多孔有机骨架:基于傅氏烷基化反应的合成与气体吸附性质
2015-12-01崔鹏景晓飞任浩元野朱广山
崔鹏 景晓飞 任浩 元野 朱广山*,
(1吉林大学化学学院,无机合成与制备化学国家重点实验室,长春130012)
(2东北师范大学化学学院,多酸科学教育部重点实验室,长春130024)
多孔有机骨架:基于傅氏烷基化反应的合成与气体吸附性质
崔鹏1景晓飞2任浩1元野2朱广山*,1
(1吉林大学化学学院,无机合成与制备化学国家重点实验室,长春130012)
(2东北师范大学化学学院,多酸科学教育部重点实验室,长春130024)
通过傅氏烷基化反应成功地合成了基于四苯锗烷构筑单元的多孔有机骨架材料PAF-9。用红外光谱,粉末X射线衍射,固体核磁共振,热重分析和低温氮气吸附-脱附表征了PAF-9材料的微结构与孔道性质。表征数据表明PAF-9具有非常高的热稳定性和化学稳定性,同时具有较高的比表面积。该PAF材料的BET比表面积为334 m2·g-1。此外,得到的PAF材料对二氧化碳具有较好的吸附能力。
傅氏烷基化;多孔有机骨架材料;气体吸附
0 Introduction
Nowadays,one of the major causes of the global warming is the excessive CO2emission.CO2capture and storage(CCS)technologies are urgently needed in twenty-first century[1-3].So it is important to synthetize novel porous materials as alternative candidates for CO2capture.Porousorganicframeworks(POFs)possess many advantage,including high surface area,low skeleton density,high chemical and thermal stability and have attracted considerable attention as a kind of sorption materials in CCS technologies.Various POFs materials,such as covalent organic frameworks (COFs)[4-7],polymers of intrinsic microporosity(PIMs)[8-9], conjugated microporous polymers(CMPs)[10-11],crystalline triazine-based organic frameworks(CTFs)[12-13],and porous aromatic frameworks(PAFs)[14-16],have been created and synthesized.In particular,POF networks derived from diamond-like building units such as tetaphenylmethane were obtained,and most of them presented ultra-high surface area and excellent gas sorption ability[17].Several pioneering and effective strategies have been employed to construct POFs, including condensation reaction of boronic acids,the dibenzodioxane-forming reaction,palladium catalyzed Sonogashira-Hagihara cross-coupling reaction,palladium-catalyzed Suzuki cross-coupling reaction and trimerization reaction of aromatic nitrile compounds etc[18].However,these cross-couple reactions are usually catalyzed by expensive noble metals or transition metalcatalystswhichlimitthedevelopmentfor extensive application of POFs in CCS technologies.It is worth to find a synthesis route with cheap catalysts to synthesize high surface area POFs.Under the circumstances,we explored to construct polymers with diamond-like structure which have high surface area via cost-effective cross-coupling reaction.Tan and Cooper group[19-22]have reported a series of polymers with heterocyclic(pyrrole,thiofuran,furan,benzene, and biphenyl etc.)as monomers and formaldehyde dimethylacetal as an external cross-linker via Friedel-Crafts alkylation reaction.This kind of reaction is catalyzed by inexpensive FeCl3or AlCl3[23-24].In the presence of Lewis acid,aromatic nuclei of monomers become active to achieve polymerization to produce polymers.Being inspired by this idea,we chose tetraphenylgermane as tetrahedral building unit and FeCl3as catalyst via Friedel-Crafts alkylation reaction under mild condition to produce the cost-effective porousaromaticframeworks(PAF-9).Afterthe synthesis of PAF-9,we carried out characterizations and discovered that PAF-9 has high surface area(334 m2·g-1).So we further studied CO2sorption ability of PAF-9 and found out that PAF-9 had good sorption ability of CO2.
1 Experimental
1.1Materials and measurements
Tetraphenylgermane(5 g,98%)and formaldehyde dimethyl acetal(FDA,500 mL,98%)were purchased from Alfa Aesar.CaH2(100 g,95%)was purchased from Aladdin.Other chemicals and reagents were purchased from commercial suppliers without further purificationunlessotherwisestated.Methylene dichloride(CH2Cl2,500 mL,99%,Beijing Chemical Works)was dehydrated with CaH2.
TheFouriertransforminfraredspectroscopy (FTIR)spectra(film)were recorded using IFS 66V/S Fourier transform infrared spectroscopy.Solid-state13C CP/MAS NMR measurements were performed on a Bruker Avance III model 400 MHz spectrometer at a MAS rate of 5 kHz.The powder X-ray diffraction (PXRD)was performed by a Riguku D/MAX2550 diffractometer using Cu Kα radiation(λ=0.154 18 nm), 50 kV,200 mA with scanning rate of 4°·min-1(2θ). Scanning electron microscopy(SEM)analysis was performed on a JEOS JSM 6700.Transmission electron microscopy(TEM)was recorded using a JEOL JEM 3010 with an acceleration voltage of 300 kV.Thermogravimetric analysis(TGA)was performed using a Netzch Sta449c thermal analyzer system at a heating rate of 10℃·min-1in air.The gas adsorption-sorption isotherms data were obtained from a Quantachrome Autosorb-iQ2 analyzer.
1.2Synthesis
Tetraphenylgermane(1 mmol,0.38 g)and FeCl3(4 mmol,0.65 g)were added into a 100 mL roundbottom-flask,and the system of mixture was degassed to vacuum and aerated with Ar and sealed by three freeze-pump-thaw cycles.Then FDA(4 mmol 0.36 mL)and CH2Cl2(10 mL)were injected into the system through a syringe.The polymerization fused aromatic monomers with 4 equivalents of FDA(depending on the number of aromatic reaction sites available).Themixture was heated at 45℃for 3 d to produce the polymer.After cooling down to room temperature,the product was washed by 1 mol·L-1hydrochloric acid solution,methanol and acetone until the filtrate liquor was nearly colorless.Then the product was Soxhlet extracted for 24 h with methanol,tetrahydrofuran and dichloromethane separately to remove monomers and catalyst residues thoroughly,and dried under vacuum to give PAF-9 as brown powder(95%yield).
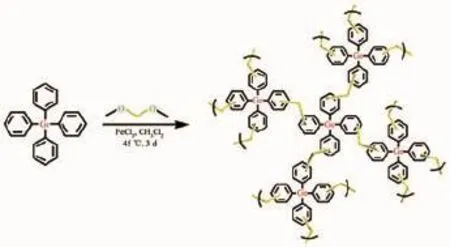
Fig.1 Synthetic pathway of PAF-9
2 Results and discussion
2.1FTIR spectra
Comparisons of the FTIR spectra of monomers and products were performed to confirm the reaction degree of the polymerization.As shown in Fig.2,the appearance of intense saturated C-H bands(at 2 904 cm-1)of PAF-9 reveal that the networks are linked by methylene groups as desired.The peaks at 1 600~1 450 cm-1belong to aromatic C=C stretching vibrations and the peaks at 900~650 cm-1belong to aromatic C-H deformation vibrations.These indicate the phenyl rings in the PAF-9 network.
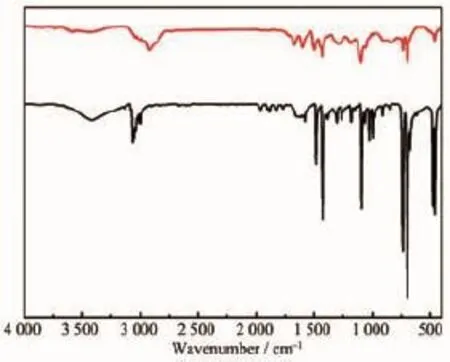
Fig.2 FTIR spectra of PAF-9(red)and tetraphenygermane(black)
2.2Solid State13C CP/MAS NMR spectra
Further investigation of the local structures of the PAF-9 was performed by solid-state13C CP/MAS NMR studies.Three different types of resonance peaks are clearly observed in the13C CP/MAS NMR spectra of PAF-9(Fig.3).The strong signals in the range of 140~125 ppm are attributed to the aromatic carbon atoms. The peak at 127 belongs to the aromatic C(C2,C3) connected to H and the peak at 138 belongs to the aromatic C(C1,C4)connected with other atom or perssad.Moreover,the signal near 37 is related to the methylene carbon atoms(C5),thus further indicating the FDA participation in the reaction.

Fig.3 13C CP/MAS NMR spectrum of PAF-9
2.3Powder X-ray diffraction
PXRD was carried out to identify the crystallinity of PAF-9 materials.No intense peaks but only a broad peak in Fig.4 indicate that the texture of the polymerisamorphouswhichiscausedbythe irreversibility of Friedel-Crafts alkylation reaction and the introduction of flexible perssad(methylene).
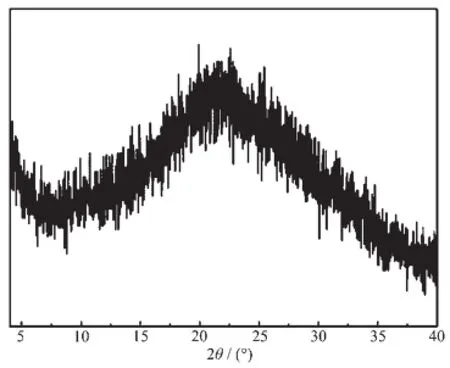
Fig.4 PXRD pattern of PAF-9
2.4SEM and TEM
The morphology and particle size of PAF-9 wereinvestigated by SEM measurement.SEM image(Fig. 5a)shows that PAF-9 is amorphous and most particles are micro-sized irregular blocks.TEM analyses(Fig. 5b)shows that PAF-9 is clearly porous textures, although the arrangement of the pores was random.

Fig.5 SEM(a)and TEM(b)images of PAF-9
2.5Thermogravimetric analysis
TGA analysis under air conditions was used to investigate the structure stability of the polymers.Fig. 6 exhibits that PAF-9 displays high thermal stability. The decomposition temperature of PAF-9 is above 400℃.In addition,PAF-9 materials cannot be dissolved and decomposed in common organic solvents,such as DMF,THF,CHCl3and so on,indicating high thermal and chemical stability.
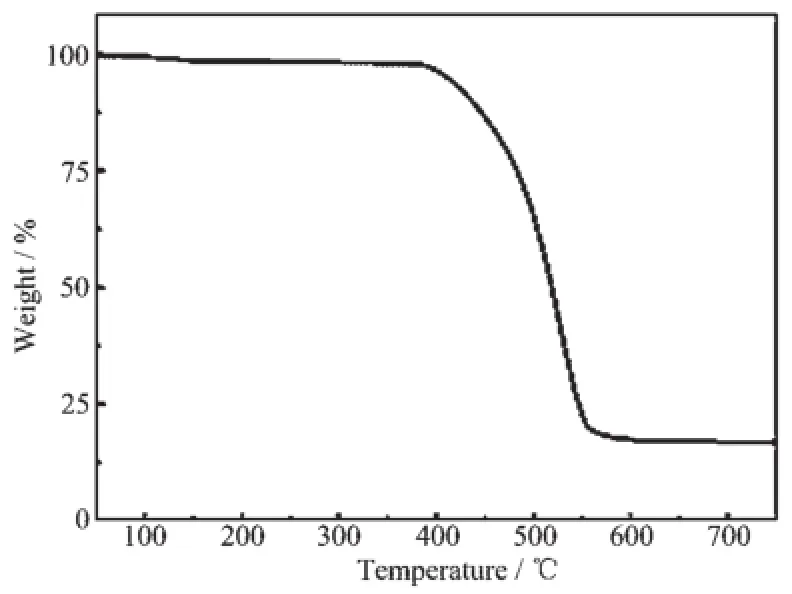
Fig.6 TGA plots of PAF-9
2.6N2sorption measurements
The pore character of PAF-9 was studied by N2sorption isotherm measured at 77 K under 1 013 kPa. As indicated in Fig.7,a sharp uptake at low relative pressuresandadistincthysteresisloopinthe desorption branch are observed indicating that PAF-9 possesses both microporous and mesoporous texture. The adsorption isotherm of PAF-9 increases rapidly at P/P0≈1.0 which might be attributed to the surface adsorption of assembled small particles.The apparent surface area of PAF-9 calculated from the Brunauer-Emmett-Teller(BET)model is 334 m2·g-1.Meanwhile, the pore size distribution calculated via nonlocal density functional theory(NLDFT)gives two narrow peaks at 1.68 and 3.80 nm,further proving the micromesoporous network of PAF-9.

Fig.7 N2sorption isotherms and pore size distribution (inset)of PAF-9
2.7CO2adsorption measurements
The high surface area and permanent porosity make PAF-9 an outstanding candidate for gas sorption such as CO2.Herein,CO2sorption isotherms are collected at 273 and 298 K.As shown in Fig.8a, under STP condition,the CO2uptakes of PAF-9 at 273 and 298 K are 28 cm3·g-1and 17 cm3·g-1respectively.The heat of adsorption(Qst)for CO2calculated from adsorption data from 273 and 298 K, using the Clausius-Clapeyron equation,is 32 kJ·mol-1(Fig.8b).The results indicate that PAF-9 has comparative CO2sorption capacity with other reported POF materials[25-26]and is possible to be a cost-effective material in CCS technologies.
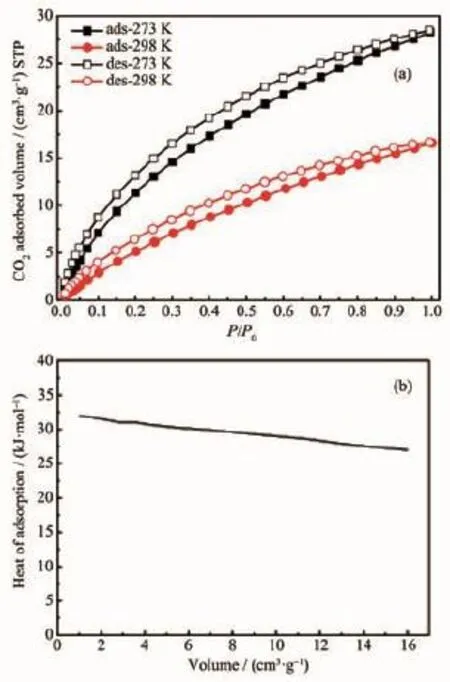
Fig.8 CO2sorption isotherms measured at 273 and 298 K(a)and Qstfor CO2(b)of PAF-9
3 Conclusions
In summary,the PAF materials(PAF-9)based on tetrahedral building blocks was synthesized by simple one-stepFriedel-Craftsalkylationreactioninthe presence of inexpensive catalyst FeCl3.The properties of PAF-9 materials were investigated and discussed. Characterizations indicate that the PAF-9 materials display high thermal and chemical stability as well as high surface area.In addition the PAF-9 materials exhibit potential application in CO2capture.
Acknowledgments:We are grateful for the financial support of National Basic Research Program of China(973 Program,GrantNo.2012CB821700andGrantNo.2014CB931804), Major International(Regional)Joint Research Project of NSFC (Grant No.21120102034).
References:
[1]YUAN Ye(元野),REN Hao(任浩),SUN Fu-Xing(孙福兴). Chinese J.Inorg.Chem.(无机化学学报),2013,29(8):1645-1648
[2]Dawson R,Cooper A I,Admas D J,et al.Polym.Int.,2013, 62:345-352
[3]LI Jin-Li(李锦丽),FU Ning(付宁),LÜ Gong-Xuan(吕功煊). Chinese J.Inorg.Chem.(无机化学学报),2010,26(12):2175-2181
[4]Cote A P,Benin A I,Ockwig N W,et al.Science,2005,310: 1166-1170
[5]Zeng Y F,Zou R Y,Luo Z,et al.J.Am.Chem.Soc.,2015, 137:1020-1023
[6]Ding S Y,Gao J,Wang Q,et al.J.Am.Chem.Soc.,2011, 133:19816-19822
[7]Medina D D,Rotter J M,Hu Y H,et al.J.Am.Chem.Soc., 2015,137:1016-1019
[8]Budd P M.Science,2007,316:210-211
[9]Carta M,Croad M,Malpass-Evans R,et al.Adv.Mater., 2014,26:3526-3531
[10]Ding X S,Han B H.Angew.Chem.Int.Edit.,2015,54:6536 -6539
[11]Du R,Zhang N,Xu H,et al Adv.Mater.,2014,26:8053-8058
[12]Hao L,Zhang S S,Liu R J,et al.Adv.Mater.,2015,27: 3190-3195
[13]Zhu X,Tian C C,Mahurin S M,et al.J.Am.Chem.Soc., 2012,134:10478-10484
[14]Ben T,Ren H,Ma S Q,et al.Angew.Chem.Int.Edit., 2009,48:9457-9460
[15]Yuan Y,Sun F X,Zhang F,et al.Adv.Mater.,2013,25: 6619-6624
[16]Yuan Y,Sun F X,Li L N,et al.Nat.Commun.,2014,5: 5260-5264
[17]Ben T,Ren H,Ma S Q,et al.Angew.Chem.Int.Edit., 2009,48:9457-9460
[18]Zou X Q,Ren H,Zhu G S,et al.Chem.Commun.,2013,49: 3925-3936
[19]Luo Y,Li B,Wang W,et al.Adv.Mater.,2012,24:5703-5707
[20]Li B,Gong R,Wang W,et al.Macromolecules,2011,44: 2410-2414
[21]Dawson R,Stevens L A,Drage T C,et al.J.Am.Chem. Soc.,2012,134:10741-10744
[22]YUAN Ye(元野),YAN Zhuo-Jun(闫卓君),REN Hao(任浩), et al.Acta Chim.Sinica(化学学报),2012,70(13):1446-1450
[23]Jing X F,Zou D L,Cui P,et al.J.Mater.Chem.A,2013,1: 13926-13931
[24]LI L N,Ren H,Yuan Y,et al.J.Mater.Chem.A,2014,2: 11091-11098
[25]Dawson R,Stockel E,Holst J R,et al.Energy Environ.Sci., 2011,4:4239-4245
[26]Jiang J X,Cooper A I.Top.Curr.Chem.,2010,293:1-33
Porous Aromatic Frameworks:Synthesis via Friedel-Crafts Alkylation Reaction and Gas Sorption Property
CUI Peng1JING Xiao-Fei2REN Hao1YUAN Ye2ZHU Guang-Shan*,1
(1State Key Laboratory of Inorganic Synthesis&Preparative Chemistry,College of Chemistry,
Jilin University,Changchun 130012,China)
(2Key Laboratory of Polyoxometalate Science of Ministry of Education,Department of Chemistry, Northeast Normal University,Changchun 130024,China)
A porous aromatic framework,PAF-9 derived from tetraphenylgermane as basic building unit,was synthesized via Friedel-Crafts alkylation reaction.The microstructure and pore property were investigated by FTIR spectroscopy,powder X-ray diffraction,solid state NMR,thermogravimetric analysis and low temperature N2adsorption-desorption measurements.The characterizations reveal that PAF-9 possess high thermal and chemical stability as well as high BET surface area of 334 m2·g-1.Additionally,the resulting PAF materials exhibit high CO2adsorption ability.
Friedel-Crafts alkylation reaction;porous aromatic framework;gas sorption
O621.25
A
1001-4861(2015)09-1855-05
10.11862/CJIC.2015.256
2015-05-31。收修改稿日期:2015-08-03。
国家重点基础研究发展计划(973计划,No.2012CB821700,No.2014CB931804)、国家自然科学基金委重大国际(地区)合作研究项目(No.21120102034)资助项目。
*通讯联系人。E-mail:zhugs@jlu.edu.cn
