Nitric oxide synthase inhibition ameliorates nicotine-induced sperm function decline in male rats
2015-12-26IPOyeyipoRajiAdeyomboBolarinwa
IP Oyeyipo, Y Raji, Adeyombo F. Bolarinwa
1Department of Physiology, College of Health Sciences, Osun State University, Osogbo, Osun State, Nigeria
2Department of Physiology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria
3Division of Medical Physiology, Department of Biomedical Sciences, Stellenbosch University, Tygerberg, South Africa
Document heading
Nitric oxide synthase inhibition ameliorates nicotine-induced sperm function decline in male rats
IP Oyeyipo1,3*, Y Raji2, Adeyombo F. Bolarinwa2
1Department of Physiology, College of Health Sciences, Osun State University, Osogbo, Osun State, Nigeria
2Department of Physiology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria
3Division of Medical Physiology, Department of Biomedical Sciences, Stellenbosch University, Tygerberg, South Africa
ARTICLE INFO
Article history:
Received 2 February 2015
Received in revised form 19 May 2015
Accepted 20 May 2015
Available online 20 September 2015
Nicotine
Nitric oxide
Inhibitor
Sperm
Rats
Objective: To evaluate the effects of inhibiting nitric oxide synthase as a means of intervention in nicotineinduced infertility in male rats. Methods: Forty-eight male and thirty female Wistar rats (180-200 g) were randomly assigned to six groups and treated orally for 30 days with saline (control), nicotine (0.5 mg/kg, 1.0 mg/kg) with or without NG Nitro-L-Arginine Methyl Ester (L- NAME, 50 mg/kg). Treated male rats were cohabited with untreated females in ratio 1:2 for fertility studies. Sperm analysis was done by microscopy. Results: There was a significant decrease in the epididymal sperm motility and count after nicotine treatment. However, the percentage of abnormality significantly increased in nicotine treatment groups. Fertility studies revealed that nicotine reduced libido in male rats and decreased litter weight and number delivered by the untreated female during the experiments. Co-treatment with L-NAME effectively reversed the nicotinemediated alterations in the sperm functional parameters, fertility indexes and hormone when compared to nicotine only. Conclusion: Taken together, the present data indicate the abilities of L-NAME to ameliorate nicotine-induced spermatotoxic effects in male rats via a mechanism dependent on the circulating testosterone level.
1. Introduction
In the last decade, nitric oxide (NO) which is a highly reactive free radical gas and reactive oxygen species (ROS) has assumed an important functional role in a variety of physiological systems and different pathways, therefore it is indisputable that such a polyvalent molecule should also play a decisive role in the reproductive system. NO is synthesized from an essential amino acid L-arginine by a family of isoenzymes known as the nitric oxide synthases (NOS) [1].
Basal generation of NO plays an important role in the physiology of several organs. Studies have shown that in the vascular system, NO inhibit platelet aggregation, induce vasodilation, prevent neutrophil/platelet adhesion to endothelial cells, maintainendothelial cell barrier function and inhibit smooth muscle cells proliferation and migration[2].
NO was first recognized in the reproductive system by Ignarroet al. [3], who demonstrated that NO was generated in response to non-adrenergic/non-cholinergic neurotransmission-mediated penile erection. Following this finding, several other studies have implicated NO in its involvement in penile erection at several neuronal levels[4]. It has long been documented that regulation of penile erection by androgens and the pituitary and its relationship with NOS activity is of special physiological interest and deficiency of male sex hormone such as testosterone, has long been associated with impotence and dysfunction of penile erection[5, 6]. Studies have shown that castration in rodents results in a significant decrease in NOS activity in the penis and significantly reduced electrical stimulation-induced penile erection[6, 7].
Furthermore, in the female reproductive system, it was demonstrated that expression of NOS was increased in the cervix, anddecreased in the uterus, during labour and preterm labour [8]. NO also seems to be involved in pre-eclamptic conditions and pregnancyrelated hypertension[8].
NO has also been shown to regulate sperm motility. Lewiset al.[9] documented that low concentrations of NO have been shown to enhance sperm motility while Rosselliet al. [10] concluded that high concentrations of NO decrease sperm motility. Interestingly, nicotine administration has been implicated with increased NO level and decrease antioxidant enzyme activities [11].
The use of nicotine seems to remain a broad public health concern since several million of humans use nicotine worldwide through smoking for a prolonged period of time and infertility among couples of child bearing age is also on the rise. In spite of the growing knowledge of effects of NO on reproduction and the association between nicotine and male reproductive dysfunction, little is known about the effect of inhibiting NOS and its effect on nicotine-induced infertility, Therefore, this present study was designed to investigate whether or not inhibition of systemic biosynthesis of nitric oxide will ameliorate nicotine-induced infertility in rat models.
2. Materials and methods
2.1. Animals
The experiments were performed on forty-eight male and thirty female Wistar rats, 2-2.5 month old and whose average weight ranged between 180 g and 200 g obtained from the Animal House, College of Medicine, University of Ibadan, Oyo State, Nigeria. Animals were divided into six equal groups with ad libitum access to rat chow and drinking water. Animals were also maintained in a well-ventilated room with a 12/12-hour light/ dark condition at room temperature. The experiment was conducted in accordance with the Guidelines of the U.S. National Institute of Health (NIH) on the care and use of laboratory animals. The male animals in the six groups were treated orally for 30 days and they included the control group that received 0.2 mL/kg normal saline, 0.5 mg/kg nicotine-treated group, 1.0 mg/kg nicotine-treated group, 50 mg/kg L-NAME, 0.5 mg/kg nicotine alongside with 50 mg/kg L-NAME and 1.0 mg/kg nicotine alongside with 50 mg/ kg L-NAME
2.2. Drug preparation
2.2.1. Nicotine preparation
Nicotine hydrogen tartrate (95% Nicotine) (BDH Chemicals Ltd., Poole, England) was used in the study. The nicotine dosage freshly prepared in normal saline for each group of animals was delivered at 0.5 mg/kg and 1.0 mg/kg per body weight. The working solutions were stored in foil-wrapped glass bottle at 4°℃ for no longer than ten days.
2.2.2. Nitric oxide (NO) synthesis inhibition
NG-nitro-L-arginine methylester (L-NAME) (Sigma Chemicals St Louis, MO, USA), a nitric oxide synthase (NOS) inhibitor was administered in the drinking water at a dose calculated to provide 50 mg/kg/day to rats. This was administered in light-proof bottles for a period of 4 weeks. It was used to determine the role of NO synthesis in nicotine induced infertility.
2.3. Sperm characteristics analysis
The left testis was removed along with its epididymis. The caudal epididymis was separated from the testis and lacerated to collect the semen with a microscope slide for semen characteristics evaluation as previously described[12].
Progressive motility was tested immediately. Semen was squeezed on a pre-warmed slide, two drops of warm 2.9% sodium citrate was added to it. This was then covered with a cover slip, examined and scored under the microscope using ×40 objective with reduced light[13]. A viability study (percentage of live spermatozoa) was done using eosin/nigrosin stain. Semen was squeezed onto a microscope slide and two drops of the stain were added. The motile (live) sperm cells were unstained while the non-motile (dead) sperms absorbed the stain. The stained and the unstained sperm cells were counted using ×40 microscope objectives and an average value for each was recorded from which percentage viability was calculated. Sperm morphology was evaluated by staining the sperm smears on microscope slides with two drops of Walls and Ewas stain after they were air dried. The slides were examined under the microscope under oil immersion with ×100 objective. The abnormal sperm cells were counted and the percentage calculated according to the method described by Wyrobek and Bruce[14]. The epididymis was immersed in 5 mL normal saline in a measuring cylinder and the volume displaced was taken as the volume of the epididymis. Sperm count was done under a microscope with the aid of the improved Neubauer hemocytometer. Counting was done in five Thoma chambers[15].
2.4. Libido test
To observe the libido-oriented mounting behaviour, non-estrous untreated female rats were paired on the 30th day at 6.00 pm. The male rats assuming the copulatory position over the female rats, but failing to achieve intromission was considered as a mount[16]. Male rats from each group were chosen and suitably marked. The rats were placed in a clear aquarium and were allowed to acclimatize for 15 minutes. Afterwards a non-estrous female rat was introduced intothe arena. The number of mounts was recorded for 15 minutes. This process was also done for the recovery groups.
2.5. Fertility studies
A total of thirty untreated fertile, prestrous female rats were used for the fertility test. Five untreated female rats were cohabited with a male rat from one of the six male groups on the 31st day of treatment. All animals were cohabited for 5 days according to earlier studies[16]. The presence of a vaginal plug was accepted as the index for a positive mating and it was taken as day one of pregnancy[17]. A fertility test was calculated using the following formula:
The number of litters delivered and their body weights were determined.
2.6. Statistical analysis
Data are expressed as means ± S.E.M. for each group. One-way analysis of variance (ANOVA) was used to analyze for significance of difference between means followed by post hoc Duncan’s multiple range test. Statistical significance was assigned to aP-value of less than 0.05.
3. Results
3.1. Effect of nicotine and L-name on semen characteristics
Administration of 0.5 mg/kg B.W and 1.0 mg/kg B.W of nicotine significantly decreased (P<0.05) the progressive motility of the sperm when compared with the control group. L-NAME treatment abrogated these alterations as shown in Figure 1. The mean epididymal sperm count of rats administered with 0.5 mg/kg B.W and 1.0 mg/kg B.W of nicotine was significantly decreased (P<0.05) when compared with their control. Co-administration of L-NAME abrogated these alterations. However, the L-NAME treated group had a significant decrease (P<0.05) in the mean sperm count when values were compared with the control as shown in Figure 2. A significant decrease (P>0.05) was recorded for the mean percentage live sperm of rats treated with both 0.5 mg/kg B.W, 1.0 mg/kg B.W of nicotine and L-NAME treated groups when compared with the control. This decrease is dose-related in the nicotine treated group. However, coadministration of L-NAME abrogated the alterations the effect observed in nicotine only treated group as shown in Figure 3.
The result showed that sperm volume was comparable in all experimental group as shown in Figure 4.
The most common abnormality encountered during the morphological examination of the sperms in the rats that received the two doses (i.e. 0.5 mg/kg B.W and 1.0 mg/kg B.W) of nicotine was the “curve tail”. Though there seems to be a dose-related morphological abnormality. 0.5 mg/kg B.W nicotine + L-NAME and1.0 mg/kg B.W nicotine + L-NAME showed a fewer occurrence of the morphological aberration as recorded in the Table 1.
3.2. Effect of nicotine and L-NAME on male fertility
3.2.1. Percentage fertility
The female rats used in mating male albino rats that had no nicotine treatment had 100% fertility rate while L-NAME, 0.5 mg/kg B.W and 1.0 mg/kg B.W of nicotine treated rats had 60%, 50% and 0% fertility rate respectively. However, 0.5 mg/kg B.W nicotine + L-NAME and 1.0 mg/kg B.W + L-NAME had a fertility rate of 80% and 70% respectively. Female rats cohabited with male rats from the high-dose group did not conceive throughout the period of the study as shown in Table 2.
3.2.2. Litter weight
Female rats used in mating the male albino rats that did not receive nicotine gave an average litter weight of 6.22±0.10. This served as the control. An average of 4.36±0.10 and 5.30±0.10 litter weight was delivered by female rats used to mate male rats that received L-NAME and 0.5 mg/kg B.W (low dose) of nicotine respectively. 0.5 mg/kg B.W nicotine + L-NAME and 1.0 mg/kg B.W nicotine + L-NAME treated rats had 6.47±0.60 and 5.05±0.48 litter weight respectively as shown in Table 2.
3.2.3. Litter number
Female rats used in mating the male albino rats that did not receive nicotine had an average litter number of 7.00±0.40. This group served as the control.
An average litter number of 4.88±0.50 and 4.86±0.50 were produced by female used in mating the L-NAME and 0.5 mg/kg B.W nicotine treated animals respectively. 0.5 mg/kg B.W nicotine+ L-NAME and 1.0 mg/kg B.W nicotine + L-NAME B.W treated rats had an average litter number of 7.20±0.38 and 7.85±0.40 respectively as shown in Table 2.

Table 1 Effect of Nicotine and L-NAME on morphological characteristics of sperm in rats.
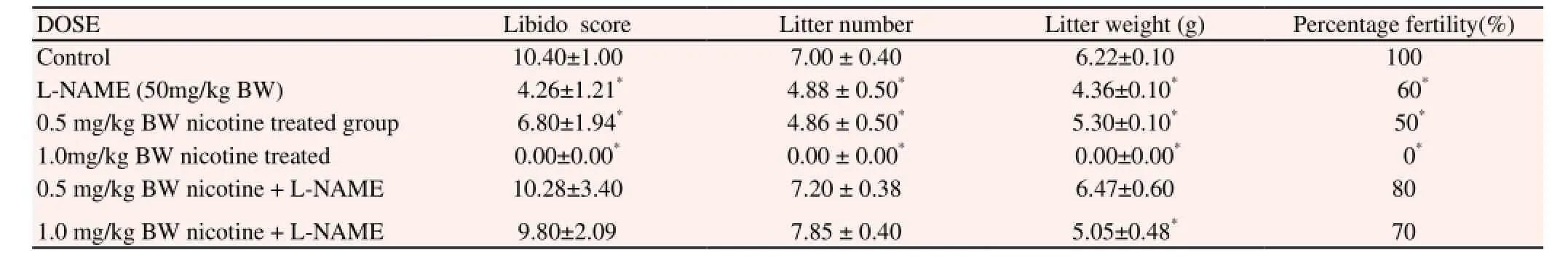
Table 2 Fertility profile of experimental rats treated with nicotine and L-NAME.
4. Discussion
This present study has demonstrated profound restoration of the adverse reproductive effects of nicotine on male reproductive competence with co- administered of NOS inhibitor; L-NAME in rats. To date, this is the first investigation for a relationship between the effects of nitric oxide synthase inhibitor (L-NAME) and nicotine on epididymal sperm count, motility and morphology in male rats. Previous studies have documented the presence of NO synthase within the epididymis and testis which includes the Leydig cells and Sertoli cells[19, 20]. The dose use to inhibit NO synthase (50 mg/kg/day) in this study has been shown to inhibit NO formation for previous reproductive studies[21, 22].
Nicotine treatment has been associated with reduced sperm function, reproductive organ weight and testosterone subsequentlyleading to elevated infertility in earlier studies[23, 24].
During the study, there were no signs of lethargy, motor noncoordination, behavioural abnormalities or toxicity in the L-NAME treated rats as observed in the nicotine treated animals. The reversibility of the antifertility effects of nicotine, coupled with the increase in testosterone earlier published [24] showed that L-NAME was acting through androgen levels increase. Thus, in the present experiments, L-NAME was probably acting by promoting testosterone synthesis and secretion probably through inhibiting the formation of NO in the reproductive organs. This result agrees with previous studies that observed increased testosterone secretion with L-NAME administration[25, 26]. The results of this study also indicate that the synthesis of NO through NOS may play a role in the regulation of the serum levels of testosterone.
It was also observed that animals treated with nicotine had decreased sperm motility, viability, count and increased abnormalities in a concentration-related manner as compared to untreated controls. A significantly higher sperm motility as well as viability was maintained in animals co-administered with nicotine and L-NAME when compared with nicotine treated group thus production of very low amounts of NO in the testicular tissue appears to play a physiological role in regulating normal sperm functions, whereas as high levels of NO endanger sperm function and viability. This result is in consonance with previous studies that observed increased sperm parameters with L-NAME incubation in-vitro[10] and others that reported that NO at supra physiological levels is harmful for both testicular and sperm function[27, 28]. Previous studies have also implicated nicotine with increase testicular NO levels[29]
Rats co-treated with nicotine and L-NAME had an improved libido when compared with the nicotine treated animals as measured by sexual and mounting behavior. The rapid onset of this effect suggests an action on the central nervous system. There is evidence that NO is important in the control of male sexual behaviour via its action in the hypothalamus[11, 30]. Similarly, the increased testosterone level previously observed by co- treating L-NAME with nicotine could also account for the improved libido since testosterone has been associated with increased sexuality, physical and mental energy, stamina and vitality [31]. However, animals treated with L-NAME only showed profound reduced male reproductive activities as evident by reduced fertility profile caused by significant adverse effect on sperm count, motility or morphology. This indicates that NO plays a significant role in male reproduction but its excess can be detrimental to fertility. This result is in consonance with previous studies[22]. Similarly, there is evidence that NO is important in the maturation of spermatozoa [32] and, therefore, L-NAME treatment alone could reduce the fertilizing ability of spermatozoa in consequence.
The decrease in the average litter number delivered by the untreated female rats mated with the nicotine treated male was not observed in the L-NAME co-treated nicotine group. This might be due to the effect of L-NAME to restore progressive epididymal sperm motility. It was observed that percentage fertility, litter weight and number was significantly increased showing an index of improved fertility in the L-NAME intervention group. The results of this investigation are in accordance with other studies in which increase in NOS activity were found in infertile patients[28, 33].
It is worth noting that there was a better restoration of fertility profile in animals in the 0.5 mg/kg BW nicotine treated group coadminister with L-NAME compared with 1.0mg/kg BW nicotine group co-administered with L-NAME.
In conclusion, our data confirms the adverse reproductive effect of nicotine on sperm cells and suggested that NO is an important mediator in the pathogenesis of infertility with nicotine treatment. NOS inhibitor and perhaps L-NAME could be useful in prevention of nicotine induced infertility in smokers.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Acknowledgement
The authors are grateful to the Education Trust fund (TETfund) Nigeria for funding this research.
[1] Marletta MA. Nitric oxide synthase structure and mechanism.J Biol Chem1993; 268: 12231-12234.
[2] Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of antioxidants in treatment of male infertility: an overview of the literature.Reprod Biomed Online2004; 8: 616-27.
[3] Ignarro LJ, Bush PA, Buga GM, Wood KS, Fukuto JM, Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle.Biochem Biophys Res Commun1990; 170: 843–850.
[4] Vanhatalo S, Klinge E, Sjostrand NO, Soinila S. Nitric oxidesynthesizing neurons originating at several different levels innervate rat penis.Neuroscience1996; 75: 891–899.
[5] Garban H, Arquez D, Cai L, Rajfer J, Gonzalez-Cadavid NF. Restoration of normal adult penile erection response in aged rats by-long term treatment with androgens.Biol Reprod1995; 53: 1365–1372.
[6] Penson DF, Ng C, Cai L, Rajfer J, Gonzalez-Cadavid NF. Androgen andpituitary control penile nitric oxide synthase and erectile function in rat.Biol Reprod1996; 55: 567–574.
[7] Lugg J, Ng C, Rajfer J, Gonzalez-Cadavid N. Cavernosal nerve stimulation in the rat reverses castration-induced decrease in penile NOS activity.Am J Physiol1996; 271: E354–E361.
[8] Buhimschi I, Ali M, Jain V, Chwalisz K, Garfield RE. Differential regulation of nitric oxide in the rat uterus and cervix during pregnancy and labour.Hum Reprod1996; 11(8): 1755-1766.
[9] Lewis SE, Donnelly ET, Sterling ES, Kennedy MS, Thompson W, Chakravarthy U. Nitric oxide synthase and nitrite production by human sperm: evidence that endogenous nitric oxide is beneficial to sperm motility.Mol Hum Reprod1996; 2: 873–878.
[10] Rosselli M, Dubey RK, Imthurn B. Effect of nitric oxide on human spermatozoa: evidence that nitric oxide decreases sperm motility and induces sperm toxicity.Hum Reprod1995; 10: 1786–1970.
[11] Oyeyipo IP, Raji Y, Bolarinwa AF. Nicotine alters serum antioxidant profile in male albino rats.North Am J Med Sci2014; 6(4): 168-71.
[12] Raji Y, Udoh US, Mewoyeka OO, Ononye FC, Bolarinwa AF. Implication of reproductive endocrine malfunction in male antifertility efficacy of Azadirachta indica extract in rats.Afr J Med Med Sci2003; 32(2): 159-165.
[13] Morrissey RE, Schwetz BA, Lamb JC, Ross MD, Teague JL, Morris RW. Evaluation of rodent sperm, vaginal cytology, and reproductive organ weight data from National Toxicology Program 13-week studies.Fundam Appl Toxicol1988; 11(2): 343-358.
[14] Wyrobek AJ, Bruce WR. The induction of sperm shape abnormalities in mice and humans. In: Hollaender A, De Serres FJ.Chemical mutagens. Vol. 5. New York: Plenum Press; 1980. p. 257-85.
[15] Freund M, Carol B. Factors affecting haemocytometer count of sperm concentration in human semen.J Reprod Fertil1964; 8: 149-155.
[16] Dhawan K, Sharma A. Prevention of chronic alcohol and nicotineinduced azospermia, sterility and decreased libido, by a novel trisubstituted benzoflavone moiety fromPassiflora incarnataLinneaus in healthy male rats.Life Sci2002; 71(26): 3059-30 69.
[17] Bolarinwa Y. Gastric acid secretion in pregnant and lactating rat.Trop Vet1993; 11: 35-38.
[18] Raji, Y, Oloyo AK, Morakinyo AO. Studies in the reproduction activities of method and extract ofRicinus communisseed in male albino rats.Asian J Androl2006; 8: 115 – 121.
[19] Burnett AL, Ricker DD, Chamness SL, Maguire MP, Crone JK, Bredt DS, et al. Localization of nitric oxide synthase in the reproductive organs of the male rat.Biol Reprod1995; 52: 1-7.
[20] Zini A, O’Bryan MK, Magid MS, Schlegel PN. Immunohistochemical localization of endothelial nitric oxide synthase in human testis, epididymis and vas deferens suggest a possible role for nitric oxide in spermatogenesis, sperm maturation and programmed cell death.Biol Reprod1996; 55: 935-941
[21] Arnal JF, Battle JT, Menard J, Michel B. The vasodilatatory effect of endogenous nitric oxide is a major counterregulatory mechanism in the hypertensive rats.J Hypertens1993; 11: 945–950.
[22] Ratnasooriya WD, Dharmasiri MG, Wadsworth RM. Reduction in libido and fertility of male rats by administration of the nitric oxide (NO) synthase inhibitor N-nitro-l-arginine methyl ester.Int J Androl2000; 23: 187-191.
[23] Oyeyipo IP, Raji Y, Emikpe BO, Bolarinwa AF. Effects of oral administration of nicotine on organ weight, serum testosterone level and testicular pathology in adult male rats.Niger J Physiol Sci2010; 25 (1): 81-86.
[24] Oyeyipo IP, Raji Y, Bolarinwa AF. NG-nitro-?-arginine methylester protects against hormonal imbalances associated with nicotine administration in male rats.North Am J Med Sci2015; 7(3): 59-64.
[25] Sharma AC, Lee LY, Hales DB, Law WR, Ferguson JL, Bosmann HB. Effect of NG-nitro-L-arginine methyl ester on testicular blood flow and serum steroid hormones during sepsis.Shock1998; 9(6): 416-21.
[26] Pinilla L, González LC, Tena-Sempere M, Bellido C, Aguilar E. Effects of systemic blockade of nitric oxide synthases on pulsatile LH, prolactin, and GH secretion in adult male rats.Horm Res2001; 55(5): 229-235.
[27] Kisa U, Basar MM, Ferhat M, Yilmaz E, Basar H. Testicular tissue nitric oxide and thiobarbituric acid reactive substance levels: evaluation with respect to the pathogenesis of varicocele.Urol Res2004; 2(3):196-199.
[28] Stefani S. De, Silingardi V, Micali S, Mofferdin A. Experimental varicocele in the rat: early evaluation of the nitric oxide levels and histological alterations in the testicular tissue.Andrologia2005; 37: 115-118.
[29] Oyeyipo IP, RajiY, Bolarinwa AF. Antioxidant profile changes in reproductive tissues of rats treated with nicotine.J Hum Reprod Sci2014; 7: 41-46.
[30] Mellis MR, Stancampiano R, Argiolas A. Role of nitric oxide in penile erection and yawning induced by 5-HT1C receptor agonists in male rats.N-S Arch Pharmacol1995; 351: 439-445
[31] Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens.Endocr Rev1987; 8: 1 – 28.
[32] Yeoman RR, Jones WD, Rizk BM. Evidence for nitric oxide regulation of hamster sperm hyperactivation.J Androl1998; 19: 58-64.
[33] Romeo C, Ientile R, Santoro P, Impellizzeri P, Turiaco N, Impala P, et al. Nitric oxide is increased in the spermatic vein of adolescents with left idiopathic varicocele.J Pediatr Surg2001; 36: 389-393.
10.1016/j.apjr.2015.06.004
*Corresponding author: Dr. Oyeyipo I.P., Department of Physiology, College of Health Sciences, Osun State University, Osun State, Nigeria.
Tel.: +234-803-414-6150
E-mail:greatibuks@yahoo.com
Foundation project: This study was funded by the Education Trust fund (TETfund) Nigeria.
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Reviewing reports of semen volume and male aging of last 33 years: From 1980 through 2013
- In vitro embryo outgrowth is a bioassay of in vivo embryo implantation and development
- Diet–induced obesity alters kinematics of rat spermatozoa
- Infant mortality in twin pregnancies following in-utero demise of the co-twin
- The preparation and culture of washed human sperm: A comparison of a suite of protein-free media with media containing human serum albumin
- Lauric acid abolishes interferon-gamma (IFN-γ)-induction of Intercellular Adhesion Molecule-1 (ICAM-1) and Vascular Cell Adhesion Molecule-1 (VCAM-1) expression in human macrophages
