JPA Prize in 2014
2015-11-17
JPA Prize in 2014
The outstanding editor awarded the 2014 JPA Prize was as follows:
Bart De Spiegeleer
Department of Pharmaceutical Analysis
Drug Quality & Registration group
Ghent University
Belgium
The two excellent referees awarded the 2014 JPA Prize were as follows:
1. Stacy Brown
Pharmaceutical Sciences
East Tennessee State University
United States of America
2. Satyanarayanaraju Sagi
School of Pharmacy
National Center for Natural Products Research
University of Mississippi
United States of America
The three top cited papers awarded the 2014 JPA Prize were as follows:
1. Joseph J. Kirkland, Stephanie A. Schuster*, William L. Johnson, Barry E. Boyes. Fused-core particle technology in high-performance liquid chromatography: An overview. Journal of Pharmaceutical Analysis, 2013, 3(5): 303-312.
2. Srinivasa Rao Polagani, Nageswara Rao Pilli,Ramakrishna Gajula, Venkateswarlu Gandu*.
Simultaneous determination of atorvastatin, metformin and glimepiride in human plasma by LC-MS/MS and its application to a human pharmacokinetic study. Journal of Pharmaceutical Analysis, 2013, 3(1): 9-19.
3. T. Satyanarayana Raju*, O. Vishweshwari Kutty,V. Ganesh, P. Yadagiri Swamy. A validated stabilityindica
ting LC method for the separation of enantiomer and potential impurities of Linezolid using polar organic mode. Journal of Pharmaceutical Analysis, 2012, 2(4): 272-278.
JPA Prize Medal

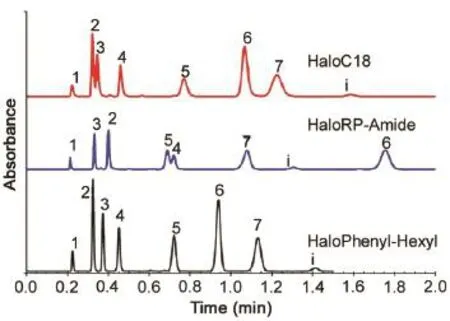
Fig. 2 Separation of anticoagulants with fused-core columns of different selectivities. Columns: 50 mm × 4.6 mm Halo C18, 50 mm × 4.6 mm Halo RP-amide, 50 mm × 4.6 mm Halo phenyl-hexyl; mobile phase: A/B = 40/60; A = 0.1% formic acid, pH 2.6; B = methanol/acetonitrile,50/50; flowrate: 2.0 mL/min; temperature: 45 ºC; pressure: 210 bar;detector, UV: 254 nm; 1 μL injection; andsolutes: (1) uracil, (2)4-hydroxycoumarin, (3) coumarin, (4) 6-chloro-4-hydroxycoumarin, (5)warfarin, (6) coumatetralyl, and (7) coumachlor, i = impurity.
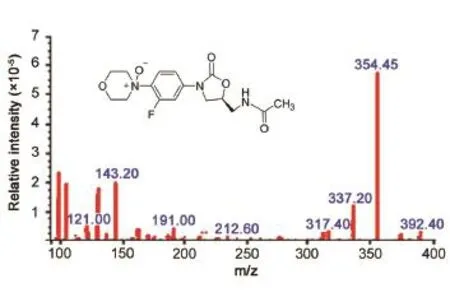
Fig. 3 Complete degradation profile of Linezolid along with the % impurity formation and corresponding mass nos. of impurities.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Application of analytical instruments in pharmaceutical analysis
- Simultaneous quantitation of folic acid and 5-methyltetrahydrofolic acid in human plasma by HPLC-MS/MS and its application to a pharmacokinetic study☆
- Identification,synthesis and characterization of process related impurities of benidipine hydrochloride,stress-testing/stability studies and HPLC/UPLC method validations☆
- Multi-spectroscopic investigation of the binding interaction of fosfomycin with bovine serum albumin☆
- Simultaneous determination of four Sudan dyes in rat blood by UFLC-MS/MS and its application to a pharmacokinetic study in rats☆
- Fabrication of multiwalled carbon nanotube-surfactant modified sensor for the direct determination of toxic drug 4-aminoantipyrine☆
