Evolutionary relationship of 5’-untranslated regions among Thai dengue-3 viruses, Bangkok isolates, during 24 year-evolution
2015-11-30WatchareeAttatippaholkunPanyupaPankhongAnandaNisalakSiripenKalayanarooj
Watcharee Attatippaholkun, Panyupa Pankhong, Ananda Nisalak, Siripen Kalayanarooj
1Department of Clinical Chemistry, Faculty of Medical Technology, Mahidol University, Bangkok 10700, Thailand
2Department of Virology, US Army Medical Component, Armed Forces Research Institute of Medical Sciences, Bangkok 10400, Thailand
3Queen Sirikit National Institute of Child Health, Bangkok 10400, Thailand
Evolutionary relationship of 5’-untranslated regions among Thai dengue-3 viruses, Bangkok isolates, during 24 year-evolution
Watcharee Attatippaholkun1*, Panyupa Pankhong1, Ananda Nisalak2, Siripen Kalayanarooj3
1Department of Clinical Chemistry, Faculty of Medical Technology, Mahidol University, Bangkok 10700, Thailand
2Department of Virology, US Army Medical Component, Armed Forces Research Institute of Medical Sciences, Bangkok 10400, Thailand
3Queen Sirikit National Institute of Child Health, Bangkok 10400, Thailand
ARTICLE INFO
Article history:
Received 15 December 2014
Received in revised form 20 January 2015
Accepted 15 February 2015
Available online 20 March 2015
Thai dengue-3 viruses
Evolutionary relationship
5'untranslated regions
24 Year-evolution
Objective: To study evolutionary relationship of the 5'untranslated regions (5'UTRs) in low passage dengue3 viruses (DEN3) isolated from hospitalized children with different clinical manifestations in Bangkok during 24 year-evolution (1977-2000) comparing to the DEN3 prototype (H87). Methods: The 5'UTRs of these Thai DEN3 and the H87 prototype were amplified by RT-PCR and sequenced. Their multiple sequence alignments were done by Codon Code Aligner v 4.0.4 software and their RNA secondary structures were predicted by MFOLD software. Replication of five Thai DEN3 candidates comparing to the H87 prototype were done in human (HepG2) and the mosquito (C6/36) cell lines. Results: Among these Thai DEN3, the completely identical sequences of their first 89 nucleotides, their high-order secondary structure of 5'UTRs and three SNPs including the predominant C90T, and two minor SNPs including A109G and A112G were found. The C90T of Thai DEN3, Bangkok isolates was shown predominantly before 1977. Five Thai DEN3 candidates with the predominant C90T were shown to replicate in human (HepG2) and the mosquito (C6/36) cell lines better than the H87 prototype. However, their highly conserved sequences as well as SNPs of the 5'UTR did not appear to correlate with their disease severity in human. Conclusions: Our findings highlighted evolutionary relationship of the completely identical 89 nucleotide sequence, the high-order secondary structure and the predominant C90T of the 5'UTR of these Thai DEN3 during 24 year-evolution further suggesting to be their genetic markers and magic targets for future research on antiviral therapy as well as vaccine approaches of Thai DEN3.
1. Introduction
Dengue is the leading cause of children hospitalization and its outbreaks continue to pose a public health problem in Thailand until present. Dengue disease incidence has increased to the largest incidence reported of 325/100 000 in 1987 and 124/100 000 in 2012[1]. In recent years, dengue fever (DF) and its more serious forms, dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS), have emerged geographic distributions and increased epidemic activities[2]. An analysis of death frequency among patients infected with each dengue serotype identified a greater than expected frequency of death among patients infected with DEN3, especially during the 1987-1988[3]. During that epidemic, DEN3 viruses were recovered from an unusual number of patients hospitalized with primary infection, further suggesting that the intra-epidemic isolates of DEN3 were more than unusually virulent.
Thailand is one of the biggest dengue-endemic countries in the world: in particular, this country has seen the largest incidence ofDHF and DSS since 1987. Metropolitan Bangkok is the epicenter of dengue in Thailand and the Queen Sirikit National Institute of Child Health (QSNICH), formerly Bangkok Children's Hospital, has admitted the greatest number of dengue cases for over 40 years because it is government-financed, accessible to all, and widely recognized center of excellence[4]. Since 1973, the QSNICH and the US Army Medical Component (USAMC), Armed Forces Research Institute of Medical Sciences (AFRIMS), Bangkok, Thailand have collaborated to conduct longitudinal surveillance of dengue in Bangkok. DEN 1, and 2 were associated with moderate severedengue epidemic years but only DEN3 involved severe dengue years especially 1987-1988. Since then, DEN3 outbreak with DHF/DSS has continued periodically in Thailand including Bangkok until present.

Table 1 List of Thai DEN3, Bangkok isolates during 1977-2000.
The pathogenesis of dengue severity remains unclear and neither specific antiviral therapy nor licensed vaccine exists. The molecular biology of dengue virus is not well understood. The dengue genome is a single-strand sense RNA of 11 kbs in length. It encodes an uninterrupted open reading frame (ORF) flanked by 5' and 3' untranslated regions (UTRs) with approximately 100 and 400 nucleotides respectively. The order of proteins encoded in the dengue genome is Cap5'-C-prM-E-NS1-NS2A-NS2B-NS3-NS4ANS4B-NS5-3'. The genomic RNA of dengue virus has a type I 5' cap of m7GpppA which exhibit no internal ribosomal entry site (IRES) region for translation initiation, but lacking a poly (A) tail at the 3' end[5,6]. Both 5' and 3'UTRs of dengue virus and flaviviruses have the potential to form secondary structures that are functional important and conserved among flaviviruses[7,8]. However, the most significant function of the 5'UTR probably resides in its reverse complement, the 3'UTR of viral minus strands, which forms the site for initiation of plus-strand synthesis[9].
There are two AUG codons on the 5'UTR of all four dengue serotypes. The first and the second AUG codons of DEN3 are located at the nucleotides 95 and 137 respectively (GenBank Accession no. M93130)[10]. The protein translation of the dengue polyprotein is preferentially initiated and started at the first AUG codon, with the exception that DEN-1, DEN-2 and DEN-3 can utilize the second initiation codon with equal efficiency[11]. The genome of positivestrand RNA viruses participates in at least three different processes in the cytoplasm of the infected host cell. They serve as mRNA to direct the synthesis of viral proteins and also act as a template for genome amplification. They are packaged along with structural proteins during viral assembly. The balance between these processes must be properly maintained to allow efficient viral proliferation. The molecular mechanisms controlling the utilization of the viral RNA in each step of the viral life cycle are still poorly understood. Very little is known, however, about the secondary structures of the noncoding and coding regions despite some evidence that the coding region might also contain functionally RNA motifs[12,13]. Thus, defining the molecular determinants that regulate utilization of the viral RNA in the infected cell is of central importance for understanding the dengue virus life cycle.
Here, we conducted a molecular epidemiology study on evolutionary relationship of the 5'UTRs among Thai DEN3 isolated from hospitalized children with different clinical manifestations in Bangkok during 24 year-evolution (1977-2000) comparing to the H87 prototype.
2. Materials and methods
2.1. Virus strains
In this study, DEN3 were isolated from children hospitalized at the QSNICH who presented with different severity grades ranging from DF, DHF and DSS, during 1977-2000 (Table 1). These low passage DEN3, having no more than 4 passages in the laboratory, including no more than 2 passages in LLC-MK2 cells (monkey kidney; for isolates made prior to 1981) and no more than 3 passages in Aedes albopictus cells (C6/36 cells), were kindly provided from Department of Virology, USAMC, AFRIMS, Bangkok, Thailand. Each isolate was characterized by date, grade of disease produced, age of the patient (infant with presumed maternal antibodies, or child), and whether the infection was primary or secondary (infection sequence). Grading of each patient was done by QSNICH physicians using WHO classification guidelines. Characterization of the dengue serotypes and serological tests was primarily done by AFRIMS. Viral nomenclature for Thai DEN3 in this study was described as the following; D stand for dengue virus, the first two numerals are the year of isolation and the last four digits indicate the sample number. All of these dengue samples were confirmed to be DEN3 by RTPCR using serotype-3 specific primers[14]. All theses Thai DEN-3 sequences reported herein have already been submitted to GenBank (accession number DQ863567 to DQ863637) shown in Table 1.
2.2. RNA extraction, RT-PCR and sequencing of the 5’UTRs
Total genomic RNA of each DEN3 isolate was extracted by using QIA amp Vira1 RNA Mini Kit (Qiagen, Germany) following the manufacturer's instructions. The extracted RNAs were stored at -80 ℃ in the presence of RNasin® ribonuclease inhibitor (Promega, USA) until used. The RT-PCR amplifications of 465 base pairs in length (nucleotides 1-465 based on the H87 prototype, GenBank accession no. M93130) were generated using the specific primers at 5'end: 5'AGTTGTTAGTCTACGTGGACCG3' and 3'end: 5'GGCTCTCCATCTCGTGA GAGTTA 3'[15]. The amplified product of each Thai DEN3 isolate was analyzed by agarose gel electrophoresis and the specific band of 465 bps was cut and purified by using QIAquick® gel extraction kit (QIAGEN, Germany) following the manufacturer's instructions. Finally, the purified band of 465 bps was used as the template for sequencing by the BigDye® Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, USA) following the manufacturer's instructions. Sequences were obtained from both strands of each RT-PCR amplicon using the same primers for amplification.
2.3. Multiple sequence alignment and RNA secondary structure prediction
Multiple sequence alignment was performed by Codon Code Aligner v 4.0.4 software (http://www.codoncode.com/aligner/ downlode.htm). The predicted RNA secondary structures were generated by MFOLD software[16] (http://www.bioinfo.rpi.edu/ applications/mfold/old/rna/form1.cgi). The sequences of other dengue viruses accessed from Genbank were used for comparison in this study as shown in Table 2.
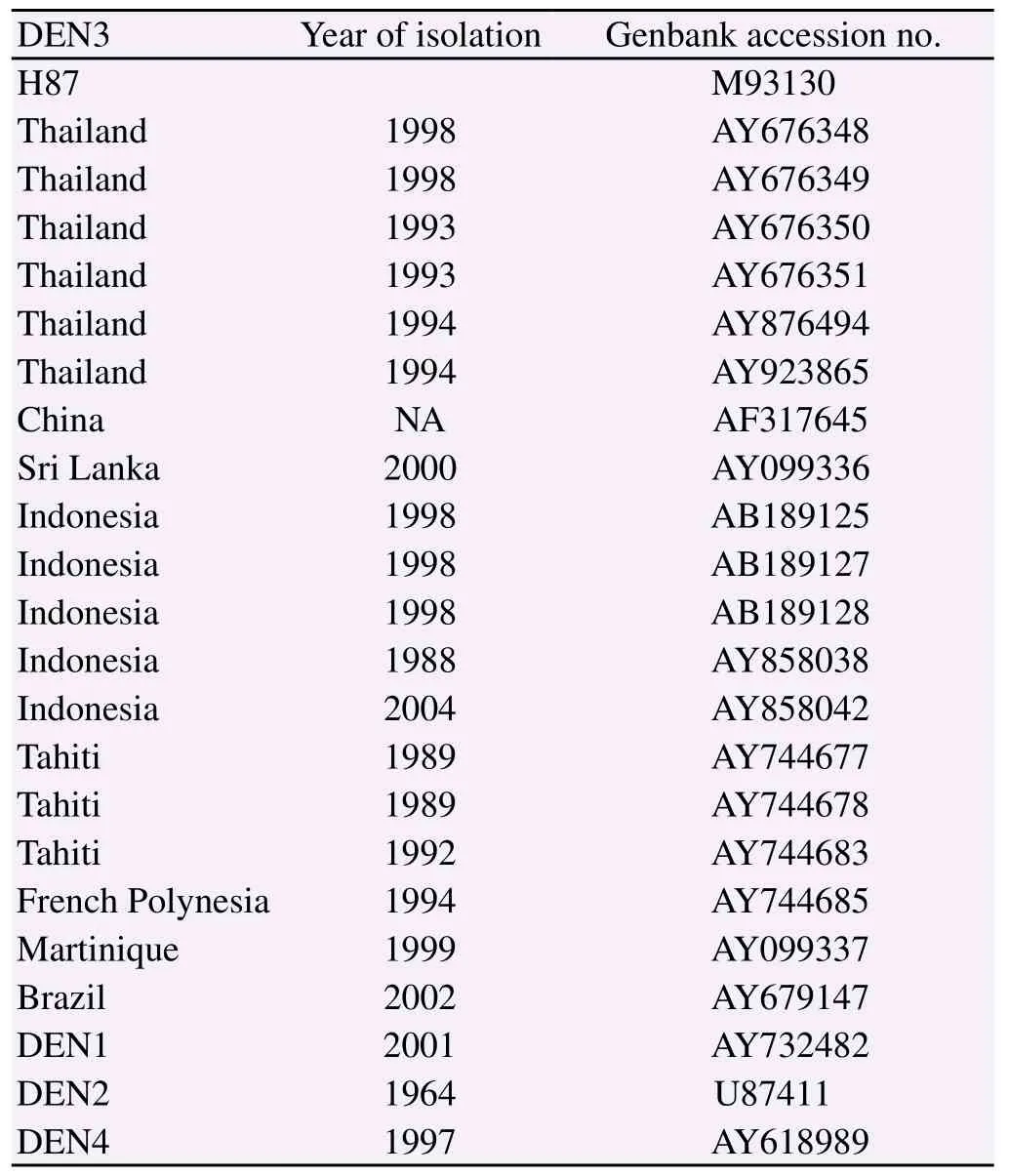
Table 2 Nucleotide sequences from other dengue viruses used in this study.
2.4. Cell culture
The human hepatocellular carcinoma cell line, HepG2, and the mosquito cell line, C6/36 were purchased from the American Type Culture Collection (Manassas, USA) and grown in minimum essential medium (GIBCO-Invitrogen, USA) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone, USA), 1% nonessential amino acids, 1mM sodium pyruvate, 100 U/mL penicillin, and 100 mg/mL streptomycin (GIBCO, USA). The HepG2 and C6/36 were maintained in the humidified incubator at 28 ℃ and 37 ℃ with 5% CO2, respectively. The African green monkey kidney cell line (Vero cell) was maintained in Dulbecco's modified Eagle's medium (GIBCO-Invitrogen, USA) containing 10% heat-inactivated fetal bovine serum (Hyclone, USA), 1% nonessential amino acids, 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM L-glutamine, and 1% OPI (GIBCO, USA), and maintained in a humidified incubator at 37 ℃ with 5% CO2[17].
2.5. Virus infection in HepG2 and C6/36cells for monitoring the replication rate
The infections were performed in the 75-cm2flask. Both cell lines, HepG2 and C6/36 were infected with each DEN3 candidate (Table 3) at a multiplicity of infection (MOI) of approximately 0.01. After adsorption for 2 h, 30 mL of growth medium was added and the cultures were incubated in 5% CO2at 28 ℃ and 37 ℃, respectively. The aliquots from viruses-infected HepG2 cells were harvested daily for 5 days while the aliquots from viruses-infected C6/36 cells were harvested at 48 h intervals for 12 days. Those aliquots were adjusted to 12.5% heat-inactivated fetal bovine serum before being stored at -80 ℃ prior to titration. The uninfected-HepG2 and the uninfected-C6/36 were performed parallely with the DEN3 infected cells[17]. The infections were performed in duplicate for each sample.
2.6. Virus titration by plaque assay
Plaque titrations were performed in six-well plates of confluent Vero cells as described previously[18]. The cells were infected with 200 μL of a 10-fold serial dilution of each virus. Plaques were counted 8-10 days after infection. Virus concentrations were determined as plaque forming units (PFU) per milliliter. Plaque titrations were performed in duplicate for each sample.
3. Results
3.1. Multiple alignments and the predicted secondary structures of the 5’UTRs
The RT-PCR product of each Thai DEN3 isolate amplified using the specific primers at the 5'end showed a single band of 465 base pairs in length on agarose gel electrophoresis staining with ethidium bromide (Figure 1). The purified amplicon was used as the template for sequencing. Nucleotide sequences of each Thai DEN3 isolate were analyzed from both strands of the pure PCR amplicon comparing to the H87 prototype. The 5'UTRs of all these Thai DEN-3, 72 Bangkok isolates during 1977-2000, showed completely identical sequences of their first 89 nucleotides. In addition, their multiple sequence alignment of 140 nucleotides presented three single nucleotide polymorphisms (SNPs) including C90T (9C/63T: 87.5%), A109G (63A/9G: 12.5%) and A112G (71A/1G: 1.1%). The C90T and the A109G of the isolate D77-0039 were detected in 1977 (DQ863566) and the A112G of the isolate D95-0023 was firstly shown in 1995 (DQ863620) (Figure 2, Table 1). Multiple alignment of Thai DEN3-5'UTRs to the available sequences in GenBank of other DEN3 from different geography (Table 2) demonstrated their highly conserved regions and the following different SNPs as G9A, A55G, G62A, and G65A, were found (Figure 4a). Onerepresentative Thai DEN3 with the predominant C90T (D95-0380) showed its predicted secondary structure of its 5'UTR identical to the H87 prototype and both of them still conserved their structure of two RNA domains as a large stem loop or stem loop A (SLA) and a short stem loop or stem loop B (SLB). Their first domains of~70 nucleotides of SLA were folded to have a Y shaped structure in the presence of three helical regions (S1, S2, and S3), a top loop, and a side stem loop. The two domains were separated by oligo (U) track spacer (Figure 3). Among the other DEN-3 with G9A, A55G, G62A, and G65A (Table 2) only the DEN3 with two SNPs of G9A and G65A (Brazil 2002: AY679147) slightly altered its predicted secondary structure but still conserved almost the same structure as Thai DEN3 and the H87 prototype (Figure 4b).
3.2. Efficiency of Thai DEN3 infection in HepG2 and C6/36 cells
Five Thai DEN3 isolates with the predominant C90T of 5'UTR in combination with their clinical history were chosen to be Thai DEN3 candidates to verify the importance of their 5'UTRs related to viral replication. Replication rates using the HepG2 and C6/36cells of five Thai DEN3 candidates (Table 3) and the H87 prototype were compared based on the hypothesis that the predominant C90T of 5'UTR of these Thai DEN3 candidates may affect their viral replication rates. The supernatant from each virus was collected at 24-h intervals for HepG2 and 48-h for C6/36. The supernatants were used to perform plaque assays in duplicate for each sample at each time point. In HepG2 cells, all the viruses reached peak viral titers on day 3 p.i. (Figure 5a). In C6/36 cells, D87-0010 reached their peak titer on day 6 p.i, while the others replicated to a peak level on day 10 p.i. (Figure 5b). The candidate D87-0010 virus reached its highest titer on day 3 p.i. and the titer suddenly dropped on day 4 p.i., while the others reached their highest titers on day 3 p.i., and gradually declined on day 4 p.i. (Figure 5b). The P-values were calculated on the day of peak viral titers, day 3 and day 10 p.i. for HepG2 (Figure 5a) and C6/36 cells (Figure 5b), respectively. The statistical data comparing to the H87 prototype indicated that all these Thai DEN3 candidates appeared to have significantly higher replication rates (P<0.05) in both host cells than the H87 prototype except the candidate D89-0833, which generated the delayed replication in C6/36 cells. The genetic relationships of their 5'UTR and their replication efficiency in human (HepG2) and the mosquito (C6/36) cell lines did not appear to correlate with their disease severity in human.
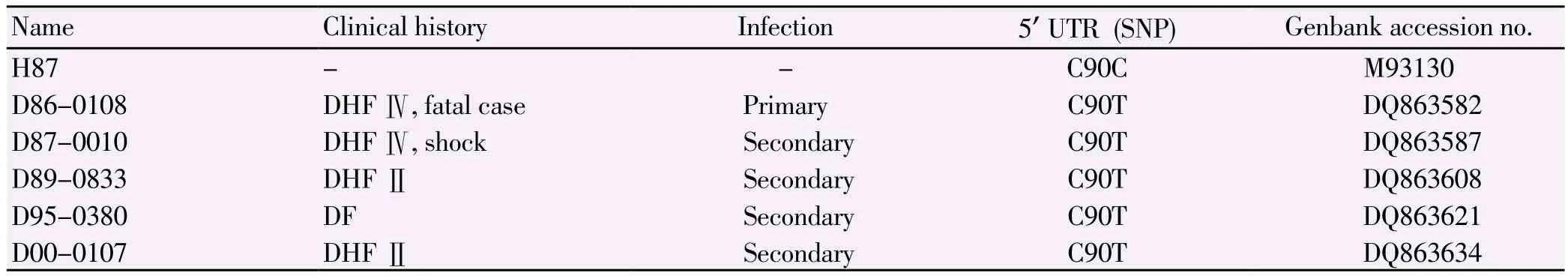
Table 3List of Thai DEN-3 candidates for tissue culture analysis.
4. Discussion
Despite long history of DEN3 associated with severe dengue epidemics, Bangkok has only limited information on this circulating virus: judging by the numbers of publications as well as nucleotide sequences in GenBank. This study on evolutionary relationship of the 5'UTRs indicated that 72 Bangkok isolates of Thai DEN3 continuously kept identical sequences of their first 89 nucleotides during the 24 year-period. The primary sequence conservation of these viruses was greater than 98%, indicating that the mutation rate of DEN3 circulating in Bangkok was not higher than 2% over 24 year-evolution. This observation highlighted the important stability of the first 89 nucleotides of their 5'UTRs. Comparing Thai DEN3 to the other DEN3 from different geography, the same regions of their 5'UTRs were highly conserved. Dengue viruses carry a common type I cap structure and thus exhibit no IRES region. The 94 nucleotides of their 5'UTRs appear to contain conserved secondary structural elements among the same serotype as well as different serotypes that influenced the viral replication, minus-strand RNA synthesis, translation initiation and genome cyclization[19]. Specific deletions within this region in the context of DEN4 showed an important role for the 5'UTR during viral replication[20]. The nucleotides located at position 70 to 80 of DEN 5'UTR became highly susceptible to RNase A cleavage when bound to the viral polymerase[21]. Comparing these Thai DEN3 to the H87 prototype, our finding of three SNPs including the predominant C90T (9C/63T), A109G (63A/9G) and A112G (71A/1G) during 1977-2000 suggested that the first DEN3 outbreak in 1958 followed by many subsequent outbreaks until 2000 in Bangkok might have caused the frequent divergence of Thai DEN3, which were better adapted to grow in the transmission cycle between human and mosquitoes in a particular environment. Different SNPs but not the C90T was found in the 5'UTRs of the other DEN3 isolates from several countries indicating genetic responses of these dengue evolution related to different hosts, mosquito vectors and geographic environments.
Both 5' and 3'UTRs have the potential to form secondary structures that are functional important and conserved among flaviviruses[7,8]. RNA secondary structures have been shown to be very sensitive to mutations: mutations in about 10% of the sequence positions already leads almost surely to unrelated structures if the mutated positions are chosen randomly[22,23]. The predicted 5'UTR secondary structure of a representative Thai DEN-3 (D95-0380) with C90T showed almost identical to the structures of the H87 prototype as well as four DEN3 isolates from other countries. All of them still conserved two RNA domains including SLA of ~70 nucleotides to form a Y shaped structure in the presence of three helical regions (S1, S2, and S3) and SLB of ~16 nucleotides separated by an oligo(U) spacer. The dengue genome is translated in the infected cell by a cap-dependent mechanism. In this process, the small subunit of the ribosome scans through SLA and SLB to reach the initiator AUG codon. Therefore, the finding that SLA plays an important role during viral replication could be attributed to a function of this RNA element in translational regulation and/or in further steps of viral replication[24]. The SLA has been proposed to act as the promoter for the RNA-dependent RNA polymerase (RdRp) of dengue viruses. Direct binding of RdRp to SLA was reported to be necessary for viral RNA synthesis[25,26]. Within dengue 5'UTRs, the S1 and S2 regions represented one of the most conserved elements whereas the sequence and structure of S3 and the side stem loop showed the most variations[9]. The role of each conserved elements within the SLA promoter have been investigated and mutations within the SLA structure in the context of full length viral RNAs allowed the identification of sequences and structures necessary for viral replication[13]. The SLB has been proposed to be essential sequences for long range RNA-RNA interaction and genome replication[25]. The oligo(U) spacer was required for proper function of the two stem loops as previously reported[8,27]. Several reports revealed that genome circularization was required for viral replication and dengue RNA genomes could circularize to regulate initiation of translation and RNA synthesis at the 5' and 3' ends of the genome[8,19]. There was a functionalevidence using in vitro assay supporting that efficient RNA synthesis by RNA-dependent RNA polymerase of dengue virus required both the 5' and 3' cyclization sequences (CS)[28,29]. The new element of 16 nts present at 5' and 3' UTR was identified as 5' upstream AUG region (5'UAR), based on their location just 5' to the initiator AUG. Both CS and 5'UAR were necessary for long range RNA-RNA interactions and RNA cyclization[30]. Although the predominant C90T of Thai DEN3-5'UTR was located at the proposed 5' UAR, this SNP did not affect its predicted secondary structure and its proposed 5'UAR (16nts in the box).
The replication efficiency of five Thai DEN3 candidates with the predominant C90T comparing to the H87 prototype were assessed in the human (HepG2) and mosquito (C6/36) cell lines as models for their replication in human host and the mosquito vector respectively. Growth in C6/36 cells was assessed because the replication kinetic in these cells might be associated with their replication in Aedes mosquitoes, which served as DEN transmission vectors. Replication in HepG2 cells was performed because these cells represented mammalian cells and also served as one target cell of DEN infection. Liver involvement in DEN pathogenesis has been documented[31,32]. Hepatomegaly has been reported in up to 98% of Thai children with DHF[2,33]. Previous studies reported that dengue infection caused apoptosis in liver cells both in vitro and in vivo[32,34]. All these Thai DEN3 candidates appeared to have significant higher replication rates (P<0.05) in both cell types than the H87 prototype except D89-0833, which generated the delayed replication in C6/36 cells. From the history of these Thai DEN3 which produced a degree of disease severity from mild to severe form, relationship of their primary sequences and their predicted secondary structures of the 5'UTRs as well as their replication efficiency in both two cell cultures did not correlate with their clinical outcomes. However, our findings supported their important requirements of the completely identical 89 nucleotides and their high-order secondary structure of the 5'UTR for viral replication. The highly conserved 89 nucleotides of 5'UTR has been proposed to be a candidate magic target for future research on antiviral therapy as well as vaccine approach of Thai DEN3.
In conclusion, our study on evolutionary relationship among Thai DEN3, Bangkok isolates during 24 year-evolution (1977-2000) highlighted that the remarkable 89 nucleotide conservation, the highorder RNA secondary structure and the predominant C90T of their 5'UTRs were found over 24 years of evolution. Therefore, the 89 nucleotides and the C90T of 5'UTR might be genetic markers of evolutionary relationship among these Thai DEN3.
Conflict of interest statement
The authors disclose no conflicts.
Acknowledgements
This work was supported by two research grants of Associate Professor Dr. W. Attatippaholkun: Grant No.493-5600-G-00-3461, Program in Science and Technology Cooperation, Human Capacity Development, Bureau for Global Programs, Field Support and Research, US Agency for International Development, Washington, DC and The Royal Golden Jubilee-Ph.D Program, Thailand Research Fund, Thailand. The authors would like to thank Dr. Bruce L. Innis, Dr. David W.Vaughn, Dr. Mammen P. Mammen, Jr. and Dr. Timothy P. Endy for kindly providing all these Thai DEN3 isolates studied in this project, which were maintained at Department of Virology, The US Army Medical Component, Armed Forces Research Institute of Medical Sciences (USAMC-AFRIMS), Bangkok, Thailand. Cell culture analysis was supported by Professor Dr. David B. Weiner's laboratory, University of Pennsylvania, Philadelphia, PA.
[1] Office of the Permanent Secretary for Public Health. Annual epidemiological surveillance report, Thailand Ministry of Public Health; 2012.
[2] Kalayanarooj S. Clinical manifestation and management of Dengue/ DHF/DSS. Trop Med Health 2011; 39: 83-87.
[3] Nisalak A, Endy T, Nimmannitya S, Kalayanarooj S, Thisayakorn U, Scott R, et al. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailandfrom 1973 to 1999. Am J Trop Med Hyg 2003; 68: 191-202.
[4] Gubler DJ. Dengue/dengue hemorrhagic fever: historical and current status. Novartis Found Symp 2006; 277: 3-16.
[5] Koraka P, William MM, Diamiatun K, Setiati TE, van Batenburg FH, et al. RNA secondary structures in the proximal 3'UTR of Indonesian dengue 1 virus strain. Virus Res 2009; 142: 213-216.
[6] Halstead S. Dengue hemorrhagic fever: two interactions of antibody dependent enhancement, a brief history and personal memoir. Rev Cubana Med Trop 2002; 54: 171-179.
[7] Filomatori CV, Lodeiro MF, Alvarez DE, Samsa MM, Pietrasanta L, et al. A 5'UTR element promotes dengue virus RNA synthesis on a circular genome. Genes Dev 2006; 20: 2238-2249.
[8] Thurner C, Witwer C, Hofacker I, Stadler P. Conserved RNA secondary structures in Flaviviridae genomes. J Gen Virol 2004; 85: 1113-1124.
[9] Simmons CP, Farrar JJ, van Vinh Chau N, Wills B. Dengue. N Eng J Med 2012; 366: 1423-1432.
[10] Thaisonthi S, Rabablert J, Yoksan S. Comparison of full-length genomics sequences between dengue virus serotype 3, parental strain, and its derivative, and B-cell epitopes prediction from envelope region. Bioinformatics 2013; 9: 622-628.
[11] Clyde K, Harris E. RNA secondary structure in the coding region of dengue virus type 2 directs translation start codon selection and is required for viral replication. J Virol 2006; 80: 2170-2182.
[12] Cuceanu NM, Tuplin A, Simmonds P. Evolutionarily conserved RNA secondary structures in coding and non-coding sequences at the 3' end of the hepatitis G virus/GB-virus C genome. J Gen Virol 2001; 82: 713-722.
[13] Tuplin A, Wood J, Evans D, Patel A, Simmonds P. Thermodynamic and phylogenetic prediction of RNA secondary structures in the coding region of hepatitis C virus. RNA 2002; 8: 824-841.
[14] Mishra B, Scharma M, Pujhari SK, Appannanavar SB, Ratho RK. Clinical applicability of single-tube multiplex reverse transcriptase PCR in dengue virus diagnosis and serotyping. J Clin Lab Anal 2011; 25: 76-78.
[15] Attatippaholkun W, Attatippaholkun M, Nisalak A, Vaughn D, Innis B. Highly conserved nucleotide sequence and its deduced amino acids of the 5'-noncoding region and the capsid protein of a Bangkok isolate dengue-3 virus. Southeast Asian J Trop Med Public Health 2000; 31(suppl.1): 119-125.
[16] Zuker M. M fold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 2003; 31: 3406-315.
[17] Pankhong P, Weiner D, Ramanathan M, Nisalak A, Kalayanarooj S, et al. Molecular genetic relationship of the 3' untranaslated region among Thai dengue-3 virus, Bangkok isolates during 1973-2000. DNA Cell Biol 2009; 28: 481-491.
[18] Sithisarn P, Suksanpaisan L, Thepparit C, Smith D. Behavior of the dengue virus in solution. J Med Virol 2003; 71: 532-539.
[19] Khromykh A, Meka H, Guyatt K, Westaway E. Essential role of cyclization sequences in flavivirus RNA replication. J Virol 2001; 75: 6719- 6728.
[20] Sirigulpanit W, Kinney RM, Leardkamolkarn V. Substitution or deletion mutations between nt 54 and 70 in the 5’noncoding region of dengue type2 virus produce variable effects on virus viability. J Gen Virol 2007; 88: 1748-1752.
[21] Filomatori C, Iglesias G, Villordo S, Alvarez D, Gamarnik A. RNA sequences and structures required for the recruitment and activity of the dengue virus polymerase. J Biol Chem 2011; 286: 6929-6939.
[22] Quarrier S, Martin JS, Neulander LD, Beauregard A, Laederach A. Evaluation of the information content of RNA structure mapping data for secondary structure prediction. RNA 2010; 16: 1108-1117.
[23] Sukosd Z, Swenson MS, Kjems J, Heitsch CE. Evaluating the accuracy of SHAPE-directed RNA secondary structure predictions. Nucl Acids Res 2013; 41: 2807-2816.
[24] Lodeiro M, Filomatori C, Gamarnik A. Structural and functional studies of the promoter element for dengue virus RNA replication. J Virol 2009 ; 83 : 993-1008.
[25] Gritsun TS, Gould EA. Origin and evolution of flavivirus 5'UTRs and panhandles: trans-terminal duplications? Virology 2007; 366: 8-15.
[26] Yu L, Nomaguchi M, Padmanabhan R, Markoff L. Specific requirements for elements of the 5' and 3' terminal regions in flavivirus RNA synthesis and replication. Virology 2008; 374: 170-185.
[27] Gebhard L, Filomatori C, Gamarnik A. Functional RNA elements in the dengue virus genome. Viruses 2011; 3: 1739-1756.
[28] Polacek C, Foley JE, Harris E. Conformational changes in the solution of the dengue virus 5' end in the presence and absence of the 3' untranslated region. J Virol 2009; 83: 1161-1166.
[29] You S, Falgout B, Markoff L, Padmanabhan R. In vitro RNA synthesis from exogenous dengue viral RNA templates requires long range interactions between 5'- and 3'-terminal regions that influence RNA structure. J Biol Chem 2001; 276: 15581-15591.
[30] Alvarez D, Lodeiro M, Luduena S, Pietrasanta L, Gamarnik A. Longrange RNA-RNA interactions circularize the dengue virus genome. J Virol 2005; 79: 6631-6643.
[31] Seneviratne SL, Malavige GN, Silva HJ. Pathogenesis of liver involvement during dengue viral infections. Transactions Royal Soc Trop Med Hyg 2006; 100: 608-614.
[32] Huerre MR, Lan NT, Marianneau P, Hue NB, Khun H, et al. Liver histopathology and biological correlates in five cases of fatal dengue fever in Vietnamese children. Virchows Arch 2001; 438: 103-115.
[33] Wichmann O, Hongsiriwon S, Bowonwatanuwong C, Chotivanich K, Sukthana Y, Pukrittayakamee S. Risk factors and clinical features associated with severe dengue infection in adults and children during the 2001 epidemic in Chonburi, Thailand. Trop Med Int Health 2004; 9: 1022-1029.
[34] Thepparit C, Khakpoor A, Khongwichit S, Wikan N, Fongsaran C, et al. Dengue 2 infection of HepG2 liver cells results in endoplasmic reticulum stress and induction of multiple pathways of cell death. BMC Res Notes 2013; 6: 372-385.
ent heading
10.1016/S1995-7645(14)60311-4
*Corresponding author: Watcharee Attatippaholkun, Department of Clinical Chemistry, Faculty of Medical Technology, Mahidol University, Bangkok 10700, Thailand.
Tel: +66 2 4197168
Fax: +66 2 412 4110
E-mail: mtwap@mahidol.ac.th
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Afebrile presentation of 2014 Western Africa Ebolavirus infection: the thing that should not be forgotten
- Dengue in pregnancy: an under-reported illness, with special reference to other existing co-infections
- Relevance of EGFR gene mutation with pathological features and prognosis in patients with non-small-cell lung carcinoma
- Influence of artificial luminous environment and TCM intervention on development of myopia rabbits
- MicroRNA-126 inhibits the proliferation of lung cancer cell line A549
- Expression and significance of netrin-1 and its receptor UNC5C in precocious puberty female rat hypothalamus
