Processes of hydrogeochemical evolution of groundwater in the Guanzhong Basin, China
2015-12-12ZHANGChunchaoWANGWenKeSUNYiboLIXiangquanHOUXinwei
ZHANG Chun-chao, WANG Wen-Ke, SUN Yi-bo, LI Xiang-quan , HOU Xin-wei
1 Institute of Hydrogeology and Environmental Geology, Shijiazhuang 050061, China.
2 School of Environmental Science and Engineering, Chang’an University, Xi’an 710054, China.
3 Key Laboratory of Subsurface Hydrology and Ecology in Arid Areas, Ministry of Education, China.
4 Engineering Research Center for Groundwater and Eco-Environment of Shaanxi Province, China.
Abstract: This paper analyzed regional hydrogeochemical evolution characteristics of groundwater with respect to hydrogeological conditions in the Guanzhong Basin, China.Coefficient variation in the subregion between the Shichuan River and Luo River of the Guanzhong Basin is larger than other subregions, reflecting the more complicated hydrogeological conditions of this subregion. The hydrochemical components and hydrodynamic conditions of this area have distinct horizontal zoning characteristics, and hydrodynamic conditions play a controlling role in the groundwater’s hydrochemistry. The relationship between ions, and between ions and TDS (total dissolved solids) can give an indication of many charteristics of grounwater such as evaporation intensity, ion exchange, and the sources of chemical components. Results indicated that for the coefficient of variation (the coefficient of variation is a statistical measure of the distribution or dispersion of data around mean. This measure is used to analyze the difference of spread in the data relative to the mean value. Coefficient of variation is derived by dividing the standard deviation by the mean), the minimum value of pH parameters is 0.03-0.07, the minimum value of 꿞parameters is 0.24,while the maximum is the 꿝 coefficinet at 1.67. A PHREEQC simulation demonstrated that different simulation paths roughly have the same trend in dissolution and precipitation of minerals. Along the direction of groundwater flow, the predminant precipitation is of calcite and gypsum and the cation exchange of Na+ and Ca2+ in some paths. However, in other paths,the precipitation of calcite and dissolution of gypsum and dolomite are the main actions, as well as the exchange of Mg2+ and Ca2+ in addition to Na+ and Ca2+.
Keywords: Hydrogeochemistry; Groundwater; Guanzhong Basin; Geochemical modelling
Introduction
Groundwater is a valuable resource and has an increasingly important impact on the development of industry, agricultural production and the quality of human life, especially in arid and semi-arid areas (WANG et al. 2006). In recent years, as a consequence of rapid development of industry,agriculture production and the intensification of human activities, groundwater is becoming severely contaminated (Jamshidzadeh Z and Mirbagheri S A, 2011; Sandow M Y et al. 2010),and environmental problems have become increasingly prominent. Fundamentally, in order to understand and solve contaminated groundwater issues, it is necessary to investigate the spatial distribution characteristics and evolutionary mechanisms of hydrogeochemistry. In recent years,cluster analysis, correlation analysis, principal component analysis and the chemical composition analysis of groundwater (Piper Plot) have been used to study the spatial zoning of groundwater hydrogeochemistry (Salifu A et al. 2012; ZHAO Min et al. 2010; LI Jun-xia et al. 2010; José J L et al. 2012; Anwar A E, 2010; Grande J A et al. 2013).Many researchers have found that spatial zoning characteristics are influenced by a combination of natural and human factors (JIANG Gui-hua et al.2009). Similarly, the hydrogeochemistry of a particular groundwater resource, evolves as a signature of its flow path, being influenced by aboveground industrial and agricultural activities in the catchment area (Alahmadi M E and Eifiky A A, 2009). PHREEQC is used by researchers to simulate the processes of hydrogeochemical evolution. Kyu-Youl Sung (2012) investigated the hydrogeochemical evolution of groundwater in the granite layer, and found that the predominant hydrochemical type varies between Cl-Ca during rain events, to HCO3-Ca in shallow groundwater to HCO3-Na in deep groundwater. Biswajeet (2011)showed that the difference in ion concentration in groundwater was controlled by aquifer lithology,flow rate, geochemical properties, ion solubility and human activities. Toran (1999) utilized reaction paths to study the evolution of HCO3-Na type groundwater in Tennessee U. S. A, and proved that groundwater with HCO3-Na type hydrochemicals was formed by ion exchange and silicate dissolution. Christian Ekberg (2001) discussed the existence of several uncertainties during calculations of the solubility of the water and rock reaction system, including experimental data uncertainty and concept uncertainty. Isotopic evolution methods have also been used to study the circulation of groundwater and hydrogeochemical evolution (Stephanie A O et al. 2012; WANG Wen-ke and WANG Yan-lin, 2012; QIN Da-ju et al.2005; HE Jian-hua et al. 2012), in which2H,18O and14C are usually used as tracer elements (XIE Xian-jun et al. 2013). Although the studies of hydrology and geochemistry are numerous, yet most focus on the regularities of spatial zoning.The mechanism is not studied very deeply at present and their methods are singular and simple.
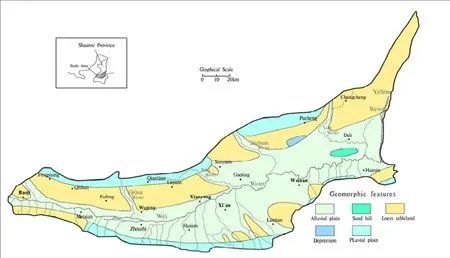
Fig.1 Geomorphological map of the Guanzhong basin
The Guanzhong Basin is approximately 20 000 km2and located in the central part of Shaanxi Province (Fig. 1), in a semi-arid region. Access to groundwater is an important provision for industrial development, agriculture and household water supplies. At present, priority is given to development and utilization of shallow groundwater (JIANG Gui-hua et al. 2009).Groundwater is rich in the Guanzhong Basin and surface water (rainfall and waterways) is the main source of supply (WANG Wen-ke et al. 2006).However, rapid economic development, increasing pollution and inadequate management of land use(HE Jin et al. 2013; WANG Xue-quan and GAO Qian-zhao, 2002) has resulted in poor and sharply deteriorating surface water quality and quantity.Consequently, the development and utilization of groundwater has become more desirable (WANG Ya and JIAO Jiu-jimmy, 2012).
This study aims to investigate the hydrogeochemical spatial zoning characteristics of groundwater in the Guangzhong Basin. Utilizing a variety of methods, we have made a qualitative and quantitative description of hydrogeochemical processes, discussed evolution mechanisms of these processes, and provided a reliable basis for the rational use of groundwater, the provision of safe drinking water and overall improvements to water quality. The broad application of our research findings may contribute to solving similar problems in other arid areas of Northwest China.
2 Materials and methods
Shallow groundwater samples (n=169) were collected from October to December 2011 from wells with a depth range between 4 and 80 meters.At each sample point, water was collected into a 500 mL polyethylene bottle which had been rinsed with deionized water and dried before sampling.One in every three samples was acidified to pH=2 with ultra-pure HNO3for cation analysis, the other two used for anion analysis without the addition of reagent. The pH, temperature and conductivity were tested at the worksite and water samples were taken for total analysis in laboratory. The spatial variability of samples was analyzed using conventional statistics and statistical software SPSS, and based on the water quality analysis data and results of previous research. Piper plots, drawn by software AQUACHEM, expounded the changes in water chemical components. The relationship between ions and between ions and total dissolved solids were analyzed, and statistical, hydrological and geochemical zoning characteristics were determined. PHREEQC software was used to simulate the hydrogeochemistry of the water-rock interaction. Quantitative analysis of the evolutionary processes and formation mechanisms of the groundwater’s hydrogeochemical characteristics were conducted.
3 Results and discussion
3.1 Spatial variability of hydrogeochemical parameters
The Guanzhong Basin is divided into two regions and seven sub-regions according to a combination of topographical, hydrological and geological conditions (WANG Wen-ke et al. 2006).The mean value of hydrogeochemical parameters and spatial variability of groundwater samples is illustrated in Table 1. Variation of the pH value between water samples was small, found in the range of 6.74-8.86, and the coefficient variation was only 0.03-0.07. Groundwater quality was mainly weak acid or weak alkaline. Acidic water mainly occurred in the loess tableland district in the western district of the study area, as well as in an area east of Qishui River, the piedmont alluvial fan and the alluvial plain to the south of the Wei River. Other areas in the study area were found to contain weak alkaline groundwater.
Although pH variation in the study area was minimal, the coefficient variation of other parameters was large. The lowest coefficient variation was(0.24) and the maximum was(1.67). In general, the largest coefficient variation was found in the Shichuan River-Luo River region, where the mean value was approximately 1, followed by the Luo River-Yellow River region. These results reflect the more complex topographical and hydrogeological conditions in these subregions,whereby the spatial variability of hydraulic conditions and water cycling conditions differ greatly between sites.
The coefficient variation of other subregions demonstrated less variability. Except forand Cl-, coefficient variation in the pluvial fan region varied less, especially in alluvial plain area. These results reflect alternating regional water cycling conditions and indicate less variability in water quality conditions overall in these sub-regions.Thus, the coefficient of spatial variation reflects the discrete degree of statistical parameters, and its value also reflects the complexity of the hydrogeological conditions.
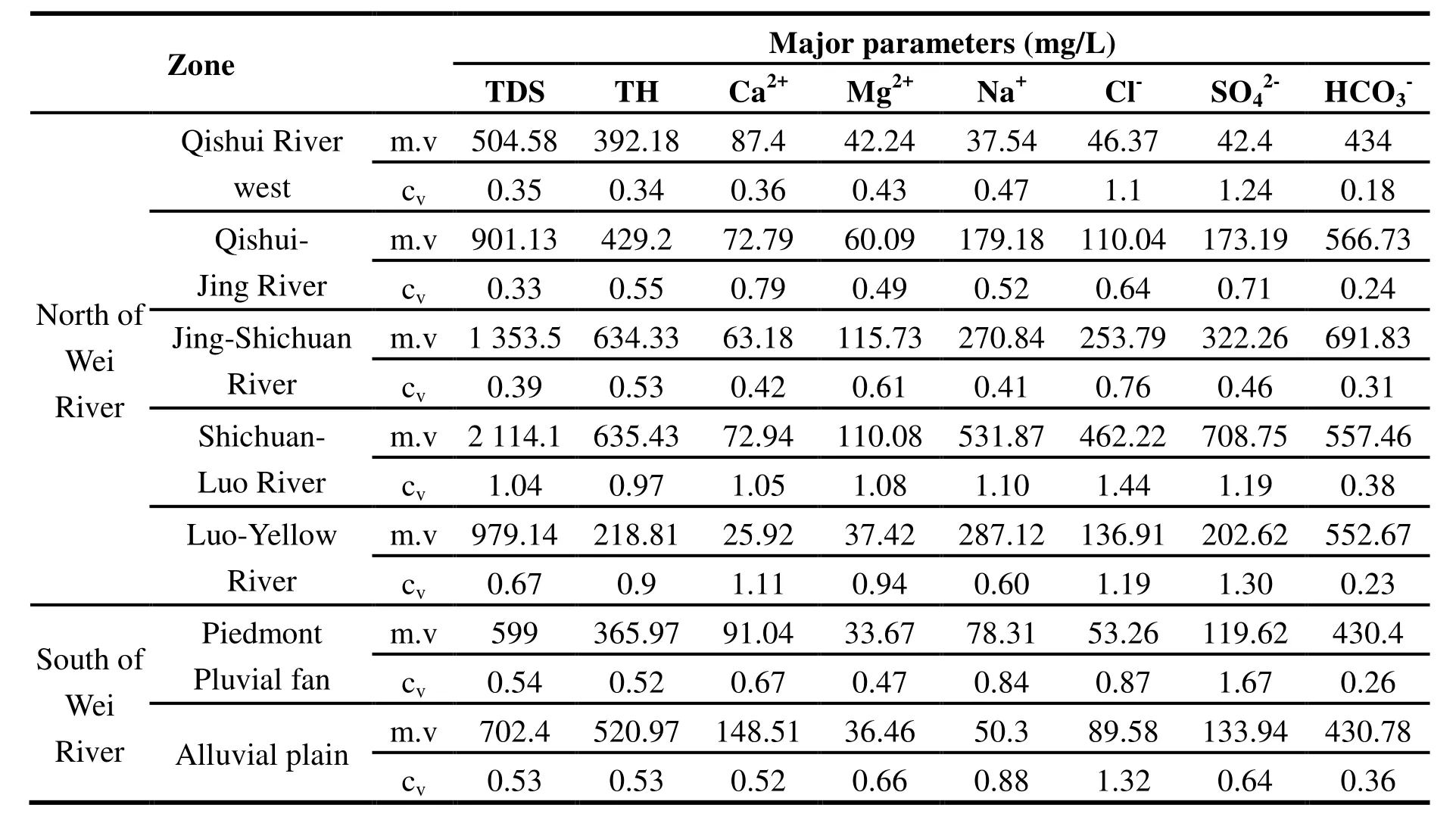
Table 1 Hydrogeochemical parameters and statistics of groundwater samples in the Guanzhong Basin study area
3.2 Hydrogeochemical evolution
3.2.1 Spatial zoning characteristics of hydrodynamic and hydrochemistry
The chemical components of groundwater are formed as water moves in and around voids in the parent rock and polymineral interactions occur between the water and rock during movement(CAO Yu-qing et al. 2009; Dickson A et al. 2011).Therefore, the hydrogeochemical zoning characteristics should be the combined effect of both hydrodynamic conditions and water chemistry.According to the groundwater levels in the study area, a diving contour map of the groundwater table was drawn (Fig. 2). The Shug Kalev method(WANG Da-chun et al. 1995) was used to classify the chemical types of groundwater, and the entire study area was divided into seven categories of hydrochemical types.
The hydrodynamic conditions of groundwater in the Guanzhong Basin have distinctive characteristics which predominantly relate to the topography and terrain of the region which control groundwater flow direction. From the periphery to the center of the Guanzhong Basin and along the entire length from of the Wei River, groundwater is eventually discharged into rivers and valleys. From sub-mountain regions to the center of the basin, the hydraulic gradient of groundwater gradually decreases, and groundwater flows from a strong to weak circulation zone. In the Shichuan River-Luo River sub-region, groundwater flows through many kinds of landforms with different geological conditions, and therefore the intensity of groundwater circulation appears to alternate. Given the difference between flow paths and geological conditions, hydrochemical types display different characteristics (DUAN Lei et al. 2007). The horizontal zoning characteristics of the groundwater hydrochemistry are illustrated in Fig.3. In Fig. 3 we can see that the regions to the south of Wei River and the west of the Jing River demonstrated good conditions for groundwater circulation, indicating the hydrochemical types were simple but predominantly HCO3-Ca (Ca·Mg).However, to the east of Jing River, as a result of slow circulation, poorer runoff conditions, low groundwater levels and high evaporation at the tableland depression, the hydrochemical types varied from simple to complex. The hydrochemical types were mainly HCO3·SO4·Cl-Na and SO4·Cl·HCO3-Na·Mg.
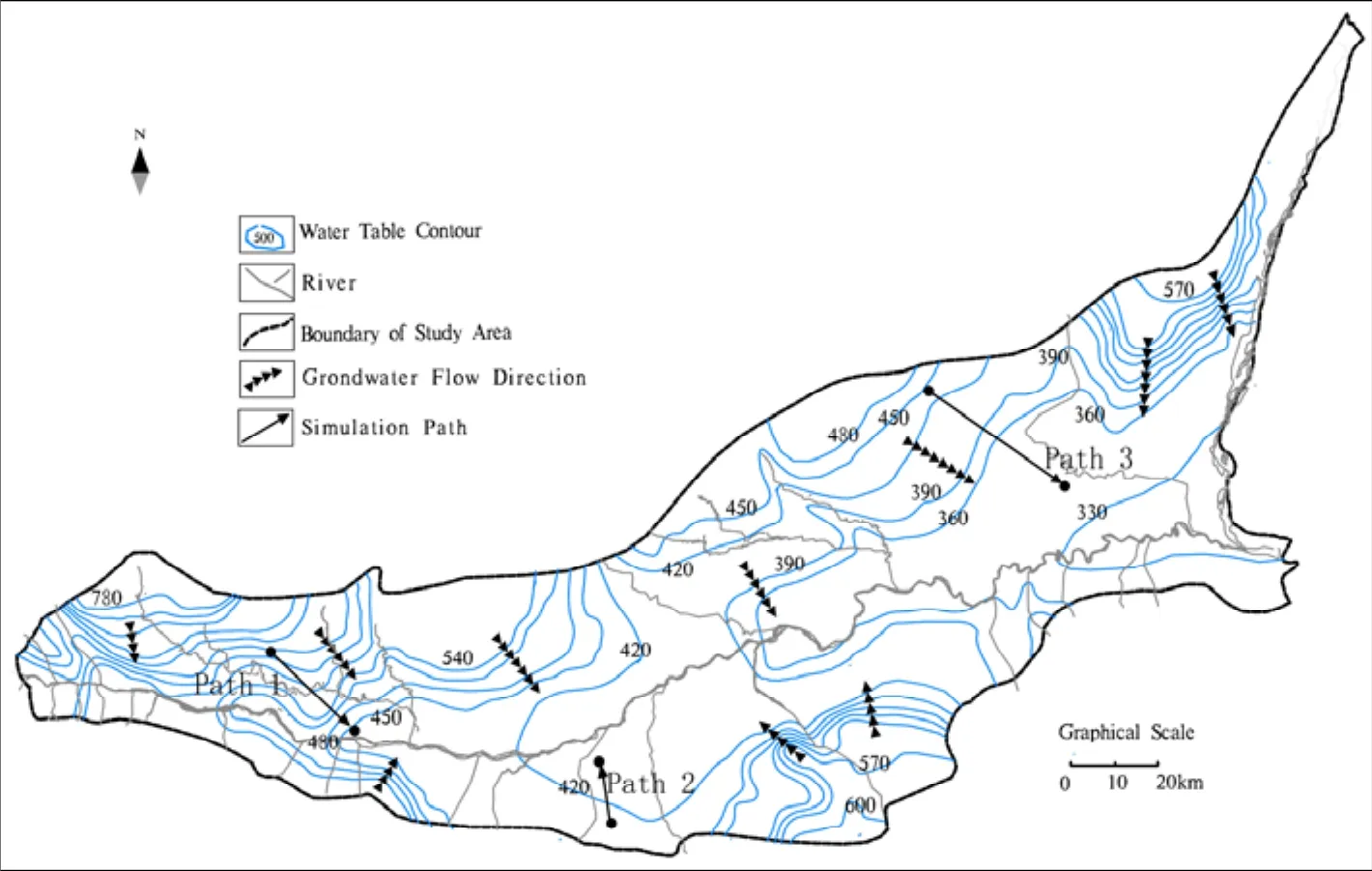
Fig. 2 Groundwater flow direction and simulation paths within the Guanzhong Basin
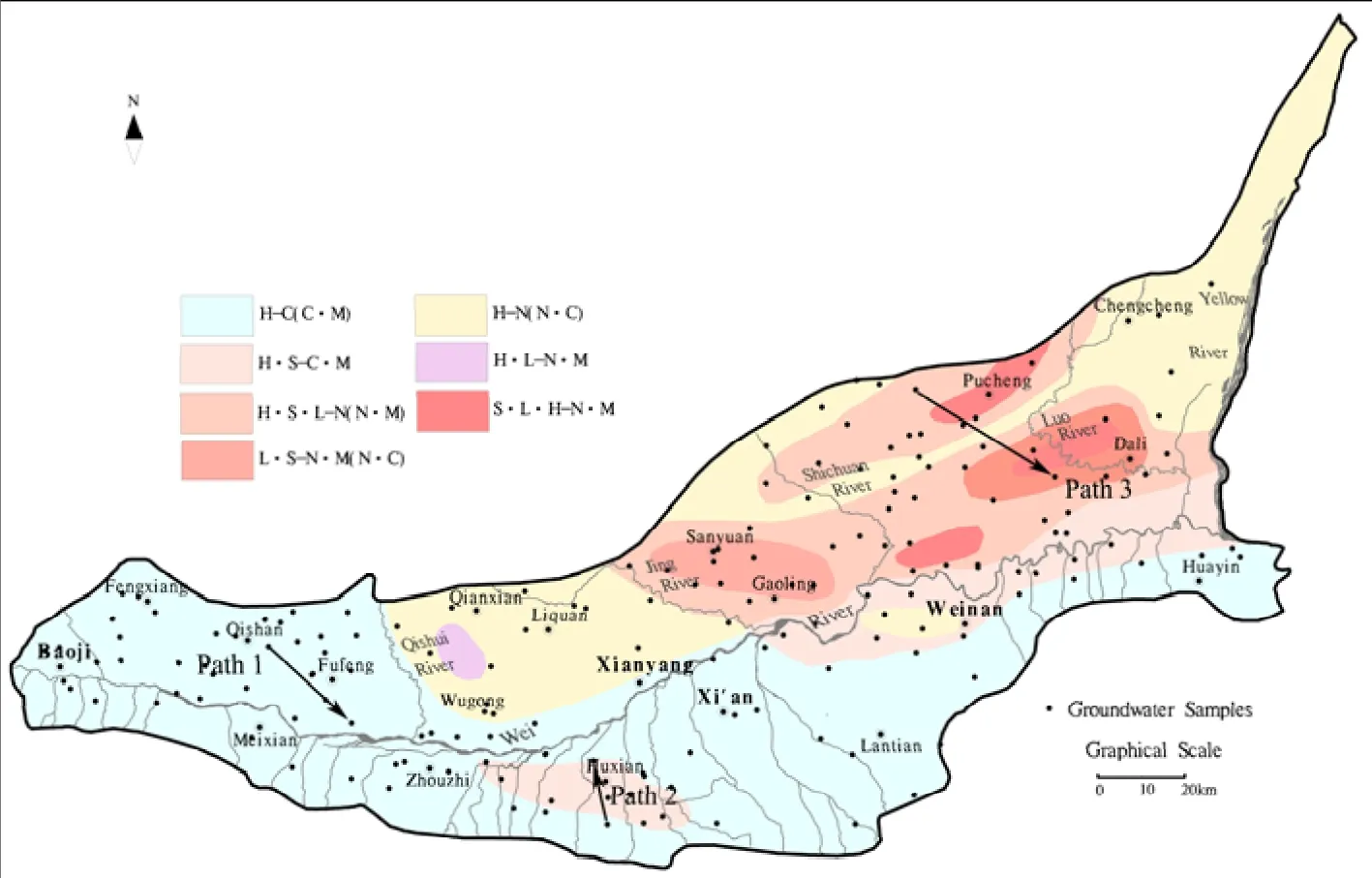
Fig. 3 Groundwater samples and hydrochemical type distribution within the Guanzhong Basin
3.2.2 Groundwater chemical components
The distribution of major ions in groundwater is illustrated clearly in the Piper plot (Fig. 4) of the Guanzhong Basin groundwater samples. On the whole, the Na+and K+were the dominant cations compared to Ca2+and Mg2+, and dominant anion wasAlong the groundwater flow direction,zone ‘A’ relates to the recharge area, located in the Piedmont diluvia fan, with a larger hydraulic gradient and good circulation of groundwater.During the hydrolysis of carbonate rock and dissolution of silicate, the concentration of Mg2+and Ca2+became higher. The TDS content of zone A was generally less than 1 000 mg/L and the maximum was 990 mg/ L. The hydrochemical type was mainly HCO3-Ca.
Zone ‘B’ refers to the runoff area, located in the leading edge of alluvial fan, the loess plateau and the river terrace. The aquifer lithology of zone B was mainly sandy clay and loess soil, in favor of Ca2+in the groundwater exchanging with Na+in loess particles, leading to Na+enriched in groundwater. The TDS content of zone B ranged from 1 020 mg/L to 2 770 mg/L and the hydrochemical type was mainly HCO3-Na. Fine particles,slow runoff and poor conditions for groundwater circulation lead to the occurrence of Cl-enriched HCO3·Cl-Na-type water in this zone.
Zone “C” relates to the discharge area, located in the plateau depressions and alluvial plain. A combination of poor permeability, slow runoff,poor discharge condition and low groundwater levels, resulted in dramatic increases in the mineralization and salinization of groundwater in Zone C, which is also strongly affected by evaporation and human activities. The TDS content was generally greater than 3 000 mg/L and the maximum was 22 490 mg/L. The hydrochemical types became more complicated consisting mainly of Cl·SO4-Na·Mg and HCO3·Cl·SO4-Na, and the water quality was unacceptable. From the recharge area to the discharge point, dominant cations changed from Ca2+and Mg2+to Na+, and anions changed fromtoand Cl-. Groundwater became more saline. Human factors such as large scale irrigation affect groundwater (Intissar, 2013),driving increased solution of ions such as chloride,sulfate and others. Evaporation then leads to the precipitation of these ions onto vadose zone soils.In our study, given that all of these factors are shown to influence groundwater quality, they were taken into account when we zoned different hydrochemical characteristics by geology, geomorphological conditions, aquifer lithology and human activities.
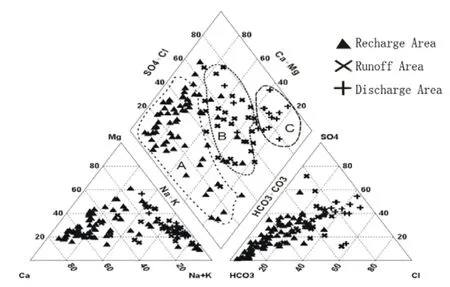
Fig. 4 Piper plot of groundwater samples in Guanzhong Basin
3.2.3 Formation of hydrochemical characteristics
Chemical substances dissolved in water are not independent; therefore the relationship between different ions and TDS could be used to study hydrochemical characteristics and groundwater mineralization in the study area (Schoeller H,1977). In arid areas, evaporation causes ions to accumulate in groundwater and is main driver controlling the salinity of groundwater (Richer B C and Kreitler C W, 1993) especially for the soluble chloride ion. The study showed the concentration of chloride ions increased with TDS (Fig. 5a),which could be measured to characterize the regional evaporation intensity in the overall groundwater system. As shown in Fig. 5b, a good relationship was detected between Na+and Cl-ions,indicating that the concentration of Na+and Clcontrols the dissolution of halite in groundwater.Another important characteristic of groundwater quality was the molar ratio of Na+and Cl-. As seen from Fig. 5c, the molar ratio of most water samples was larger than 1. When TDS was relatively low,most molar ratios were around or larger than 2. But when the molar ratio is nearby 2 at high TDS, the fact of groundwater with high TDS would be covered by this phenomenon in a certain extent.Fig. 5c also illustrates that different geochemical processes occur in fresh and salt groundwater. This may be due to the weathering of mirabilite(Na2SO4) which exchanges ions and precipitates Na+ion as the compound dissolves in fresh water.
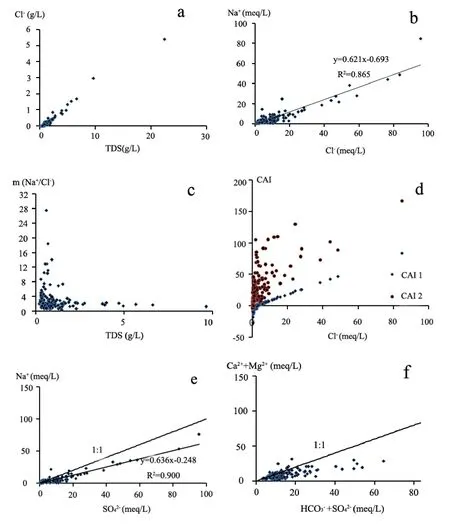
Fig. 5 The relationship between ions, and between ions and TDS
A variety of indices have been used historically to determine ion exchange, and Schoeller (1965)defined two chloride alkaline indices (CAI):
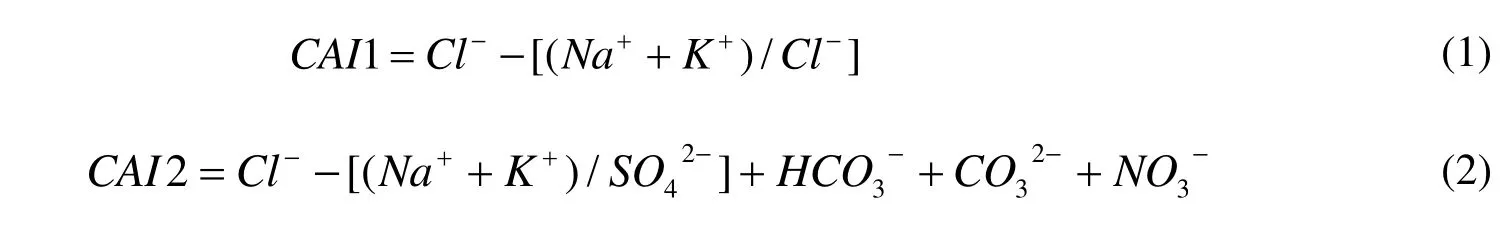
An exchange between Na+and K+ions in groundwater with Ca2+or Mg2+in alluvium or weathered rock indicates two positive indices(Schoeller H, 1965). As was shown in Fig. 5d, out of all water samples CAI2 were positive, while CAI1 were negative in the low Cl-concentration and positive in high, indicating that ion exchange in the study area mainly occurs in groundwater with high TDS. There was also a good correlation between Na+and(Fig. 5e), reflecting the dissolution of mirabilite (Na2SO4) in groundwater.However the location of the correlation line in Fig.5e under the 1:1 ratio line suggests substances other than mirabilite were dissolved in the groundwater, and that additional sources ofwere present. Fig. 5f represents the relationship between Mg2++Ca2+(meq) and(meq). Some water samples lie below the 1:1 line,indicating that the study area had mixed dissolution of calcite, dolomite and gypsum. The deficiency of Mg2+and Ca2+relative toandwas compensated by Na+.
3.3 Hydrogeochemical simulation
Hydrogeochemical simulation required the start and end water sample of each measured path with the same flow path. The path we chose has the same direction with groundwater flow. Therefore,three different paths (Fig. 2) were simulated by PHREEQC software.
Table 2 The primary ion content (mg/L) in water samples along each groundwater flow path
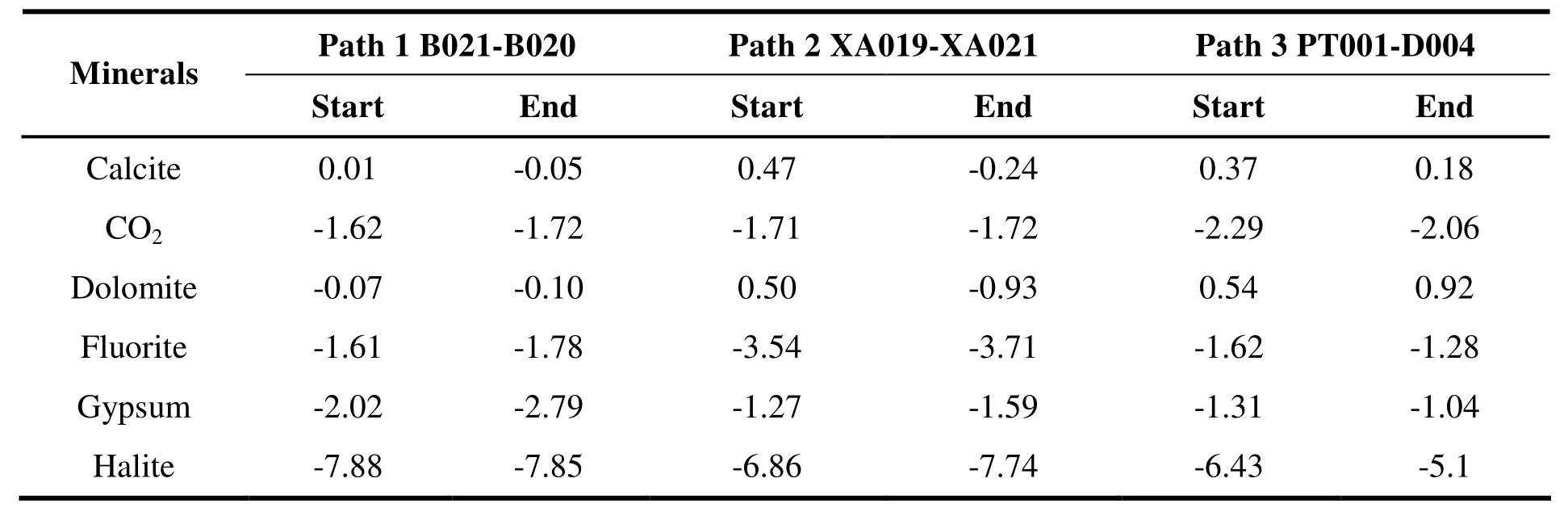
Table 3 Saturation index of the start and end water samples alongside each groundwater path
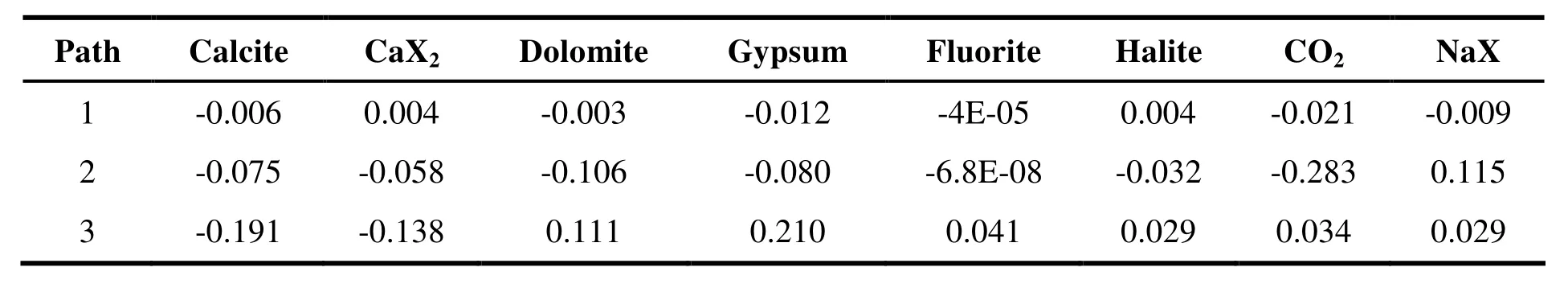
Table 4 The amount of mineral precipitation/dissolution per unit of groundwater path length (mmol/ (L·km))
According to the aquifer mineral analysis and the chemical components and conditions of the groundwater (CUI Xian-wei et al. 2010), possible mineral phases can be selected for a particular study area. The main chemical elements of the mineral phases should be consistent with the chemical components detected in the groundwater.According to the identification results of mineral composition and water chemistry analysis, the PHREEQC simulation selected calcite, dolomite,fluorite, gypsum and halite as possible mineral phases. In addition, given that all water samples derived from shallow groundwater and were relatively open, CO2was selected as one of the possible mineral phases. Cation exchange being common in the groundwater system, it was also taken into account. The saturation index of the start and end points of typical paths are shown in Table 2, and the amount of minerals dissolved or precipitated over the unit length path is shown in Table 3. The length of path 1, 2 and 3 was 25.10 km, 6.01 km and 41.36 km respectively. The direction from start water sample to end is the same with groundwater flow.
Dissolution and precipitation of minerals roughly followed the same trend over the three simulation paths. At the beginning of simulated paths, calcite and dolomite were saturated and had a tendency to precipitate, while CO2, fluorite,gypsum and halite were unsaturated with a tendency to dissolve.
From the start to end of Path 1 and 2 (Table 4),the hydrodynamic conditions were deteriorating.Groundwater flowing through the mountain areas and the piedmont alluvial fan contained large amounts of Ca2+and Mg2+, resulting in the precipitation of calcite, dolomite and gypsum. The minerals precipitated were mainly calcite and gypsum, but the amount of precipitation was very small. Concurrently, cation exchange occurred along both Path 1 and 2, but the nature of the exchange differed. The cation exchange of Path 1 centered around exchange of the Ca2+in the rock with Na+in the water, Ca2+dissolving into the water and Na+precipitating on the surface of rock.However, Path 2 was quite the opposite-the Na+dissolving and the Ca2+precipitating. During Path 3, most minerals dissolved while calcite precipitated, the primary exchange for calcite precipitation being the dissolution of gypsum and dolomite. Cation exchange in Path 3 occurred in a similar fashion to Path 2 but in Path 1 and 2 the exchange capacity of Na+was clearly greater than that of Ca2+-being approximately two times greater and demonstrating these paths conduct the greatest cation exchange. However, the exchange capacity of Na+in Path 3 was less than Ca2+, indicating that exchange of Mg2+and Ca2+also occurs. Extensive dissolution of dolomite proved this. The dissolution and precipitation of minerals and ion exchange therefore had a demonstrable influence on the hydrochemical characteristics and variation laws of groundwater chemistry.
4 Conclusions
Across the region west and east of the northern Wei River, the southern Wei River and from the periphery to the centre of the Guanzhong Basin,the hydrodynamic conditions of groundwater vary between good and bad quality, and hydrochemical types change gradually from simple bicarbonate waters to complex ones. The hydrodynamic conditions and hydrochemical types in the study area clearly demonstrated horizontal zoning characteristics. When runoff passes through from an unobstructed zone to a more sluggish environment, water circulation conditions deteriorate, the discharge modes of groundwater change from horizontal discharge to vertical evaporation, and hydrochemical actions change from lixiviation to evaporation. The different hydrochemical actions and discharge modes result in different hydrochemical characteristics. The hydrodynamic conditions of groundwater therefore play a controlling role in hydrochemistry.
The relationship between ions, and between ions and TDS, reflect the features of groundwater.The evaporation which controls groundwater salinity for example and the relationships between Cl-and TDS could be used to characterize the evaporation intensity. Two chloride alkaline indices of the Guanzhong Basin indicate ion exchange mainly occurred in groundwater with high salinity. For the coefficient variation, the minimum value of conventional parameters was pH (0.03-0.07). The minimum of other parameters was(0.24), while the maximum was(1.67). Coefficient variation of Shichuan River-Luo River region was larger than others,followed by the Luo River-Yellow River region,reflecting the more complicated hydrogeological conditions of these regions compared to others.
Variations in hydrogeochemistry were closely related to aquifer lithology, water-rock interaction and mineral precipitation and dissolution. The formation and evolution processes of hydrogeochemistry were further complicated by the influence of human activities. All of these factors influenced the zoning characteristics and variation laws of groundwater hydrogeochemistry in the Guanzhong Basin.
Acknowledgements
This research work was supported by the China Geological Survey (No.1212010634700) through groundwater exploration to ensure the water supply security of endemic disease areas.
杂志排行
地下水科学与工程(英文版)的其它文章
- Progress on the mapping of groundwater resources and environment in Asia
- Groundwater ecological environment and the mapping of Asia
- Groundwater system division and compilation of Groundwater Resources Map of Asia
- Governance of protected areas: Kara Kara National Park, Victoria
- Comparison of 1,2,3-Trichloropropane reduction and oxidation by nanoscale zero-valent iron, zinc and activated persulfate
- Evaluation on water resources carrying capacity of Changchun-Jilin Region
