Elastic modulus aff ects the growth and diff erentiation of neural stem cells
2015-12-15XianfengJiangKaiYangXiaoqingYangYingfuLiuYuanchiChengXuyiChenYueTu
Xian-feng Jiang, Kai Yang, Xiao-qing Yang, Ying-fu Liu, Yuan-chi Cheng Xu-yi Chen, Yue Tu
1 Affi liated Hospital of Logistics University of People’s Armed Police Force, Tianjin, China
2 Liaoning Medical University, Jinzhou, Liaoning Province, China
3 Logistics University of People’s Armed Police Force, Tianjin, China
Elastic modulus aff ects the growth and diff erentiation of neural stem cells
Xian-feng Jiang1,#, Kai Yang1,2,#, Xiao-qing Yang1,#, Ying-fu Liu3, Yuan-chi Cheng1,2, Xu-yi Chen1,*, Yue Tu1,*
1 Affi liated Hospital of Logistics University of People’s Armed Police Force, Tianjin, China
2 Liaoning Medical University, Jinzhou, Liaoning Province, China
3 Logistics University of People’s Armed Police Force, Tianjin, China
It remains poorly understood if carrier hardness, elastic modulus, and contact area aff ect neural stem cell growth and diff erentiation. Tensile tests show that the elastic moduli of Tiansu and SMI silicone membranes are lower than that of an ordinary dish, while the elastic modulus of SMI silicone membrane is lower than that of Tiansu silicone membrane. Neural stem cells from the cerebral cortex of embryonic day 16 Sprague-Dawley rats were seeded onto ordinary dishes as well as Tiansu silicone membrane and SMI silicone membrane. Light microscopy showed that neural stem cells on all three carriers show improved adherence. After 7 days of diff erentiation, neuron specifi c enolase, glial fi brillary acidic protein, and myelin basic protein expression was detected by immunofl uorescence. Moreover, fl ow cytometry revealed a higher rate of neural stem cell diff erentiation into astrocytes on Tiansu and SMI silicone membranes than on the ordinary dish, which was also higher on the SMI than the Tiansu silicone membrane. These fi ndings confi rm that all three cell carrier types have good biocompatibility, while SMI and Tiansu silicone membranes exhibit good mechanical homogenization. Thus, elastic modulus aff ects neural stem cell diff erentiation into various nerve cells. Within a certain range, a smaller elastic modulus results in a more obvious trend of cell diff erentiation into astrocytes.
nerve regeneration; neural stem cells; carrier; mechanical properties; elastic modulus; cell diff erentiation; neurons; immunofl uorescence; astrocytes; neural regeneration
Funding: This study was supported by the National Natural Science Foundation (Youth Project) of China, No. 11102235; a grant from the Key Project of Tianjin Science and Technology Support Plan in China, No. 14ZCZDGX00500; the Key Project of Natural Science Foundation of Tianjin City of China, No. 12JCZDJC24100; the Science and Technology Foundation Project of Tianjin Municipal Health Bureau of China, No. 2013KZ134, 2014KZ135; the Seed Foundation Project of Affi liated Hospital of Logistics University of People’s Armed Police Force of China, No. FYM201432.
Jiang XF, Yang K, Yang XQ, Liu YF, Cheng YC, Chen XY, Tu Y (2015) Elastic modulus aff ects the growth and diff erentiation of neural stem cells. Neural Regen Res 10(9):1523-1527.
Introduction
Neural stem cells (NSCs) can differentiate into neurons and glia cells, and NSC transplantation promotes recovery of neurological function following traumatic brain injury and spinal cord injury (Ma et al., 2004; Caprini et al., 2013). A number of extracellular factors involving the biological and physical environment impact upon proliferation, differentiation, maturity, and turnover of NSCs (McBeath et al., 2004; Wrage et al., 2008; Evans et al., 2009). Biochemical approaches combined with growth factors have limitations in inducing directional differentiation of stem cells e.g., diff erentiated cells do not have all the characteristics of mature neurons, a low survival rate in vivo, and uncertainty in the type of diff erentiated cells (Cattaneo and McKay, 1990; Wrage et al., 2008; Qian et al., 2010). The physical microenvironment is strongly associated with proliferation and differentiation of NSCs (Engler et al., 2006; Guilak et al., 2009; Dado et al., 2012). Substrate height can be altered using three-dimensional nanofi ber meshes and induce neuronal differentiation of NSCs (Kshitiz et al., 2012). Elastic modulus of the extracellular matrix (ranging from 1–100 Pa) noticeably contributes to expression of tubulin III and 18S ribosomal RNA in NSCs. Moreover, the diff erentiation rate is decreased with an increase in elastic modulus. Previous studies have shown that migration, proliferation and diff erentiation of NSCs is suppressed with a substrate strength of 10 Pa. In addition, with substrate strengths of 100–500 Pa, NSCs diff erentiate into neurons, while at 1,000–10,000 Pa, they diff erentiate into glial cells (Engler et al., 2006; Guilak et al., 2009; Dado et al., 2012). The elastic modulus is an important parameter of engineering materials, and specifi cally, a number that measures an object or substance’s resistance to being deformed elastically, which is slightly aff ected by the external environment. The elastic modulus can aff ect cell proliferation and the direction of diff erentiation (Banerjee et al., 2009), but current studies have only investigated the
eff ect of high elastic modulus on cells, with controversial results (Engler et al., 2006; Guilak et al., 2009; Dado et al., 2012). Indeed, there are no detailed studies regarding the eff ect of low elastic modulus on growth and diff erentiation of NSCs. Therefore, in this study, we sought to compare the eff ect of diff erent materials with low elastic modulus on the growth and diff erentiation of NSCs, and to further improve directional diff erentiation of NSCs, investigated the eff ect and subsequent mechanisms of physical factors on proliferation and diff erentiation of NSCs.
Materials and Methods
Determination of mechanical properties
To measure the mechanical properties of Tiansu silicone membrane (Tianjin Plastics Research Institute, Tianjin, China) and SMI silicone membrane (SMI, Denver, CO, USA), tensile tests were performed using a mechanical tester (INSTRON 5865; Instron, Boston, MA, USA) (Figure 1). Silicone membrane thickness was measured. The tensile parameters were: length of stretching, 50 mm; stretching rate, 10 mm/s; preload, 0.1 N; and maximum compressive strain, 2,000%; for a total of 3 cycles. Stress-strain curves were generated, and elastic moduli of both silicone membranes were obtained. Before experiments, both silicone membranes received Cobalt-60 irradiation for sterilization, and were then cut into circles of 5 cm diameters and fi xed in autoclaved culture chambers with black aprons to prevent air leakage.
NSC culture
Embryonic day 16 (E16) Sprague-Dawley rats were provided by the Experimental Animal Center of Academy of Military Medical Sciences in China (license No. SCXK (Army) 2012-0004). Experiments were approved by the Ethics Committee of Affiliated Hospital of Logistics University of People’s Armed Police Force in China. Rats were immersed and sacrifi ced in 75% ethanol. Fetal rat cerebral cortex was cut into approximately 1 mm3blocks, triturated, fi ltered with a 200-mesh sieve, and centrifuged at 100 × g for 5 minutes. After removal of the supernatant, samples were precipitated and digested with 0.25% trypsin. Digestion was terminated by addition of 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA). Following centrifugation and supernatant removal, cells were resuspended in serum-free Dulbecco’s modifi ed Eagle’s medium (DMEM)/ F12 medium (Gibco), single cell suspensions were obtained and seeded in 25 cm2culture dishes, and then incubated at 37°C. Once every 3 days, half the medium was replaced. Primary cultures were maintained for approximately 6 days. After supernatant removal, cultured NSCs were collected, centrifuged in sterile centrifuge tubes, digested with 0.25% trypsin, and terminated with 10% FBS. Following centrifugation and supernatant removal, fresh medium was added for agitation, resuspension, and quantitation. All samples were then incubated in 25 cm2culture fl asks, with half the medium replaced once every 3 days. Cells were subcultured approximately once every 6 days. Third passage NSCs (1 × 105cells/mL) were incubated on diff erent carriers: ordinary dish (Corning, Shanghai, China) (control group), Tiansu silicone membrane (Tiansu silicone membrane group), and SMI silicone membrane (SMI silicone membrane group). Twenty-four hours later, DMEM/F12 medium containing 10% FBS was replaced by serum-free DMEM/F12 medium with B27, recombinant human epidermal growth factor, and recombinant human basic fibroblast growth factor (Gibco).
Biocompatibility of silicone membranes
NSCs received mechanical separation and enzymatic digestion, and single cell suspensions were prepared. Three days later, cell morphology was observed. Cells were subcultured approximately every 6 days. Third passage NSCs were incubated in DMEM/F12 medium supplemented with 10% FBS, which promotes adherence and diff erentiation of NSCs. At 24 hours and 7 days after culture, alterations in cell morphology were observed using an inverted phase contrast microscope (Olympus, Tokyo, Japan).
Immunofl uorescence staining
Immunofl uorescence staining for neuron specifi c enolase (NSE), glial fibrillary acidic protein (GFAP), and myelin basic protein (MBP) was performed after 7 days of adherent growth. Cells from each group were fi xed, permeabilized, blocked with bovine serum albumin, and incubated with rabbit anti-rat NSE monoclonal antibody (1:150; Pharmingen, San Diego, CA, USA), rabbit anti-rat GFAP monoclonal antibody (1:1,000; Pharmingen), and rabbit anti-rat MBP monoclonal antibody (1:100; Abcam, Cambridgeshire, UK) at 4°C overnight, and then incubated with green fl uorescent protein-labeled goat anti-rabbit secondary antibody (1:200; Sigma, San Francisco, CA, USA) at 37°C for one hour. 4′,6-Diamidino-2-phenylindole (DAPI) dye was used for nuclear staining. Coverslips were mounted using antifade fl uorescence mounting medium. Specimens were observed using an inverted fl uorescence microscope (Leica, Solms, Germany). Images were processed using Image-Pro Plus 7.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Flow cytometry
Cells from each group were induced for 48 hours. Cells at a density of 3 × 106cells/cm2were seeded onto 6-well plates, digested, centrifuged, and fi xed. After membrane disruption, cells were placed in tubes, and incubated with goat anti-rat GFAP antibody (1:500; Sigma) at 4°C overnight. The rate of NSC diff erentiation into astrocytes was determined by fl ow cytometery (BD, Frankfort, NJ, USA).
Statistical analysis
Data were processed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Measurement data are expressed as the mean ± SD. Intergroup comparison was performed by oneway analysis of variance and independent-samples t-test. P values < 0.05 were considered statistically signifi cant.
Results
Mechanical properties of Tiansu and SMI silicone membranes
The ordinary dish was made of polystyrene, with an elastic modulus ranging from 3,000–3,600 MPa (Wang et al., 2007). The elastic moduli of Tiansu silicone membrane and SMI silicone membrane were 4.255 ± 0.344 and 2.256 ± 0.096 MPa, respectively. Stress-strain curves showed that Tiansu and SMI silicone membranes behaved according to Hooke’s law (i.e., a linear relationship between stress and strain). Moreover, elastic modulus variability was smaller in the SMI silicone membrane group than in the Tiansu silicone membrane group. SMI silicone membrane exhibited good stability (Figure 2).
Biocompatibility of Tiansu and SMI silicone membranes
After 3 days of culture, inverted phase contrast microscopy showed that in the control group, NSCs proliferated and formed neurospheres containing tens to hundreds of cells. Twenty-four hours after adherent differentiation, NSCs proliferated and formed neurospheres. Gradually, cells extended from the edge of neurospheres and formed dendritic processes. Neurospheres connected with each other through processes. Compared with the control group, many regular processes were visible between neurospheres in both the Tiansu and SMI silicone membrane groups. After 7 days of adherent growth, NSCs diff erentiated into nerve cells with diff erent shapes (Figure 3).
Eff ect of Tiansu and SMI silicone membranes on NSC diff erentiation
NSE, GFAP, and MBP are specifi c immune markers for neurons, astrocytes, and oligodendrocytes, respectively (Sommer and Schachner, 1981; Noetzel and Agrawal, 1985; Sterk et al., 1999). After 7 days of culture, immunofl uorescence expression of NSE, GFAP, and MBP was detected in cells from each group, thereby demonstrating NSC diff erentiaton (Figure 4). Flow cytometery showed a signifi cantly higher rate of NSC diff erentiation into astrocytes after 7 days of adherent culture in the Tiansu and SMI silicone membrane groups compared with the control group (P < 0.05, P < 0.01). Moreover, the rate of cell diff erentiation was greater in the SMI silicone membrane group than in the Tiansu silicone membrane group (P < 0.01; Figure 5).
Discussion
In neural tissue engineering, adhesion, migration, proliferation, and diff erentiation of stem cells are aff ected by physical and chemical factors and carrier surface properties (Chen et al., 2012; Lu et al., 2012). As cell carriers, tissue-engineered scaff olds provide a three-dimensional environment for nerve cells and aff ect cell turnover (Chen et al., 2013). The eff ects of elastic modulus, as inherent physical scaff old properties, have been gradually attracting the attention of scientists.
In this study, NSCs from the cerebral cortex of fetal Sprague-Dawley rats were incubated on carriers with diff erent elastic moduli, and found to adhere to diff erent carriers. Twenty-four hours after adherence, NSCs proliferated and formed neurospheres. Cells then gradually extended from the edge of neurospheres and formed dendritic processes. After 7 days of adherent growth, immunofl uorescence detected specifi c immune markers for neurons, astrocytes, and oligodendrocytes. Morevoer, fl ow cytometry found that the majority of NSCs diff erentiated into astrocytes. These fi ndings show that NSCs can grow and diff erentiate on three different carriers with different elastic moduli. Thus, the cultured NSCs are pluripotent, with both types of silicone membrane exhibiting good biocompatibility.
Carrier micromorphology has a guiding role on cell growth and impacts upon biological properties of the cells (Noetzel and Agrawal, 1985). The present results showed that the elastic modulus of SMI silicone membrane was smaller and more stable than that of Tiansu silicone membrane, which indicates that SMI silicone membrane has good mechanical deformation characteristics with a uniform nature. After 7 days of adherent diff erentiation, the fl ow cytometry results suggest that elastic modulus aff ects NSC diff erentiation into astrocytes. Within a certain range, a smaller elastic modulus results in a more obvious trend of cell diff erentiation into astrocytes.
The extracellular matrix-integrin-cytoskeleton-nuclear matrix system is an important mechanical signaling pathway infl uencing cell turnover, while cell fl exibility determines the sensitivity of cells to force (Wang et al., 2010). Diff erent cells have diff erent fl exibilities. Within a certain range, during cell adherence, cells on carriers with small elastic modulus are easily deformed and a great tension force produced. Astrocyte fl exibility may make them relatively sensitive to tension force (within a certain range), which indirectly indicates that cells on silicone membrane with a small elastic modulus are deformed, and a large local tension force generated. Thus, the direction of NSC diff erentiation into astrocytes can be aff ected, but these fi ndings still be confi rmed by a larger number of studies.
Author contributions: XFJ ensured the integrity of the data and participated in statistical analysis. KY wrote the paper and performed a part of the cell experiments. XQY performed experiments and provided the data. YT was in charge of study concept and design. XYC obtained funding, provided material support, and served as a principle investigator. YFL took responsibility for cell culture and immunofl uorescence. YCC was responsible for mechanics of materials and biocompatibility testing. All authors approved the fi nal version of the paper. Confl icts of interest: None declared.
Banerjee A, Arha M, Choudhary S, Ashton RS, Bhatia SR, Schaff er DV, Kane RS (2009) The infl uence of hydrogel modulus on the proliferation and diff erentiation of encapsulated neural stem cells. Biomaterials 30:4695-4699.
Caprini A, Silva D, Zanoni I, Cunha C, Volontè C, Vescovi A, Gelain F (2013) A novel bioactive peptide: assessing its activity over murine neural stem cells and its potential for neural tissue engineering. N Biotechnol 30:552-562.
Cattaneo E, McKay R (1990) Proliferation and diff erentiation of neuronal stem cells regulated by nerve growth factor. Nature 347:762-765.
Figure 1 Carriers with diff erent elastic moduli.

Figure 2 Stress-strain curves of Tiansu silicone membrane (A) and SMI silicone membrane (B).
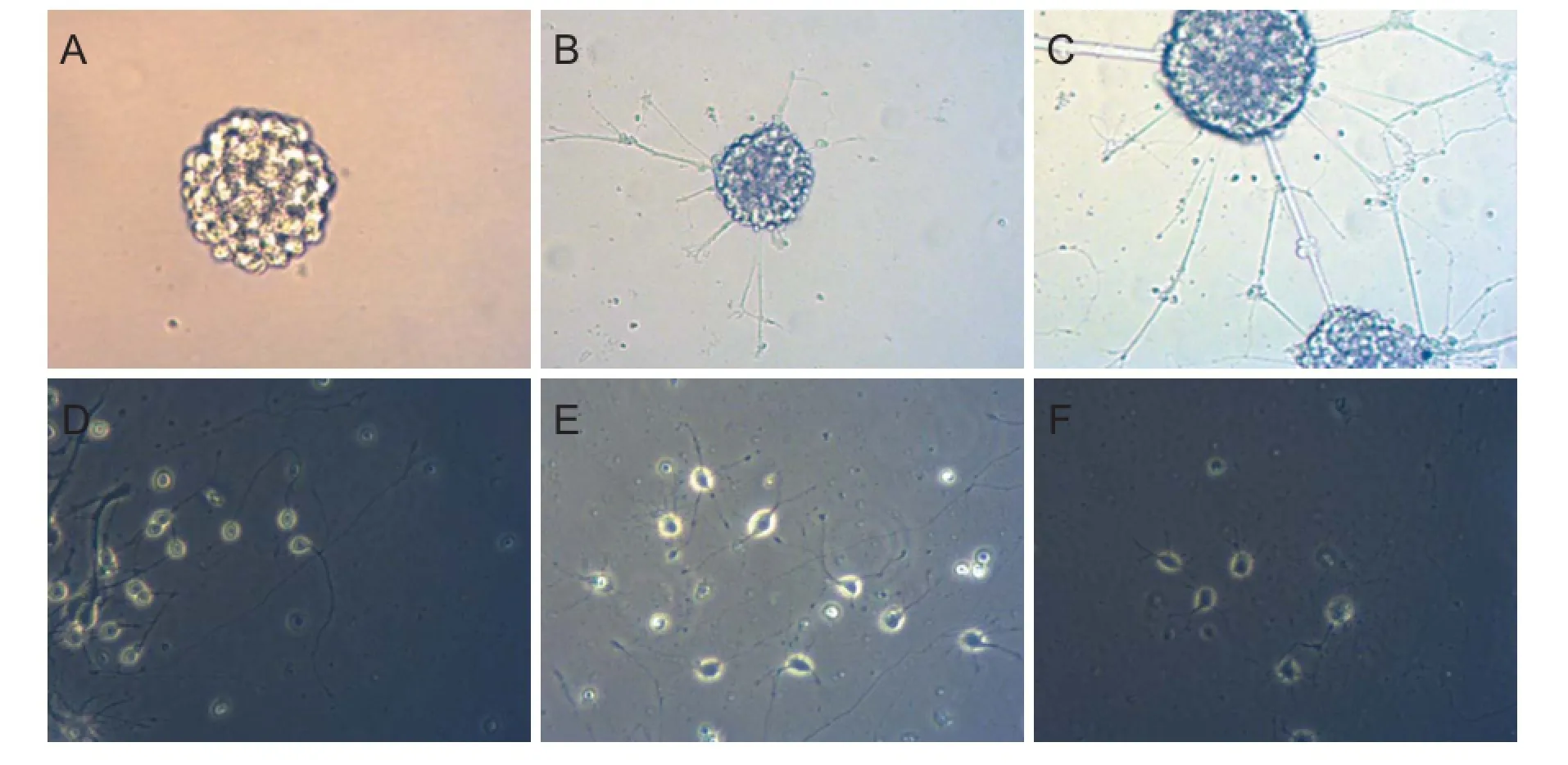
Figure 3 Morphology and diff erentiation of NSCs.
Chen G, Lv Y, Guo P, Lin C, Zhang X, Yang, Xu Z (2013) Matrix mechanics and fl uid shear stress control stem cells fate in three dimensional microenvironment. Curr Stem Cell Res Ther 8:313-323.
Chen J, Irianto J, Inamdar S, Pravincumar P, Lee DA, Bader DL, Knight MM (2012) Cell mechanics, structure, and function are regulated by the stiff ness of the three-dimensional microenvironment. Biophys J 103:1188-1197.
Dado D, Sagi M, Levenberg S, Zemel A (2012) Mechanical control of stem cell diff erentiation. Regen Med 7:101-116.
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specifi cation. Cell 126:677-689.
Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, Chen X, Roberts CJ, Stevens MM (2009) Substrate stiff ness aff ects early diff erentiation events in embryonic stem cells. Eur Cell Mater 18:1-13.
Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS (2009) Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5:17-26.
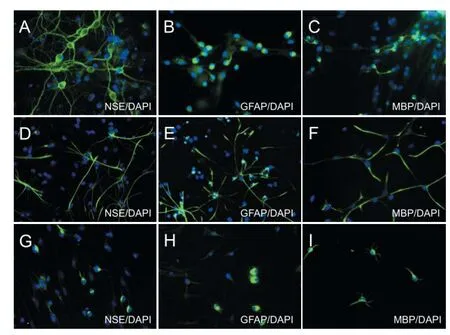
Figure 4 Eff ect of Tiansu and SMI silicone membranes on NSC diff erentiation (immunofl uorescences staining, × 400).
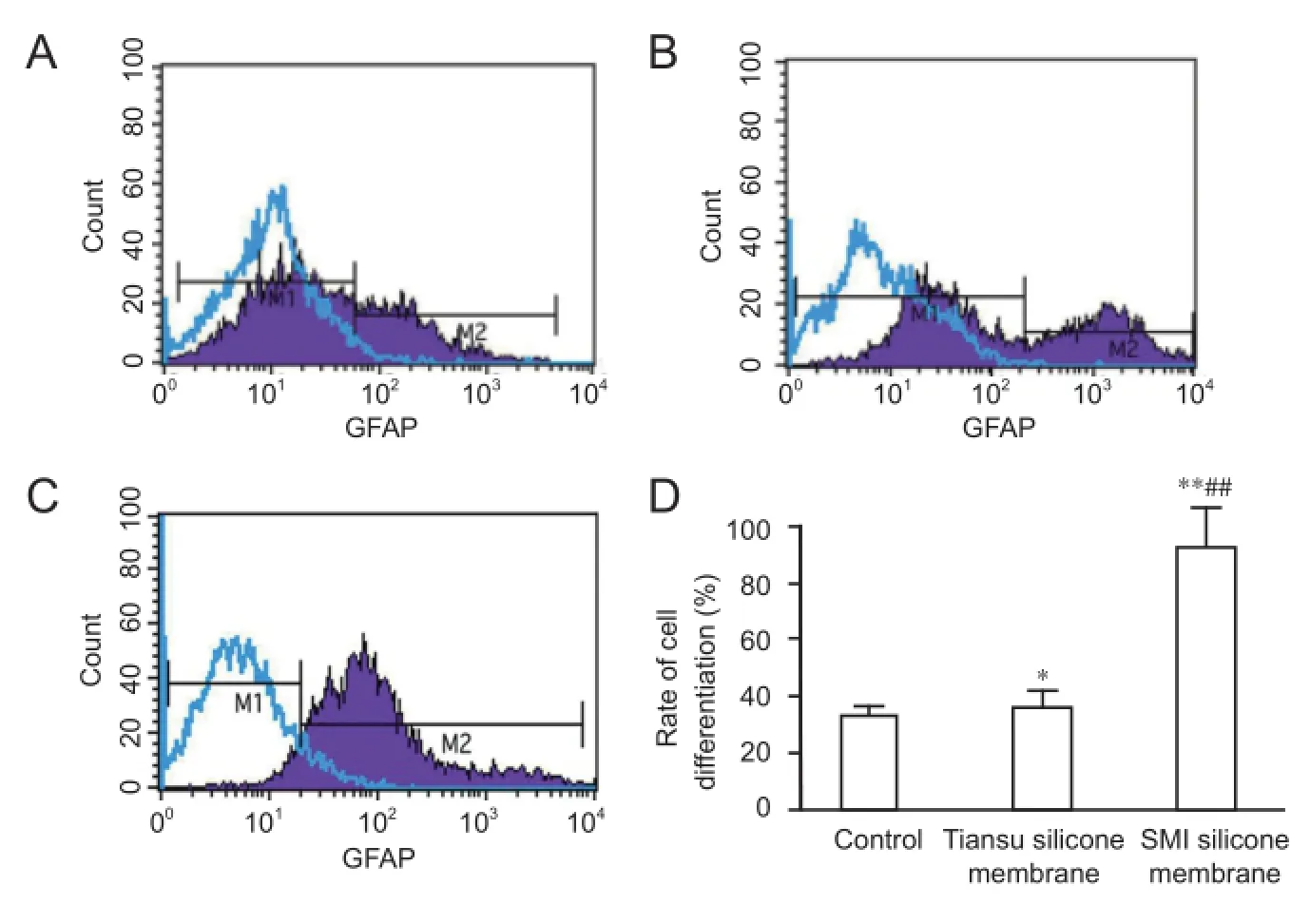
Figure 5 Eff ect of Tiansu and SMI silicone membranes on NSC diff erentiation into astrocytes (fl ow cytometry).
Kshitiz, Park J, Kim P, Helen W, Engler AJ, Levchenko A, Kim DH (2012) Control of stem cell fate and function by engineering physical microenvironments. Integr Biol (Camb) 4:1008-1018.
Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH (2012) Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150:1264-1273. Ma W, Fitzgerald W, Liu QY, O’Shaughnessy TJ, Maric D, Lin HJ, Alkon DL, Barker JL (2004) CNS stem and progenitor cell diff erentiation into functional neuronal circuits in three-dimensional collagen gels. Exp Neurol 190:276-288.
McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS (2004) Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6:483-495.
Noetzel MJ, Agrawal HC (1985) Immunoblot identification of glial fi brillary acidic protein in rat sciatic nerve, brain, and spinal cord during development. Neurochem Res 10:737-753.
Qian DX, Zhang HT, Ma X, Jiang XD, Xu RX (2010) Comparison of the effi ciencies of three neural induction protocols in human adipose stromal cells. Neurochem Res 35:572-579.
Sommer I, Schachner M (1981) Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol 83:311-327.
Sterk M, Oenings A, Eymann E, Roos W (1999) Development of a new automated enzyme immunoassay for the determination of neuron-specifi c enolase. Anticancer Res 19:2759-2762.
Wang M, Shen JB, Du Q, Xu SX, Li J, Chen GS, Guo SY (2007) Mechanical properties of EPDM/PS alternative multilayer composites. Fuhe Cailiao Xuebao 24:36-43.
Wang X, Xia Y, Liu L, Liu M, Gu N, Guang H, Zhang F (2010) Comparison of MTT assay, fl ow cytometry, and RT-PCR in the evaluation of cytotoxicity of fi ve prosthodontic materials. J Biomed Mater Res B Appl Biomater 95:357-364.
Wrage PC, Tran T, To K, Keefer EW, Ruhn KA, Hong J, Hattangadi S, Treviño I, Tansey MG (2008) The neuro-glial properties of adipose-derived adult stromal (ADAS) cells are not regulated by Notch 1 and are not derived from neural crest lineage. PLoS One 3:e1453.
Copyedited by James R, Frenchman B, Yu J, Qiu Y, Li CH, Song LP, Zhao M
*Correspondence to:
Yue Tu, M.D. or Xu-yi Chen, M.D.,
ytumail@vip.126.com or
chenxuyi1979@126.com.
# These authors contributed equally to this work.
orcid:
0000-0001-5592-4751 (Yue Tu)
0000-0002-0420-8349 (Xu-yi Chen)
10.4103/1673-5374.165527
http://www.nrronline.org/
Accepted: 2015-06-26
杂志排行
中国神经再生研究(英文版)的其它文章
- Lactulose enhances neuroplasticity to improve cognitive function in early hepatic encephalopathy
- Optimal concentration and time window for proliferation and diff erentiation of neural stem cells from embryonic cerebral cortex: 5% oxygen preconditioning for 72 hours
- Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a case report of improvement in relapsing auto-immune optic neuropathy
- Repair of peripheral nerve defects with chemically extracted acellular nerve allografts loaded with neurotrophic factors-transfected bone marrow mesenchymal stem cells
- Polylactic-co-glycolic acid microspheres containing three neurotrophic factors promote sciatic nerve repair after injury
- Transplantation of erythropoietin gene-modifi ed neural stem cells improves the repair of injured spinal cord
