周边硅笼取代的混杂酞菁卟啉三层铽单分子磁体
2015-12-01张璐曾涑源刘涛孙君善窦建民姜建壮
张璐 曾涑源 刘涛 孙君善 窦建民*, 姜建壮*,
(1北京科技大学化学与生物工程学院,功能分子与晶态材料科学与应用北京市重点实验室,北京100083)
(2聊城大学化学系,聊城252004)
(3大连理工大学,精细化工国家重点实验室,大连116012)
周边硅笼取代的混杂酞菁卟啉三层铽单分子磁体
张璐1曾涑源2刘涛3孙君善1窦建民*,2姜建壮*,1
(1北京科技大学化学与生物工程学院,功能分子与晶态材料科学与应用北京市重点实验室,北京100083)
(2聊城大学化学系,聊城252004)
(3大连理工大学,精细化工国家重点实验室,大连116012)
通过在铽的酞菁卟啉混杂三层的卟啉周边共价连接体积庞大的笼型倍半硅氧烷(POSS),得到了首个包含POSS的混杂三层Tb2(Pc)[T(OPOSS)4PP]2(1)[H2Pc=phthalocyanine;H2T(OPOSS)4PP=5,10,15,20-tetra{[[N-[heptakis(isobutyl)propoxy]phenyl] octasiloxane]}porphyrin]。为了对比研究,同时合成了类似的三层化合物Tb2(Pc)(TPP)2(2)(H2TPP=5,10,15,20-tetraphenyporphyrin)。尤其值得注意的是,在没有外加磁场的条件下,Tb2(Pc)[T(OPOSS)4PP]2(1)和Tb2(Pc)(TPP)2(2)分别表现出单分子磁体和非单分子磁体的性质,这充分说明了共价连接均匀分布的POSS基团有效地分离了磁性核心,从而改善了酞菁卟啉混杂三层的磁性。
四吡咯化合物;三明治;POSS;分子杂化;单分子磁体
0 Introduction
Single moleculemagnets(SMMs)were first noticed in early 1990s when a molecular metal coordination compound Mn12Ac was found to retain magnetization for a long time without an external field at liquid-helium temperature[1].In recent years,SMMs have attracted increasing interest because of their potential to develop new technological applications including storage and process of digital information with high density and unprecedented speed,the molecular-scalespintronicdevicesandquantum computing at the molecular level[2].
Investigations reveal that the energy barrier in reversing magnetization for SMMs is mainly related with the magnetic anisotropy projected on the ground exchange and the multiplet projection of the total spin onthesymmetryaxis[1c,3].Inaddition,the intermolecularmagneticdipole-dipoleinteraction, whichmayresultinquantumtunnelingofthe magnetization(QTM),can also influence the magnetic properties of SMMs as exemplified by lowering of the block temperature[4].To solve this problem,a physical dilutionmethodbymeansoftheisostructural diamagneticanaloguestodopetheparamagnetic SMMs is usually employed[5].However,a large amount of diamagnetic analogues is necessary for the purpose of effectively dispersing the diamagnetic counterparts around the paramagnetic molecules to weaken the intermolecular magnetic dipole-dipole interaction[4a,6]. An alternative method of such a physical dilution way appearstointroducebulkydiamagneticmoieties around the magnetic core by covalently linking.This, however,has not been employed to diminish the magnetic dipole-dipole interaction and in turn to suppress the QTM effect in this field,to the best of our knowledge.
In the present paper,we describe the design and synthesis of the first POSS-involved hybrid tripledecker complex Tb2(Pc)[T(OPOSS)4PP]2(1)[H2Pc= phthalocyanine;H2T(OPOSS)4PP=5,10,15,20-tetra{[[N-[heptakis(isobutyl)propoxy]-phenyl]octasiloxane]}porphyrin].Forthepurposeofcomparativestudies, corresponding triple-decker analogueTb2(Pc)(TPP)2(H2TPP=5,10,15,20-tetraphenyporphyrin)(2)was also prepared and structurally characterized.In particular, comparative studies clearly reveal the effect of the covalentlylinked,homogenouslydispersedbulky POSS moieties on effectively separating the magnetic cores and improving the magnetic property of tripledecker compounds as indicated by the intrinsic SMM and non-SMM nature of Tb2(Pc)[T(OPOSS)4PP]2(1)and Tb2(Pc)(TPP)2(2),respectively,at zero Oe.

Scheme 1Schematic molecular structure of POSS-involved hybrid trilple-decker complex Tb2(Pc)[T(OPOSS)4PP](1)
1 Experimental
1.1General Remarks
The trisilanollsobutyl POSS was purchased from HybridPlastics(Hattiesburg,MS,USA). Tetrahydrofuran(THF)was freshly distilled using Na withdiphenylketonecolorationundernitrogen atmosphere.N,N-dimethylformamide(DMF)was freshly distilled with CaH2under nitrogen atmosphere. 1,2,4-Trichlorobenzene(TCB)was freshly distilled withCaH2undernitrogenatmosphere.Column chromatography was carried out on silica gel(Merck, Kieselgel 60,63 μm~210 μm(70~230 mesh))with the indicated eluents.All other reagents and solvents were used as received.The compounds of Y(acac)3· nH2O(acac=acetylacetone),Tb(acac)3·nH2O,Li2Pc (H2Pc=phthalocyanine),chloropropylisobutyl POSS,5, 10,15,20-tetrakis(4-hydroxyphenyl)porphyrin and 5,10, 15,20-tetraphenyporphyrin,and Y2(Pc)(TPP)2(H2TPP= 5,10,15,20-tetra-phenyporphyrin)were prepared according to the literature procedure[7-9].
The1H NMR spectra were recorded on a Bruker DPX 400 spectrometer in CDCl3with shifts referenced to SiMe4(0.00 ppm).Electronic absorption spectra were recorded on a Hitachi U-4100 spectrophotometer.IR spectra were recorded in KBr pellets with 2 cm-1resolution using a Bruker Tensor 37 spectrometer.MALDI-TOF mass spectra were taken on a BrukerBIFLEXIIIultra-highresolutionFourier transform ion cyclotron resonance(FT-ICR)mass spectrometerwithalpha-cyano-4-hydroxycinnamic acid as the matrix.Elemental analyses were performed on an Elementar Vavio ElⅢ.Crystal data for compound 2 were determined by X-ray diffraction analysis at 150 K using Oxford Diffraction Gemini E system with Cu Kα radiation λ=0.154 18 nm,and details of the structure refinement are given in Table 1.Magneticmeasurementswereperformedona Quantum Design MPMS XL-5 SQUID magnetometer on multicrystalline samples.Data were corrected for thediamagnetismofthesamplesusingPascal constants and of the sample holder by measurement.
CCDC:989974,2.
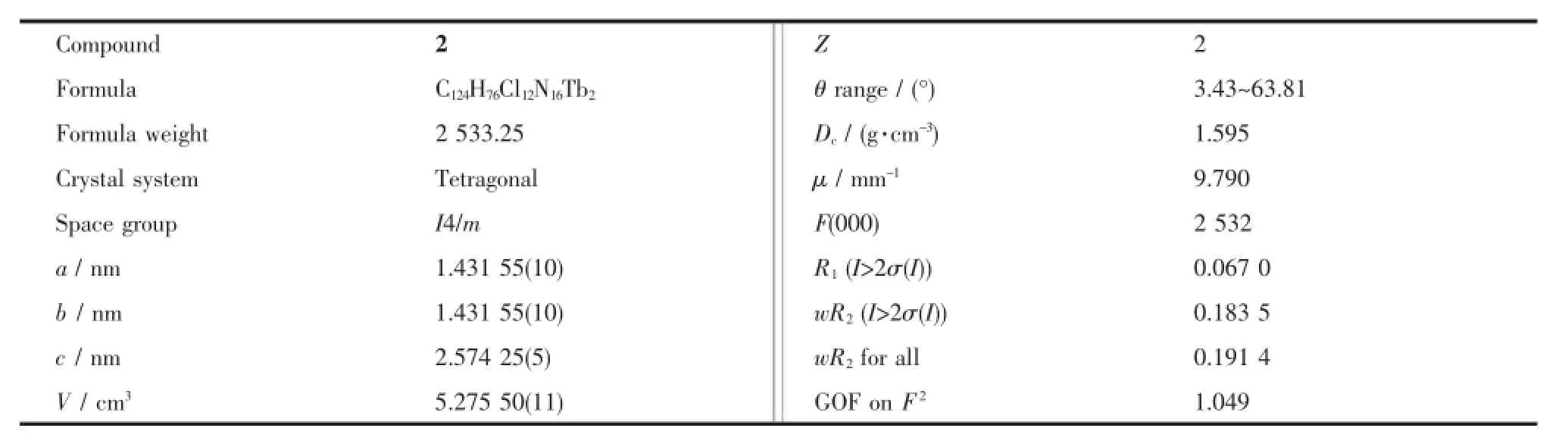
Table 1Crystal data and structure refinements of compound 2
1.2Synthesis of 5,10,15,20-tetra{[[N-[heptakis (isobutyl)propoxy]phenyl]octasiloxane]} porphyrin H2[T(OPOSS)4PP]
The mixture of5,10,15,20-tetrakis(4-hydroxyphenyl)porphyrin(34 mg,0.050 mmol)and K2CO3(60 mg,0.40 mmol)in dry DMF(200 mL)was refluxed under~3 cm3·min-1of N2stream at 80℃for 1 h.To which were added chloropropylisobutyl POSS(284 mg, 0.32 mmol)dissolved in dry THF(20 mL)and NaI(68 mg,0.32 mmol).The resulting mixture was stirred at 80℃for 24 h.After being cooled to room temperature, the reaction mixture was extracted with chloroform and then chromatographed on a silica gel column using CHCl3as the eluent to give a violet band,which was further purified using gel chromatography with CHCl3as eluent followed by recrystallization from CHCl3and MeOH,providing a dark violet solid with the yield of 19.1 mg(9%).1H NMR(400 MHz, CDCl3):δ 0.612~0.689(d,56H,iBu-CH2),0.965~0.981 (t,8H,γ propoxy-CH2),1.000~1.017(d,168H,iBu-CH3),1.868~1.973(m,28H,iBu-CH),2.067~2.124 (m,8H,β propoxy-CH2),4.216~4.249(t,8H,α propoxy -CH2),7.260~8.114(d,16H,Ph-H),8.855(d,8H,pyrrole-H).MALDI-TOFMS:anisotopiccluster peaking at m/z 4 110.77;Calcd.for C168H202N4O52Si32, 4 108.38.Anal.Calcd.(%)for C168H202N4O52Si32:C, 49.11;H,7.41;N,1.36;Found:C,49.20;H,7.04;N, 1.08
1.3Synthesis of Tb2(Pc)[T(OPOSS)4PP]2(1)
A mixture of H2[T(OPOSS)4PP](41 mg,0.01 mmol)and[Tb(acac)3]·nH2O(8 mg,0.02 mmol)in TCB(1.5 mL)was refluxed at 210℃under~3 cm3· min-1of N2stream for 4 h.The resulting dark cherryred solution was cooled to room temperature,then Li2Pc(2.6 mg,0.005 mmol)was added.The mixture was refluxed at 210C for a further 6 h,then the solvent was removed under reduced pressure.The reddish brown residue was applied on a silica gel column with CHCl3/hexane(2∶1,V/V)as the eluent to give a dark violet band,which was further purified usinggelchromatographywithCHCl3aseluent followed by recrystallization from CHCl3and MeOH, giving a dark green solid with the yield of 17.6 mg (29%).1H NMR(400 MHz,CDCl3):-92.658(s,8H, Pc-αH),-90.178(s,8H,endo-ortho phenyl-H),-47.517 (s,8H,Pc-βH),-42.527(s,16H,pyrrole-H),-31.500 (s,8H,endo-meta phenyl-H),-5.886 and-5.657(s, 48H,propoxy-H),-2.911(s,56H,iBu-CH2),-2.339 (s,168H,iBu-CH3),-1.605(s,28H,iBu-CH),-1.424 (s,56H,iBu-CH2),-0.741~-0.734(d,168H,iBu-CH3), -0.258~-0.241(d,28H,iBu-CH),12.771(s,8H,exometa phenyl-H),52.937(s,8H,exo-ortho phenyl-H). MALDI-TOF MS:an isotopic cluster peaking at m/z 9 059.98;Calcd.for C368H616N16O104Si64Tb2,9 044.20. Anal.Calcd.(%)for C368H616N16O104Si64Tb2:C,48.87;H, 6.87;N,2.48;Found:C,48.71;H,6.72;N,2.09.
1.4Synthesis of Tb2(Pc)(TPP)2(2)
By means of the above-mentioned procedure employed to prepare triple-decker 1 with a mixtureof H2(TPP)(6.1 mg,0.01 mmol)instead of H2[T(OPOSS)4PP]asstartingmaterial,thetargettriple-decker compound Tb2(Pc)(TPP)2(2)(5.0 mg,39%)was obtained.Single crystals of 2 suitable for X-ray diffraction analysis were grown from slow diffusion of methanol into the CHCl3solution of this compound.1H NMR(400 MHz,CDCl3):-94.308(s,8H,Pc-αH), -92.655(s,8H,endo-ortho phenyl-H),-48.691(s,8H, Pc-βH),-42.987(s,16H,pyrrole-H),-32.120(s,8H, endo-meta phenyl-H),-6.537(s,8H,para phenyl-H) 13.638(s,8H,exo-meta phenyl-H),54.820(s,8H,exoortho phenyl-H).MALDI-TOF MS:an isotopic cluster peaking at m/z 2 056.90;Calcd.for C120H72N16Tb2, 2 055.81.Anal.Calcd.(%)for C120H72N16Tb2·CHCl3· 2CH3OH:C,65.97;H,3.65;N,10.00;Found:C, 65.78;H,3.45;N,9.65.
2 Results and discussion
2.1Synthesis and Characterization
As a new class of condensed three-dimensional oligomericorganosiliceouscompoundswithcage framework,polyhedraloligomericsilsesquioxanes (POSS)have attracted increasing interests in recent years for the synthesis of organic-inorganic molecular hybrid materials[10].However,despite the recent preparation of quite a number of novel POSS-containing molecular hybrid materials including MCM-POSS[11a], C60-POSS[11b],and graphene-POSS[11c],the POSS-containing molecular hybrid materials with porphyrin as organic component still remain extremely rare,limited toZn[T(POSS)4PP](5,10,15,20-tetrakis{[[N-[heptakis (isobutyl)propyl]benzamidato]octasiloxane]phenyl} porphyrinato zinc)and Zn[M(POSS)PP](5-{[[N-heptakis (isobutyl)propyl]benzamidato]phenyl}octasiloxane-10, 15,20-tris(4-tert-butylphenyl)porphyrinato zinc)[12],to the best of our knowledge.In the present case,the metal free 5,10,15,20-tetrakis(4-hydroxyphenyl)porphyrin H2[T(OH)PP]was prepared according to published procedures[7].Treatment of H2[T(OH)PP]with K2CO3followed by reaction with chloropropylisobutyl POSS[8]affords the porphyrin-POSS molecular hybrid,namely metal free 5, 10,15,20-tetra{[[N-[heptakis(isobutyl)propoxy]phenyl] octasiloxane]}porphyrinH2[T(OPOSS)4PP].Reaction between H2[T(OPOSS)4PP]and[Tb(acac)3]·nH2O in situ generates the monomeric intermediate Tb[T(OPOSS)4PP](acac),which reacts with Li2Pc in 1,2,4-trichlorobenzene(TCB)to give mixed(phthalocyaninato) (porphyrinato)triple-deckers Tb2(Pc)[T(OPOSS)4PP]2(1) in the yield of 39%(Scheme 1).For comparative study, mixed(phthalocyaninato)(porphyrinato)triple-deckeranalogue Tb2(Pc)(TPP)2(2)(H2TPP=5,10,15,20-tetraphenyporphyrin)was also prepared following the same procedure.Satisfactory elemental analysis results were obtained for these newly prepared triple-decker compounds after repeatedly column chromatographic purification followed by recrystallization(Table 2).The MALDI-TOF mass spectra of these compounds clearly show intense signals for the molecular ion[M]+.The isotopic patterns closely resemble those of the simulated ones given in Fig.1.
2.2Structure of 2
Compound 2 crystallizes in the tetragonal system with an I4/m space group with two triple-deckers per unit cell.The crystal data are summarized in Table 1.
Fig.2 shows the molecular structure of 2 in two differentperspectiveviews.Inthistriple-decker compound,each terbium center is octa-coordinated by the pyrrole and isoindole nitrogen atoms of an outer TPP and the inner Pc rings,respectively,confirming the ligand arrangement of[(TPP)Tb(Pc)Tb(TPP)]in the triple-decker molecule.The two terbium centers are identical in terms of their coordination geometry and separation from the ligands.The twist angle,defined as the rotation angle of one macrocycle away from the eclipsed conformation of the two macrocycles,between Pc ring and TPP ring is 3.59°.The Pc ring locates exactly in the middle of the two terbium atoms with the distance between the outer TPP and the inner Pc rings of 0.310 2 nm.Both terbium centers lie closer to the TPP ring due to its larger central cavity in comparison with the Pc ligand,0.123 9 vs.0.186 3 nm,Table 3,with the intramolecular Tb…Tb distance amounting to 0.373 6 nm,indicating the presence of significantintramoleculardipole-dipoleinteractionbetween the two Tb atoms.
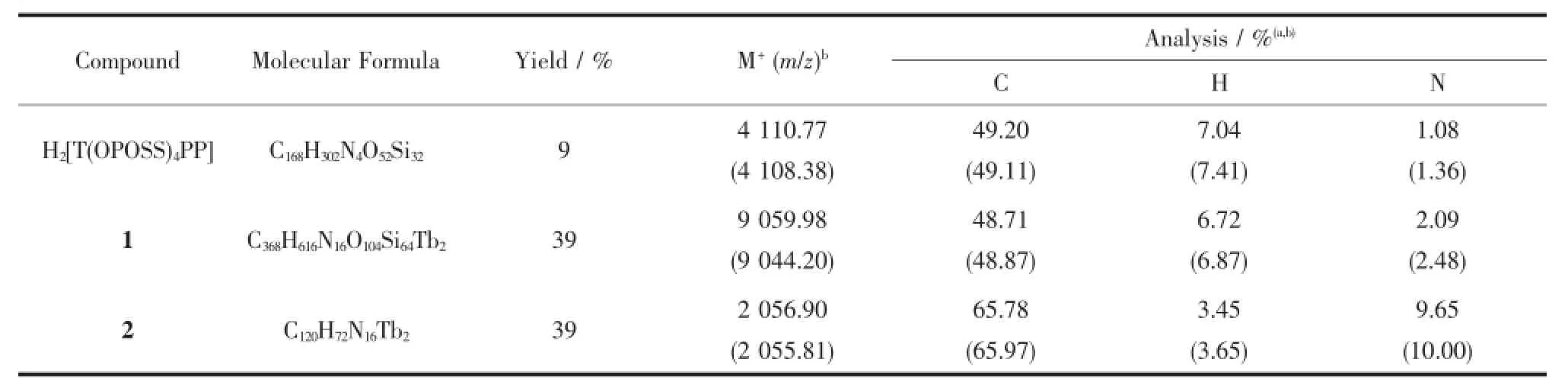
Table 2Mass spectroscopic and elemental analysis data for the compounds H2[T(OPOSS)4PP], Tb2(Pc)[T(OPOSS)4PP]2,Tb2(Pc)[TPP]2a
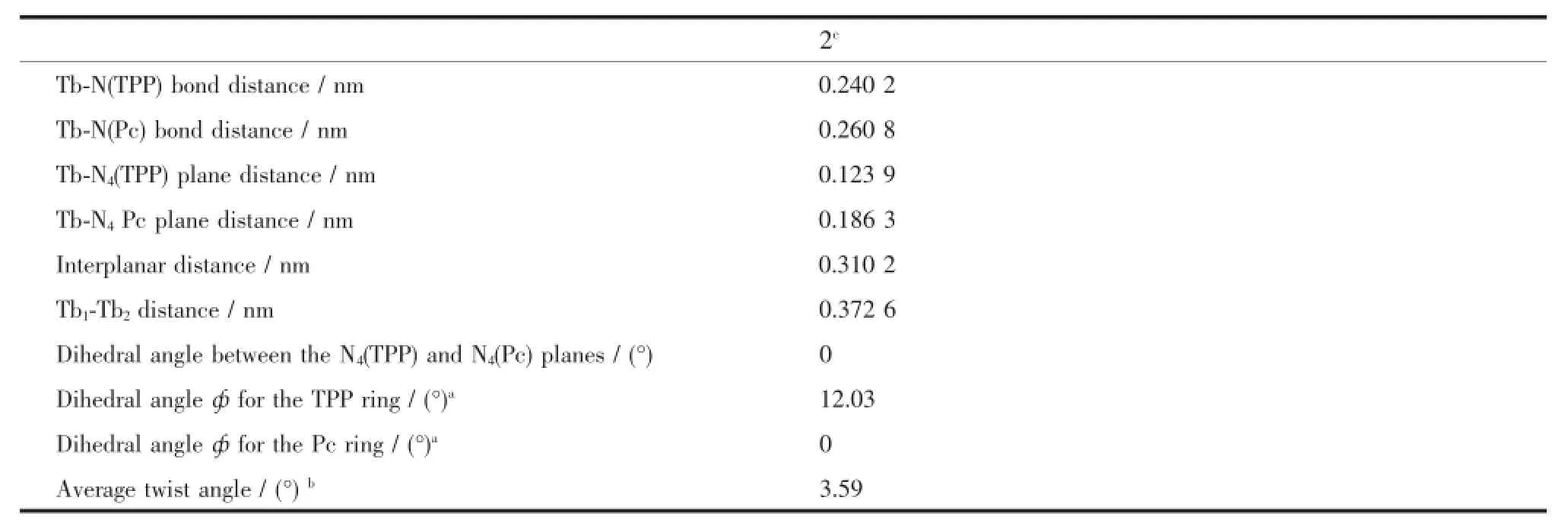
Table 3Comparison of the structural data for 2
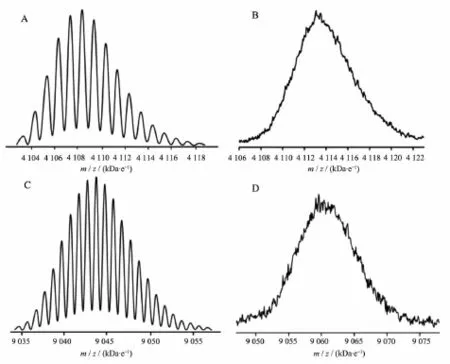
Fig.1 (A)Experimental and(B)simulated isotopic pattern shown in the MALDI-TOF mass spectrum of H2[T(OPOSS)4PP],(C) Experimental and(D)simulated isotopic pattern shown in the MALDI-TOF mass spectrum of Tb2(Pc)[T(OPOSS)4PP]2

Fig.2 Molecular structure of 2 in side view and top view with hydrogen atoms omitted and the ellipsoids drawn at the 30%probability levels(Tb(Ⅱ)green,C gray,N blue)
In the crystal of Tb2(Pc)(TPP)2(2),the adjacent triple-decker molecules are connected by the solvent CHCl3molecule to form a two dimensional(2D) structuredependingontheC-H…Clhydrogen bonding interaction between the C-H bond of the porphyrin phenyl group and the chlorine atom of CHCl3withthenearestintermolecularTb…Tb distance of 1.431 6 nm,Fig.3.These 2D structures arefurtherpackedintothreedimensional(3D) structure depending on Van der Waals interaction (Fig.4),with the nearest inter-molecular Tb…Tb distance of 1.364 2 nm.These long intermolecular terbium ionic distances seem to suggest the lack of the intermolecular dipole-dipole interaction between Tb ions.However,as detailed below,the non-SMM nature revealed for this compound at zero Oe,in combination with the typical SMM characteristic of the same compound after dilution with the diamagnetic Y2(Pc)(TPP)2,clearly indicates the presence of intermolecular dipole-dipole interaction between Tb ions and its effect on its magnetic properties.This appears in line with the previous investigation results that the dipole-dipole interaction between Tb ions with thedistance of 1.16 nm still cannot be neglected[4b,13].

Fig.3 Two dimensional packing plots of 2 in side view
Repeated trials fail to afford single crystals of the POSS-involved hybrid triple-decker complex Tb2(Pc)[T (OPOSS)4PP]2(1)suitable for X-ray diffraction analysis despite the great efforts paid thus far.
As can be seen in Figs.5 and 6,involvement of two paramagnetic terbium ions in these compounds induces observation of significantly broadened signals. For the triple-decker compound 2 without the POSS moieties,the signal observed at δ=-94.308 and -48.691 can be assigned to the non-peripheral and peripheral protons of the Pc ring respectively,the signal at δ=-42.987 is attributed to the pyrrole protons of the TPP ligand,while the signals at δ= -92.655,-32.120,-6.537,-13.638,and 54.820 are due to the protons in the phenyl moieties of the porphyrin ligand,Fig.5.This seems also true for the triple-decker analogue 1 with POSS moieties attached at the porphyrin ligand through the meso-phenyl groups,Fig.6,with the signals of the Pc protons at δ=-92.658 and-47.678,the signal of the pyrrole protons of the porphyrin ligand observed at δ= -43.368,and the signals of the protons in the aryl moieties of T(OPOSS)4PP resonating at δ=-90.178, -31.591,12.775and53.302.Additionalsignals observedatδ=-2.911,-1.424,-2.339,-1.605, -0.741~-0.734,and-0.258~-0.241 in the1H NMR spectrum of 1 are assigned to the protons in the isobutylsubstituentsofPOSSandthesignals appearing at δ=-5.891 and-5.660 to the protons in the propoxy linkers which connect the POSS and porphyrin moieties.

Fig.4 Three dimensional packing plots of 2 in side view
The electronic absorption spectra of the two complexes 1 and 2 recorded in CHCl3are shown in Fig. 7 and 8.Both triple-decker compounds 1 and 2 exhibit typical feature of the electronic absorption spectra for mixed(phthalocyaninato)(porphyrinato)rare earth triple-decker complexes in the form of(TAP)M(Pc)M(TAP)(H2TAP=5,10,15,20-tetraarylporphyrin)with the tetrapyrrole Soret bands appearing at 352~353 and 418~420 nm and Q bands at 492~493,605~606,and 976~977 nm,respectively[14].
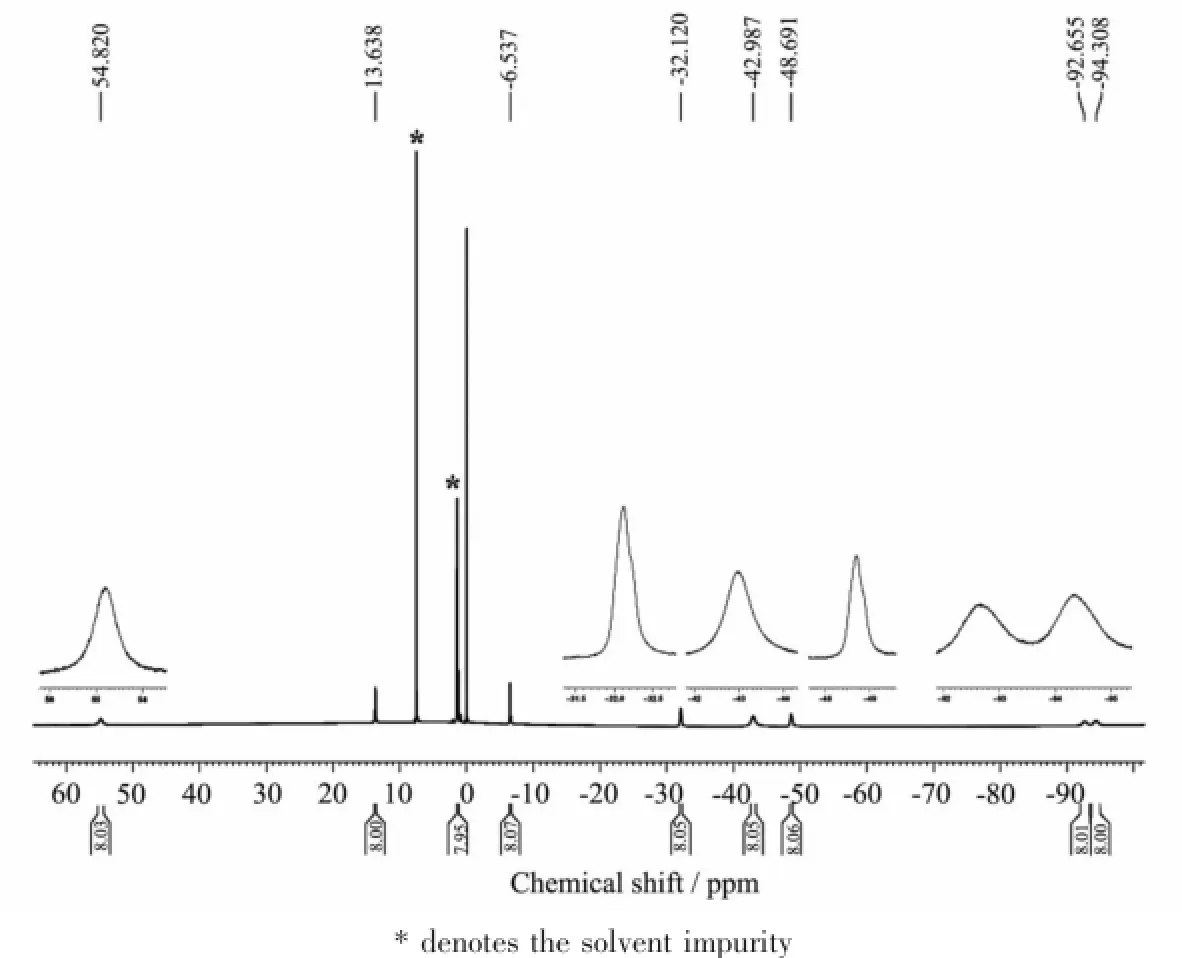
Fig.5 1H NMR spectra of compound 2 recorded in CDCl3

Fig.6 Part of1H NMR spectra of compound 1 recorded in CDCl3
In the IR spectra of triple-decker compounds Tb2(Pc)[T(OPOSS)4PP]2(1)and Tb2(Pc)(TPP)2(2)(Fig.9), the moderately strong band observed at 1 330~1 333 cm-1is attributed to the characteristic IR band for Pc2-[15]. In addition to the absorption bands contributed from the central aromatic Pc and Por macrocycle(including the wagging and torsion vibrations of C-H groups,and the C=N aza group stretching vibrations that are commonly appearing in the spectra of both compounds), the strong absorptions observed at 1 102~1 109 cm-1for Tb2(Pc)[T(OPOSS)4PP]2(1)are contributed by the Si-O-Si stretching vibrations and the absorptions at 1 231~1 238 cm-1by the symmetric C-O-C stretching vibrations.Itisworthnotingthatboththese absorptions could be observed in the IR spectrum of Tb2(Pc)(TPP)2(2).This is also true for the most intensebands observed at 2 924~2 927 cm-1and 2 954~2 959 cm-1in the IR spectrum of 1 due to the antisymmetric C-H stretching vibrations of the-CH2-and-CH3groups,respectively[16].
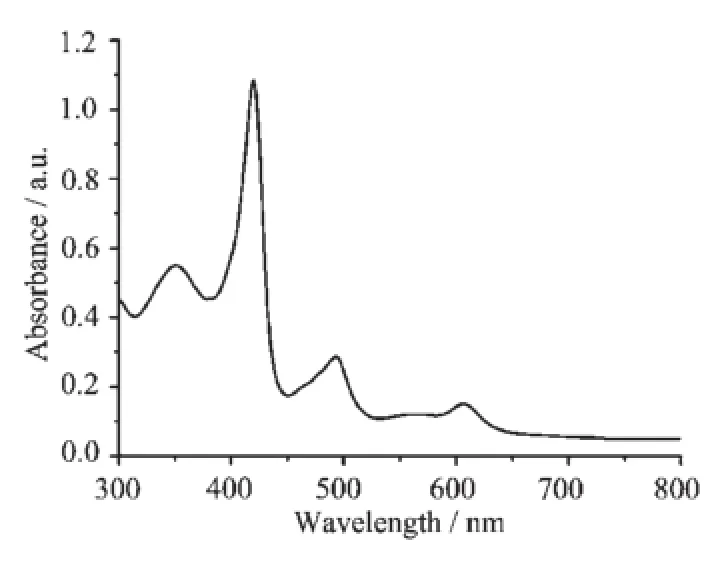
Fig.7 Electronic absorption spectrum of 1 in CHCl3

Fig.8 Electronic absorption spectrum of 2 in CHCl3

Fig.9 IR spectra of T(OPOSS)4PP,1 and 2
2.3Magnetic Properties
The dc magnetic properties of triple-deckers 1 and 2 are shown in Fig.10 under a 1 000 Oe field in the temperature range of 2~300 K.The effect of covalentlyincorporatedPOSSmoietiesatthe porphyrin periphery on the magnetic behavior of the terbium triple-decker complexes is shown in Fig.10 by magnetic measurements over the sample of Tb2(Pc) (TPP)2(2)diluted by Y2(Pc)(TPP)2in the molar ratio of 1∶9,isostructual to 2 according to XRD analysis(Fig. 11).The χmT value for per mol Tb2unit at 300 K is 23.68 cm3·K·mol-1for 1,23.88 cm3·K·mol-1for 2, and 23.81 cm3·K·mol-1for the diluted sample of 2, respectively,whichareallconsistentwiththe expected value of 23.62 cm3·K·mol-1for two Tb(Ⅱ) ions(7F6,S=3,L=3,g=3/2)[4b].When the temperature is lowered,the χmT value of all the three samples decreases slowly until about 20 K,resulting from the crystal-field effects such as thermal depopulation of the lanthanide metal(Ⅱ)Stark sublevels.Then the χmT value increases quickly to reach a value of 22.04, 23.66,and 23.65 cm3·K·mol-1for 1,2,and diluted sample of 2 at 2 K,respectively,indicating the presence of ferromagnetic interaction between two Tb (Ⅱ)ions in the triple-decker molecules[17].However,at low temperature,the curve of 1 increases more quickly than that of 2,probably owing to the weak antiferromagnetic interaction between the neighboring triple-deckers in 2 caused by the intermolecular dipole-dipole interaction.This is further verified by a qualitative manner according to the ΔχmT tendency by subtracting the χmT of 1 from that of 2,ΔχmT1,as well as the ΔχmT tendency by subtracting the χmT of diluted sample of 2 from that of 2,ΔχmT2.As shown in Fig.10, as the temperature lowers,the χmT value increases slowly until about 20 K,and then decreases quickly, confirming the presence of antiferromagnetic interaction between the neighboring triple-decker molecules of 2.The change tendency of ΔχmT1is much faster than that of ΔχmT2,indicating that connecting bulky inorganic POSS components at the porphyrin periphery of triple-decker compound leads to more obviously increase in the distance between neighboring tripledecker molecules and results in a more significant diminishmentintheintermolecularinteractionbetween the triple-decker molecules than the physical dilution method.Fig.12 displays the magnetization(M vs H/T)curves for 1 and 2 at different temperatures(2, 3,5 K),which shows a rapid increase tendency at low field and eventually reach the maximum value of 8.93μBfor 1 and 6.09μBfor 2 at 2 K,respectively,without achieving the magnetization saturation in terms of the expected saturation value of 18μBfor two Tb(Ⅱ)ions(9μB for each Tb(Ⅱ)ion),indicating the presence of magnetic anisotropy and the crystal-field effect for the Tb(Ⅱ) ions.
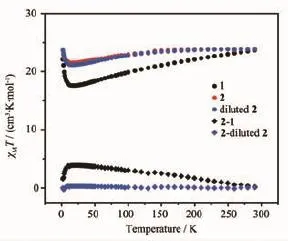
Fig.1 0Temperature(T)dependence of χmT for compounds 1,2,diluted 2,2-1,and 2-diluted 2 at 1000 Oe
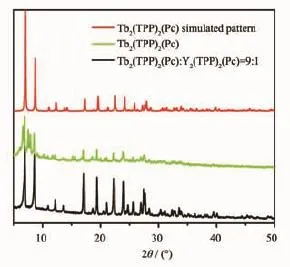
Fig.1 1XRD patterns of 2 and diluted 2 together with simulatedpatternfromsinglecrystalXRDdataof2
Fig.13 shows temperature dependence of the inphase(χ)and out-of-phase(χ)ac susceptibility of Tb2(Pc)(TPP)2(2)(A),diluted Tb2(Pc)(TPP)2(2)with Y2(Pc)(TPP)2at a molar ratio of 1∶9(B),and Tb2(Pc)[T (OPOSS)4PP]2(1)(C),respectively,under zero Oe dc magnetic field in a 3.0 Oe ac field oscillating at 1.0~870 Hz.The in-phase signal(χ)of both Tb2(Pc)[T (OPOSS)4PP]2(1)and diluted sample of Tb2(Pc)(TPP)2(2)with diamagnetic Y2(Pc)(TPP)2shows the frequency -dependent character,revealing their SMM nature. This,however,is nottrue for Tb2(Pc)(TPP)2(2). Obviously,introduction of the bulky POSS moieties onto the porphyrin periphery in the triple-decker compound 1 leads to the increase in the distance between neighboring triple-decker molecules,inducing a diminished intermolecular interactions between the triple-decker molecules and therefore showing the same effect over the magnetic behavior of tripledecker compound as done by diluting method.This result clearly indicates the advantage of connecting bulky inorganic POSS components at the porphyrin periphery of triple-decker compound in improving their magnetic properties.
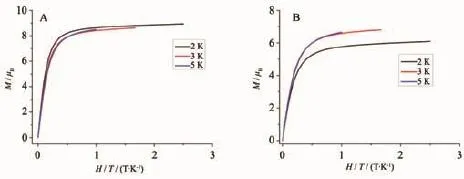
Fig.1 2M vs.H/T curves for 1(A)and 2(B)at different temperatures
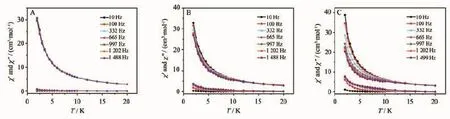
Fig.1 3Temperature dependence of the in-phase(χ′)and out-of-phase(χ″)ac susceptibility of Tb2(Pc)(TPP)2(2)(A),diluted Tb2(Pc)(TPP)2(2)with Y2(Pc)(TPP)2at a molar ratio of 1∶9(B),and Tb2(Pc)[T(OPOSS)4PP]2(1)(C),respectively, under zero Oe dc magnetic field
As expected,with the help of the external direct current(dc)magnetic field,the frequency-dependent character in the in-phase signal(χ)and out-of-phase signal(χ)becomes more obviously for both 1 and the diluted sample of 2.Nevertheless,pure sample of triple-decker compound 2 without slow relaxation behavior under zero Oe dc magnetic field does show the frequency-dependent character under the external dc magnetic field,indicating its field-induced SMM nature[3c,18].On the basis of a thermallyactivated mechanism,τ=τ0exp[-Ueff/(kT)]and τ=1/(2πυ),the Arrhenius law fitting for these picked peaks in χ vs.T curves for compounds 1,2,and diluted sample of 2 under 3 000 Oe dc magnetic field is shown in Fig.14. A linear relationship exists between ln(τ)and 1/T for 1,2,and diluted sample of 2,which in turn results in thequitesimilareffectiveenergybarrierof magnetization relaxation Ueff=14.4 cm-1(20.7 K),13.4cm-1(19.3 K),and 13.9 cm-1(20.0 K)for 1,2,and the diluted sample of 2(but different relaxation time τ0= 0.58,8.2,and5.3μ s)duetotheeffectively suppressing of the QTM with external direct current (dc)magnetic field in particular for the triple-decker compound 2.This actually gives additional evidence for the effect of the covalently connecting bulky inorganicPOSScomponentsattheporphyrin periphery of triple-decker compound on separating the magnetic cores and diminishing the intermolecular interaction,which in turn improves the magnetic properties of the triple-decker SMM.

Fig.1 4Temperature dependence of the in-phase(χ′)and out-of-phase(χ″)ac susceptibility of Tb2(Pc)[T(OPOSS)4PP]2(1)(A,B), Tb2(Pc)(TPP)2(2)(C,D)and diluted Tb2(Pc)(TPP)2(2)with Y2(Pc)(TPP)2at a molar ratio of 1∶9(E,F),respectively, under 3 000 Oe dc magnetic field
3 Conclusions
Intrinsic and field-induced SMM nature were revealed for the POSS-involved hybrid triple-decker sandwich-type complex Tb2(Pc)[T(OPOSS)4PP]2and analogue Tb2(Pc)(TPP)2,respectively,indicating the significant effect of covalently-linked,homogenously dispersed POSS moieties around the triple-decker core on improving the magnetic property of corresponding compounds.
Acknowledgements:Financial support from the Natural Science Foundation of China,National Key Basic Research ProgramofChina(GrantNo.2013CB933402and 2012CB224801),National Ministry of Education of China,and BeijingMunicipalCommissionofEducationisgratefully acknowledged.
References:
[1](a)Sessoli R,Tsai H,Schake A R,et al.J.Am.Chem.Soc., 1993,115:1804-1816
(b)Sessoli R,Gatteschi D,Caneschi A,et al.Nature, 1993,365:141-143;
(c)Woodruff D N,Winpenny R E P,Layfield R A.Chem. Rev.,2013,113:5110-5148
[2](a)Leuenberger M N,Loss D.Nature,2001,410:789-793
(b)Ardavan A,Rival O,Morton J J L,et al.Phys.Rev.Lett., 2007,98:057201(1/2/3/4)
(c)Mannini M,Pineider F,Danieli C,et al.Nature,2010,468: 417-421
(d)Urdampilleta M,Nguyen N V,Cleuziou J P,et al.Int.J. Mol.Sci.,2011,12:6656-6667;
(e)Stamp P C E,Gaita-Arino A.J.Mater.Chem.,2009,19: 1718-1730
[3](a)Habib F,Lin P,Long J,et al.J.Am.Chem.Soc.,2011, 133:8830-8833
(b)Fukuda T,Matsumura K,Ishikawa N.J.Phys.Chem.A, 2013,117:10447-10454
(c)Kan J,Wang H,Sun W,et al.Inorg.Chem.,2013,52: 8505-8510
(d)Wang H,Cao W,Liu T,et al.Chem.Eur.J.,2013,19: 2266-2270
(e)Zhang P,Guo Y,Tang J.Coord.Chem.Rev.,2013,257: 1728-1763;
(f)Wang H,Qian K,Wang K,et al.Chem.Commun.,2011, 47:9624-9626
[4](a)Titos-Padilla S,Ruiz J,Herrera J M,et al.Inorg.Chem., 2013,52:9620-9626
(b)Morita T,Katoh K,Breedlove B K,et al.Inorg.Chem., 2013,52:13555-13561
(c)Aronica C,Pilet G,Wernsdorfer W,et al.Angew.Chem. Int.Ed,.2006,45:4659-4662
(d)Guo Y,Xu G,Wernsdorfer W,et al.J.Am.Chem.Soc., 2011,133:11948-11951
[5](a)Jiang S,Wang B,Su G,et al.Angew.Chem.Int.Ed., 2010,49:448-7451
(b)Ishikawa N,Sugita M,Wernsdorfer W.J.Am.Chem. Soc.,2005,127:3650-3651
(c)Wang H,Liu C,Liu T,et al.Dalton.Trans.,2013,42: 15355-15360
[6](a)Meihaus K R,Long J R.J.Am.Chem.Soc.,2013,135: 17952-17957
(b)Lopez N,Prosvirin A.V,Zhao H,Wrnsdorfer W,et al. Chem.Eur.J.,2009,15:11390-11400
(c)Meihaus K R,Rinehart J D,Long J R.Inorg.Chem. 2011,50:8484-8498
(d)Vergnani L,Barra A,Neugebauer P,et al.Chem.Eur. J.,2012,18:3390-3398
[7]Kumar A,Maji S,Dubey P,et al.Tetrahedron Lett., 2007,48:7287-7290
[8]ZhangW,MullerAHE.Polymer,2010,51:2133-2139
[9]Sun X,Li R,Wang D,et al.Eur.J.Inorg.Chem.,2004,19: 3806-3813
[10]Cordes D B,Lickiss P D,Rataboul F.Chem.Rev.,2010, 110:2081-2173
[11](a)Jang K S,Kim H J,Johnson J R,et al.Chem.Mater., 2011,23:3025-3028
(b)ClarkeDJ,MatisonsJG,SimonGP,etal.Appl.Organomet. Chem.,2010,24:184-188
(c)Xue Y,Liu Y,Lu F,et al.J.Phys.Chem.Lett.,2012,3: 1607-1612
[12]Sun J,Chen Y,Zhao L,et al.Chem.Eur.J.,2013,19: 12613-12618
[13]Katoh K,Horii Y,Yasuda N,et al.Dalton Trans.,2012,41: 13582-13600
[14]Sun X,Cui X,Arnold D P,et al.Eur.J.Inorg.Chem., 2003,18:1555-1561
[15](a)Jiang J,Arnold D P,Yu H.Polyhedron,1999,18:2129-2139
(b)Lu F,Bao M,Ma C,et al.Spectrochim.Acta A,2003, 59:3273-3286
(c)Bao M,Pan N,Ma C,et al.Vib.Spectrosc.,2003,32: 175-184
[16]Shang H,Wang H,W.Wang K,et al,Dalton Trans., 2013,42:1109-1115
[17](a)Katoh K,Kajiwara T,Nakano M,et al.Chem.Eur.J., 2011,17:117-122
(b)Wang H,Liu T,Wang K,et al.Chem.Eur.J.,2012,18: 7691-7694
[18](a)Yamashita A,Watanabe A,Akine S,et al.Angew. Chem.Int.Ed.,2011,50:4016-4019
(b)Feltham H L C,Klower F,Cameron S.A,et al.Dalton Trans.,2011,40:11425-11432
(c)Koo B H,Lim K S,Ryu D W,et al.Chem.Commun., 2012,48:2519-2521
(d)Ruiz J,Lorusso G,Evangelisti M,et al.Inorg.Chem., 2014,53:3586-3594
(e)Chen L,Wang J,Wei J,et al.J.Am.Chem.Soc., 2014,136:12213-122
Mixed Tetrapyrrole Terbium Triple-Decker Single Molecule Magnets with Bulky Inorganic Polyhedral Oligomeric Silsesquioxanes Moieties at Outer Porphyrin Peripheries
ZHANG Lu1ZENG Su-Yuan2LIU Tao3SUN Jun-Shan1DOUJian-Min*,2JIANG Jian-Zhuang*,1
(1Beijing Key Laboratory for Science and Application of Functional Molecular and Crystalline Materials,Department of Chemistry, University of Science and Technology Beijing,Beijing 100083,China)
(2Department of Chemistry,Liaocheng University,Liaocheng,252004,China)
(3State Key Laboratory of Fine Chemicals,Dalian University of Technology,Dalian,116012,China)
Bulky inorganic polyhedral oligomeric silsesquioxanes(POSS)moieties were introduced onto the porphyrin periphery in mixed(phthalocyaninato)(porphyrinato)terbium molecule,giving POSS-involved hybrid triple-decker complex Tb2(Pc)[T(OPOSS)4PP]2(1)[H2Pc=phthalocyanine;H2T(OPOSS)4PP=5,10,15,20-tetra{[[N-[heptakis(isobutyl)propoxy]phenyl]octasiloxane]}porphyrin].For com-parative study,triple-decker analogue Tb2(Pc) (TPP)2(2)(H2TPP=5,10,15,20-tetraphenyporphyrin)was also prepared and structurally characterized.In particular, Tb2(Pc)[T(OPOSS)4PP]2(1)and Tb2(Pc)(TPP)2(2)were revealed to display intrinsic single molecule magnet(SMM) and non-SMM(or field-induced)characteristic,respectively,at zero Oe(or a 3.0 Oe ac field),clearly indicating the effect of the covalently linked,homogenously dispersed POSS moieties on effectively separating the magnetic cores and improving the magnetic property of triple-decker compounds.CCDC:989974,2.
tetrapyrrole;sandwich POSS;molecular hybrid;single molecule magnet
O614.341;O645.16+2
A
1001-4861(2015)09-1761-13
10.11862/CJIC.2015.190
2015-04-01。收修改稿日期:2015-05-22。
国家自然科学基金(No.21290174),国家重点基础研究发展计划(973计划)(No.2013CB933402和No.2012CB224801)。
*通讯联系人。E-mail:jianzhuang@ustb.edu.cn,jmdou@lcu.edu.cn
