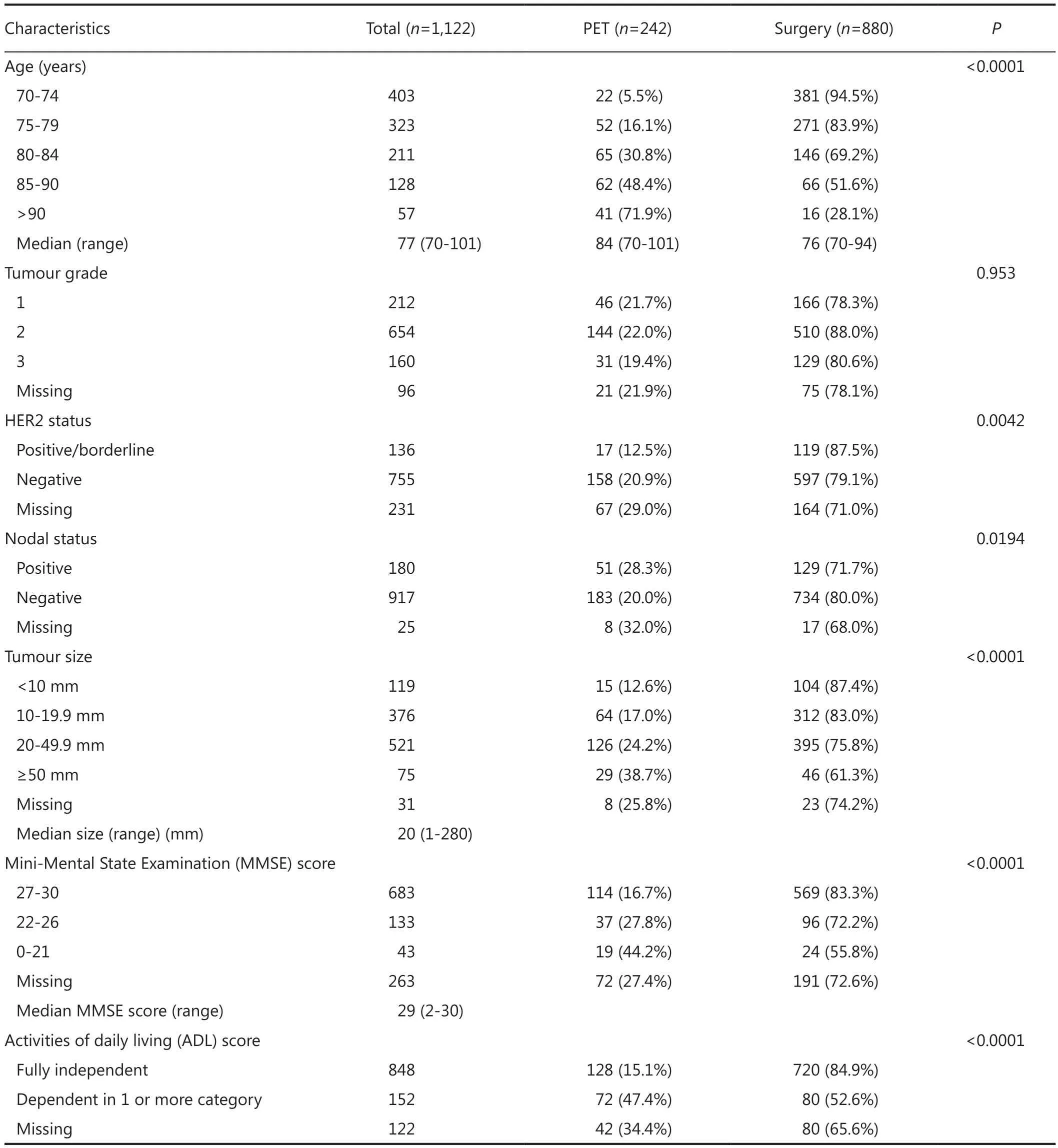
Table 1 Characteristics of the study population
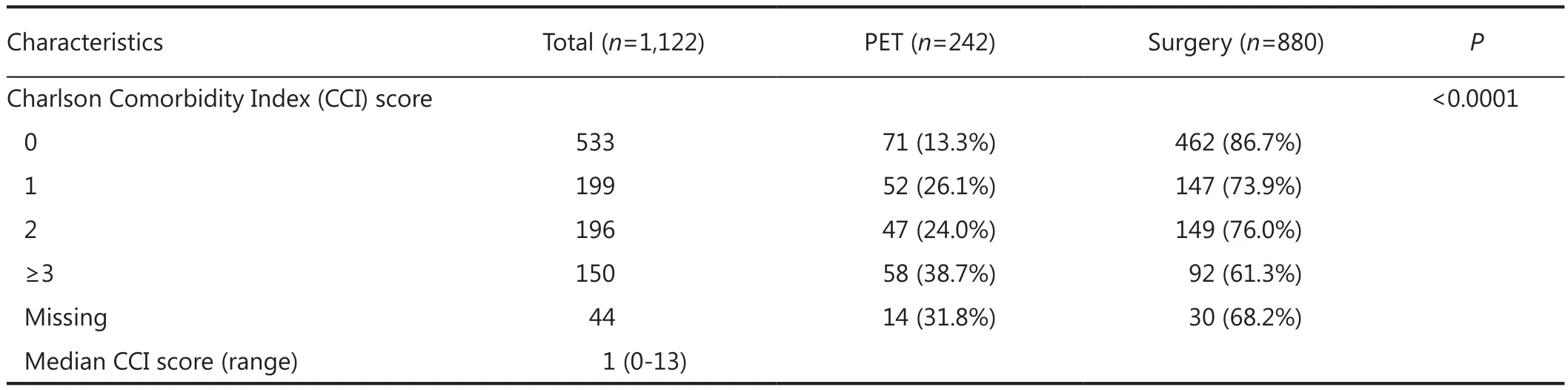
Table 1 Characteristics of the study population
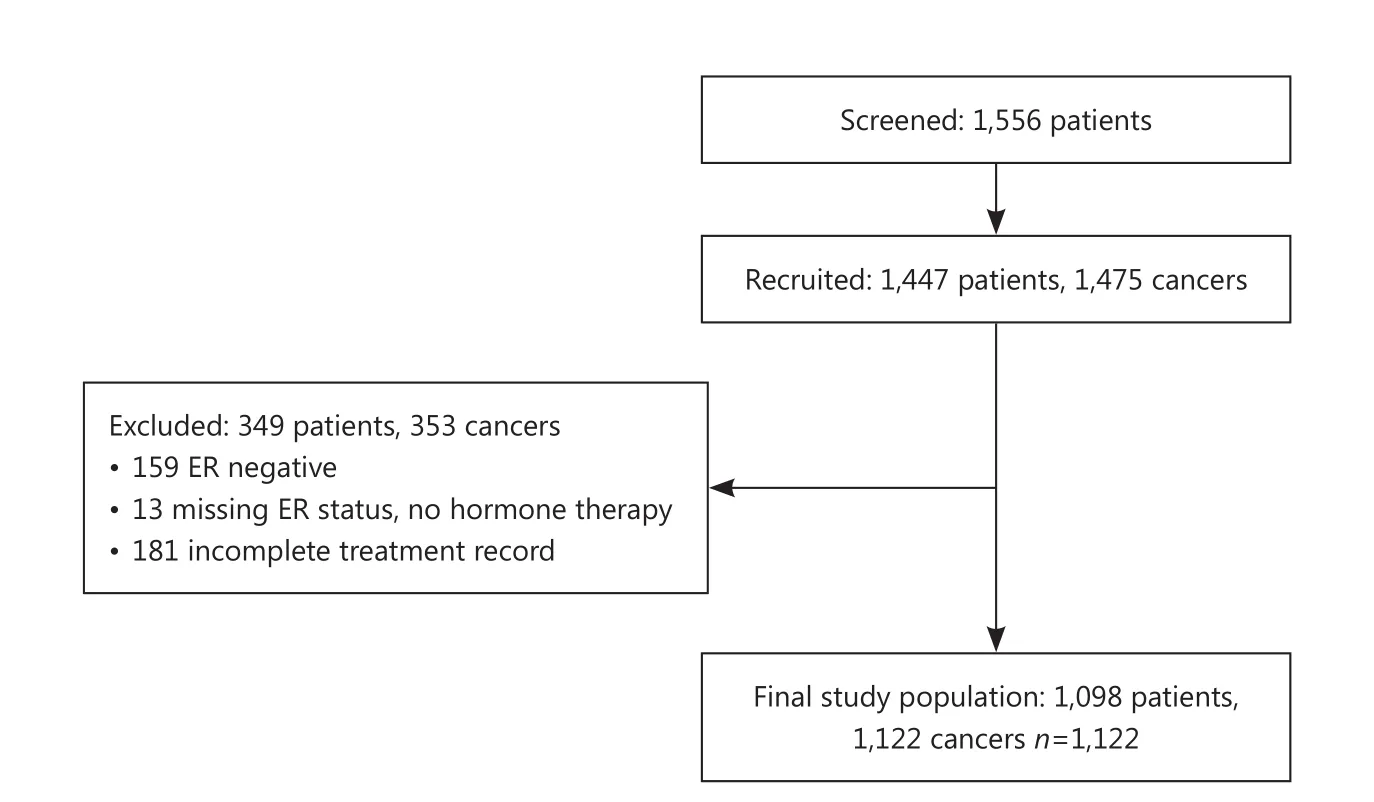
Figure 1 Determining the study population.
The performance of the regression model was assessed by considering the receiver-operating characteristic (ROC) curve of the model, along with its corresponding area under the curve (AUC) statistic. Tis is a measure of the ability of the model to discriminate between individuals treated with surgery and PET21. Te AUC can range from 0.5 to 1, where a value of 1 indicates perfect discrimination, and a value of 0.5 less indicates a model which performs no beter than random chance.
All analyses were conducted using the open source statistical programming language R (version 3.0.1). Multiple imputation was implemented using the “mi” package (version 1.0).
Ethics and research governance
Te study protocols were approved by the UK National Research Ethics Committee (12/LO/1808) and institutional approvals were granted at each site. Writen informed consent was obtained from the patient, or patient consultee in the case of patients with cognitive impairment.
Results
Data were available on 1,122 ER positive breast cancers in 1,098 patients with a treatment record of either primary surgery with adjuvant therapy as deemed appropriate by the treating clinician (“surgery”) or endocrine therapy without surgery(“PET”). Characteristics of the study population can be seen in Table 1.
About 78% of the study population were treated surgically, with the remainder being treated with PET. Te patients treated with surgery were younger overall than those treated with PET (Table 1). Te imputation algorithm converged successfully, and exploratory analysis of the distributions of the imputed values did not raise concerns about their validity or plausibility.
Te multivariable logistic regression model is shown in Table 2.Increased age at diagnosis, increasing levels of comorbidity, large tumor size (5 cm or more) and dependence in one or more ADL categories are all strongly associated with non-surgical treatment (Table 2). Te ROC curve shows that the model discriminates well between individuals treated with PET and surgery, with the AUC statistic equal to 0.824 (95% CI, 0.792-0.855) (Figure 2).
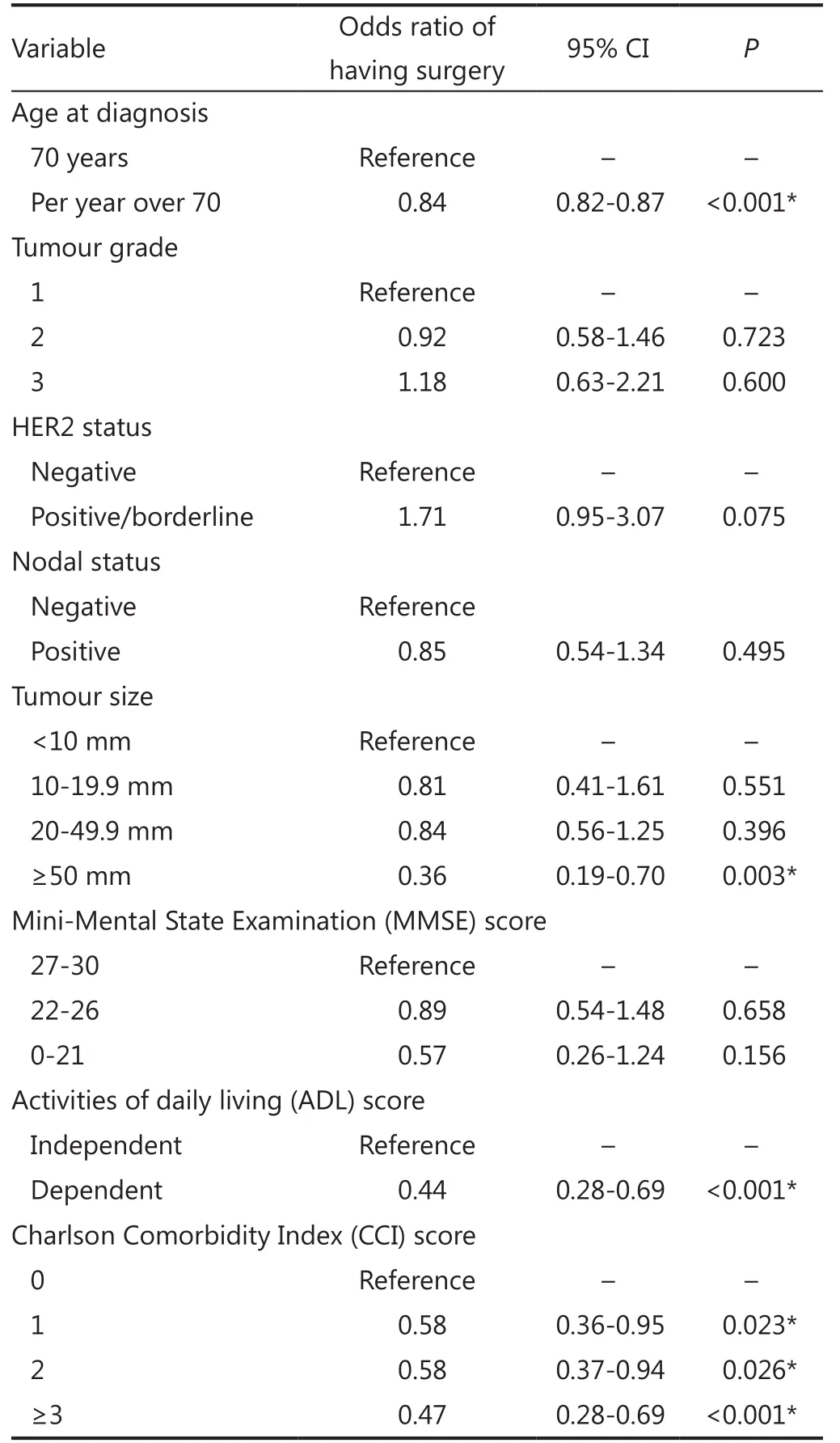
Table 2 Multivariable logistic regression of surgical treatment vs. nonsurgical treatment, conditional on individual patient characteristics
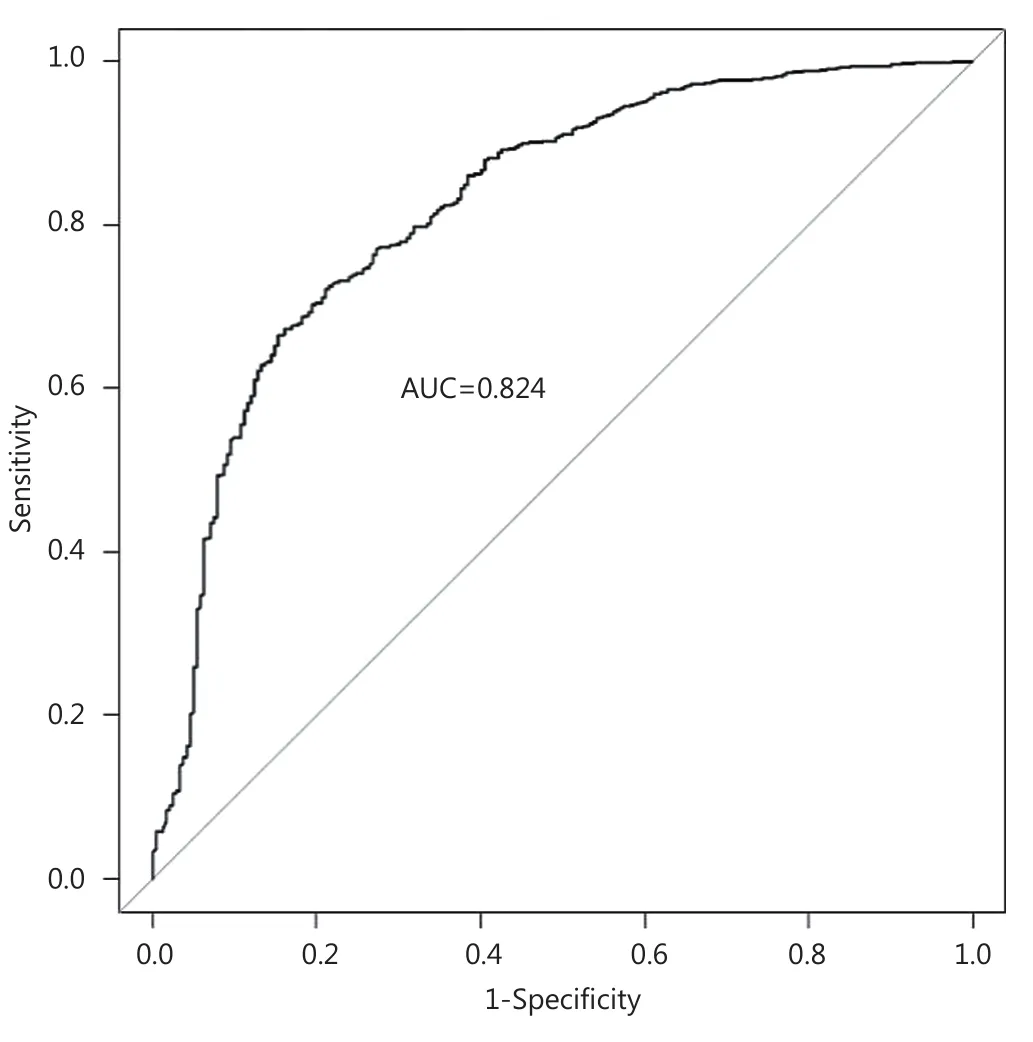
Figure 2 Receiver-operator characteristic (ROC) curve for the multivariable logistic regression model (Table 2), with area under the curve statistic (AUC).
Discussion
Of the 1,122 ER positive operable breast cancers enrolled into the study to date, 78% were treated with surgery. Age was confrmed as one of the most important factors in determining treatment in older women with operable ER positive breast cancer, with increasing age associated with higher rates of PET, even accounting for other patient and disease characteristics. This is consistent with other studies7,22,23despite current UK guidelines, which state that patients should be treated with surgery and appropriate adjuvant therapy “irrespective of age”14.
Increasing age was associated with increasing rates of comorbidity and multi-morbidity24, this in turn may reduce the survival beneft of more aggressive breast cancer therapies, such as surgery4. It was therefore unsurprising that higher levels of comorbidity in the study population are strongly associated with lower levels of surgical treatment, however, this does not accountfor the efect seen with age.
Functional dependence in one or more of the ADL categories was strongly associated with non-surgical treatment in this study. Rates of functional dependence also increase with age and have been shown to afect treatment in a small population of older breast cancer patients22. Functional dependence has been associated with increased rates of post-operative complications and longer hospital stays in elderly cancer patients25, possibly explaining why it has an impact on treatment allocation here.
Additionally, large tumor size (5 cm or more) was also associated with higher rates of PET. Tumors of this size usually mandate a mastectomy26, and so these findings may represent patients and clinicians trying to avoid more major surgery.
The rate of surgical treatment was higher in this study than some of the published fgures for the UK, which range from 55%-83%8,16,17,27-29. Tis may result from a recruitment bias as patients within this study had to be recruited prior to commencing treatment, which was logistically more difficult with patients treated with PET as there is a shorter time period between diagnosis and treatment compared with surgery. Another possible bias could be that clinicians may be more likely to ‘follow the guidelines’ knowing that their data were being captured. Tis will ultimately result in an under-representation of PET patients, and may explain why Lavelle and colleagues found a similar rate of 83% having surgical treatment in their prospective cohort study16.
Tis study is limited by missing data, particularly with relation to ADL and MMSE, which is due, in part of patients opting out of completing the questionnaires. We have used multiple imputation to account for these missing data as it is less prone to bias than other commonly used methods to account for missing data. However, whilst exploratory analysis of the imputed data suggested that the values were plausible, it is not possible to verify the extent to which the distribution of the imputed data accurately represents that of the missing values. By using 25 imputations, uncertainty due to missing data is incorporated into the estimated effects of covariates on the probability of surgery, which mitigates against any small biases due to problems with the imputation model.
Te fndings of this study confrm existing research showing that age remains a significant predictor of non-surgical treatment after adjusting for disease characteristics and the underlying health status of the patient. However, it remains unclear as to whether or not current practice resulted in suboptimal outcomes. An attempt to compare outcomes under PET and surgery with adjuvant endocrine therapy using a randomised clinical trial failed due to lack of recruitment, which was explained by an unwillingness on behalf of both patients and clinicians to choose treatment at random30. As a result, observational research is needed to investigate the efects of nonsurgical treatment in this population. As recruitment and follow up of this cohort matures, it will be possible to investigate the relationship of treatment and individual characteristics with outcomes such as survival and treatment failure. Tese data will be crucial in the development of evidence based guidance for patients and clinicians to help target treatments efectively.
Acknowledgements
Te authors would like to thank all the members of the Bridging the Age Gap in Breast Cancer Trial Management Group: Charlene Martin, Jenny Newhouse, Rosie Cooper, Alistair Ring, Stephen Walters, Tim Stephenson, Kirsty Pemberton, Tim Chater, Jaqui Gath, Lisa Caldon, Kate Lifford, Kate Brain. In addition, the authors would like to acknowledge the Principal Investigators and staf working at each of the recruiting sites: Ms. J Dicks (Barnsley), Ms. C Rogers (Doncaster and Bassetlaw), Ms. S Edwards (Milton Keynes), Mr. R Vijh (North Lincolnshire and Goole), Miss M Kaushik (Leicester), Ms. S Ashworth (Burnley), Ms. A Norton (Harrogate), Ms. R Nasr (York), Mr. C Holcolmbe (Royal Liverpool), Mr. A Nejum (Airedale), Ms. S Hartup (Leeds), Mr. R Linford (Bradford), Ms. C Morris (Cardif and Vale), Ms. H Sweetland (Cardif), Ms. A Williams (Nevill Hall Hospital), Mr. S Parker (Coventry), Mr. A Modi (Grantham), Ms. E Gullaksen (Hull), Ms. L Whisker (Notingham), Mr. A Haq (Southport), Miss V Pope (Leighton), Ms. Karen Brooks (Royal Marsden), Ms. J Forkes (Cheltenham), Mr. M Douek (Guy’s and St Tomas), Ms. C Osborne (Dorset County), Ms. S Miles-Dua (Broomfield), Ms. S Buckley (Pinderfelds), Ms. K Kirby (Southmead, Bristol), Miss I Azmy (Chesterfield), Mr. S Nicol (Rotherham), Miss S Seetharam (Dartford), Ms. C Osborne (Yeovil), Mr. S Joshi (Croyden), Ms. A Chilvers (North Tees), Mr. Cheema (James Cook South Tees), Ms. L Rivett (Luton and Dunstable), Miss R Ainsworth (Great Weston). This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (Grant No. RP-PG-1209-10071). Te views expressed are those of the authors and not necessarily those of the National Health Service (NHS), the NIHR or the Department of Health.
Conflict of interest statement
No potential conficts of interest are disclosed.
1. New cases of cancer diagnosed in England, 2010: selected sites by age group and sex. In: Cancer Registrations in England 2010. Available online: htp://www.ons.gov.uk/ons/rel/vsob1/cancerregistrations-in-england/2010/rf-cancer-registrations-inengland--2010.xls
2. Alberg AJ, Singh S. Epidemiology of breast cancer in older women: implications for future healthcare. Drugs Aging 2001;18:761-772.
3. Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst 2000;92:550-556.
4. Satariano WA, Ragland DR. Te efect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med 1994;120:104-110.
5. Ring A, Reed M, Leonard R, Kunkler I, Muss H, Wildiers H, et al. Te treatment of early breast cancer in women over the age of 70. Br J Cancer 2011;105:189-193.
6. Muss HB, Berry DA, Cirrincione C, Budman DR, Henderson IC, Citron ML, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. J Clin Oncol 2007;25:3699-3704.
7. Lavelle K, Todd C, Moran A, Howell A, Bundred N, Campbell M. Non-standard management of breast cancer increases with age in the UK: a population based cohort of women > or =65 years. Br J Cancer 2007;96:1197-1203.
8. Wyld L, Garg DK, Kumar ID, Brown H, Reed MW. Stage and treatment variation with age in postmenopausal women with breast cancer: compliance with guidelines. Br J Cancer 2004;90:1486-1491.
9. BCCOM. Breast Cancer Clinical Outcome Measures (BCCOM) Project: Analysis of the management of symptomatic breast cancers diagnosed in 2004. 3rd Year Report December 2007. Available online: htp://www.associationofreastsurgery.org.uk/ media/2327/bccom_ar09.pdf
10. Preece PE, Wood R, Mackie CR, Cuschieri A. Tamoxifen as initial sole treatment of localised breast cancer in elderly women: a pilot study. Br Med J (Clin Res Ed) 1982;284:869-870.
11. Hind D, Wyld L, Beverley CB, Reed MW. Surgery versus primary endocrine therapy for operable primary breast cancer in elderly women (70 years plus). Cochrane Database Syst Rev 2006;(1):CD004272.
12. Morgan JL, Reed MW, Wyld L. Primary endocrine therapy as a treatment for older women with operable breast cancer - a comparison of randomised controlled trial and cohort study fndings. Eur J Surg Oncol 2014;40:676-684.
13. Chakrabarti J, Kenny FS, Syed BM, Robertson JF, Blamey RW, Cheung KL. A randomised trial of mastectomy only versus tamoxifen for treating elderly patients with operable primary breast cancer-fnal results at 20-year follow-up. Crit Rev Oncol Hematol 2011;78:260-264.
14. NICE. Early and locally advanced breast cancer: full guideline. Cardif: National Collaborating Centre for Cancer; 2009.
15. Biganzoli L, Wildiers H, Oakman C, Maroti L, Loibl S, Kunkler I, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol 2012;13:e148-e160.
16. Lavelle K, Sowerbuts AM, Bundred N, Pilling M, Degner L, Stockton C, et al. Is lack of surgery for older breast cancer patients in the UK explained by patient choice or poor health? A prospective cohort study. Br J Cancer 2014;110:573-583.
17. Morgan J, Richards P, Ward S, Francis M, Lawrence G, Collins K, et al. Case-mix analysis and variation in rates of non-surgical treatment of older women with operable breast cancer. Br J Surg 2015;102:1056-1063.
18. Su YS, Yajima M, Gelman AE, Hill J. Multiple Imputation with Diagnostics (mi) in R: Opening Windows into the Black Box. J Stat Sofw 2011;45:1-31.
19. Horton NJ, Kleinman KP. Much ado about nothing: A comparison of missing data methods and sofware to ft incomplete data regression models. Am Stat 2007;61:79-90.
20. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: J Wiley & Sons; 1987.
21. Hanley JA, McNeil BJ. Te meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29-36.
22. Lavelle K, Moran A, Howell A, Bundred N, Campbell M, Todd C. Older women with operable breast cancer are less likely to have surgery. Br J Surg 2007;94:1209-1215.
23. Ali AM, Greenberg D, Wishart GC, Pharoah P. Patient and tumour characteristics, management, and age-specifc survival in women with breast cancer in the East of England. Br J Cancer 2011;104:564-570.
24. Barnet K, Mercer SW, Norbury M, Wat G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43.
25. PACE participants, Audisio R, Pope D, Ramesh HS, Gennari R, van Leeuwen BL, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol 2008;65:156-163.
26. National Mastectomy and Breast Reconstruction Audit 2011. Available online: htp://www.hscic.gov.uk/catalogue/PUB02731/ clin-audi-supp-prog-mast-brea-reco-2011-rep1.pdf
27. Lavelle K, Downing A, Tomas J, Lawrence G, Forman D, Oliver SE. Are lower rates of surgery amongst older women with breast cancer in the UK explained by co-morbidity? Br J Cancer 2012;107:1175-1180.
28. Syed BM, Johnston SJ, Wong DW, Green AR, Winterbotom L, Kennedy H, et al. Long-term (37 years) clinical outcome of older women with early operable primary breast cancer managed in a dedicated clinic. Ann Oncol 2012;23:1465-1471.
29. Dordea M, Jones R, Nicolas AP, Sudeshna S, Solomon J, Truran P, et al. Surgery for breast cancer in the elderly--how relevant? Breast 2011;20:212-214.
30. Reed MW, Wyld L, Ellis P, Bliss J, Leonard R; ACTION and ESTEeM Trial Management Groups. Breast cancer in older women: trials and tribulations. Clin Oncol (R Coll Radiol) 2009;21:99-102.
Cite this article as: Morgan JL, Richards P, Zaman O, Ward S, Collins K, Robinson T, Cheung KL, Audisio RA, Reed MW, Wyld L; on behalf of the Bridging the Age Gap in Breast Cancer Trial Management Team. The decision-making process for senior cancer patients: treatment allocation of older women with operable breast cancer in the UK. Cancer Biol Med 2015;12:308-315. doi: 10.7497/j.issn.2095-3941.2015.0080
Jenna L. Morgan
E-mail: j.morgan@sheffeld.ac.uk
Received September 18, 2015; accepted November 4, 2015.
Available at www.cancerbiomed.org
Copyright © 2015 by Cancer Biology & Medicine
杂志排行
Cancer Biology & Medicine的其它文章
- Mechanistic basis and clinical relevance of the role of transforming growth factor-β in cancer
- Advances in drug delivery system for platinum agents based combination therapy
- Upgrading the definition of early gastric cancer: better staging means more appropriate treatment
- Dermatofibrosarcoma protuberans: from translocation to targeted therapy
- Synergistic suppression of the PI3K inhibitor CAL-101 with bortezomib on mantle cell lymphoma growth
- Pathological clavicular fracture as first presentation of renal cell carcinoma: a case report and literature review
