Concentration and Chemical Speciation of K,Na,Ca,Mg and Sr in Sediment from a Upstream of the Pearl River,China
2015-11-08SilinYANGDaweiWANGLiqiongTANGBinYANG
Silin YANG,Dawei WANG,Liqiong TANG,Bin YANG*
1.Faculty of Environmental Science&Engineering,Southwest Forestry University,Kunming 650224,China;
2.Faculty of Life Science,Southwest Forestry University,Kunming 650224,China
Concentration and Chemical Speciation of K,Na,Ca,Mg and Sr in Sediment from a Upstream of the Pearl River,China
Silin YANG1,Dawei WANG2,Liqiong TANG1,Bin YANG2*
1.Faculty of Environmental Science&Engineering,Southwest Forestry University,Kunming 650224,China;
2.Faculty of Life Science,Southwest Forestry University,Kunming 650224,China
The concentration and chemical speciation of metal elements (K,Na,Ca,Mg and Sr)in sediments were investigated from upstream of the Pearl River,China.The result showed that Total concentrations of metal elements in sediments were in wide range,the ranges of K,Na,Ca,Mg and Sr were 3 016-12 742,135-565,6 794-12 968,2 060-6 229 and 4.01-35.4 mg/kg in sediments.K was dominated by the residual fraction,Sr was dominated by weak acid soluble fraction. The bioavailability of Sr in the area is higher than K.The order of bioavailability was Sr>Na>Ca>Mg>K.
Metal elements;Speciation;Sediments;Pearl River
P earl River Basin(102°14′-115° 53′E,21°31′-26°49′N)is a complex basin,which comprises Xijiang,Beijiang,Dongjiang and Pearl River Delta rivers.This area covers less than 5%of the total land area in China,but its GDP accounts for 13%of the total of China.Thus,it plays a rather important role in Chinese economic development.In recent years,with the rapid industrial and agricultural development,together with the speeding up of urbanization,the eco-environment has been worsening,along with the worsening pollution in Pearl River Basin,especially metal pollution,which has become an important factor restricting social sustainable development.Therefore much research has been conducted on heavy metals and organic pollutants in sediments from Pearl River catchments[1-5]. However,the study on sedimentary characteristics of alkali metals and alkaline earth metals in sediments is insufficient.The concentration and distribution of the alkali metals and alkaline earth metals in river sediments not only reflected their deposition process and hydrodynamic characteristics of the river,but also impacted on the distribution and mobility of heavy metals and other pollutants in the river[6].In this paper,the total concentrations and chemical speciation of metal elements(K,Na,Ca,Mg and Sr)were investigated in sediments from upstream of the Pearl River.This study will provide fundamental information for understanding geochemical characteristics of the alkali metals and alkaline earth metals.
Materials and Methods
Sample collection and preparation
The samples area is located at Nanpan River(25°24′-25°46′N,103° 51′-103°55′E)the upstream of Pearl River.Twelve sites were set along Nanpan River(A1-A12)and were indicated in Fig.1 in our previous study[5]. Sediment samples of 3 random points with 5 m away from sample sites were collected using a grab sampler and mixed as one sample.The sediments were collected only with depth of 0-10 cm and about 1 kg were collected for each sample from November 12 to 13 in 2011.The collected sediment samples were put into clean polyethylene sample bags,labeled and then stored in the lab.The sediments were dried at room temperature,grilled into pieces with a wood stick,and then filtered with a 2-mm nylon sieve.The gravels andplantresiduesweremanually picked out.The sediments were furthergrounded and filtered with a 0.149-mm nylon sieve for the analysis of total metal elements concentrations and the metal elements fractions.
Sample analysis
Sediment pH and TOC were determined as described in our previous study[7].The sediment particles were digested using HCl-HNO3-HClO4and total concentrations of the metal elements in sediments were determined using ICP-OES(VISFA-MPX).
Sediment samples(0.5 g)were introduced in a Teflon crucible (50 ml)containing 15 mL of aqua regia.The solutions were heated on a hot plate at 140-160℃ for about 10 min.After that,5 ml of HClO4were added to the crucible,and heated until complete evaporation of the solution. The residues were dissolved in 10 ml 5% HNO3.The solutions were then transferred to volumetric polypropylene tubes (50 ml)and diluted to 50 ml. Quantification of metal elements concentrations in these solutions stands for total metal concentrations.
The metal elements in all the sediment samples were also fractionated(weak acid soluble,reducible,oxidiz-able and residual fractions)according to the sequential extraction procedure modified by Bureau Communautaire de Référence(BCR)[8-9].
Statistic analysis
The correlation matrix of five elements total concentrations in sediments were analyzed by bivariate correlation using the Pearson coefficient in a two-tailed test,P≤0.05.All statistical analyses were performed using SPSS13.0 software.
Results and Discussions
Total concentration and distribution of the metal elements
The statistical summary of total concentration parameters for the metal elements in sediments were given in Table 1.Total concentrations of metal elements in sediments were in wide range,the ranges of K,Na,Ca,Mg and Sr were 3 016-12 742,135-565,6 794-12 968,2 060-6 229 and 4.01-35.4 mg/kg in sediments.The average K,Na,Ca and Mg concentrations were significantly lower than those of other large rivers in China such as the Yangtze River(K,21 000;Na,9 200;Ca,33 000 and Mg,16 900 mg/kg)and the Yellow River(K,18300;Na,17 100;Ca,36 900 and Mg,9 900 mg/kg)[10-11].Inter-element relationships can provide interesting information on element sources and pathways.The correlation data of the sediments were given in Table 2.The data showed that there were significantly positive correlations among K,Na,Ca,Mg and Sr in sediments,this result suggests that their sources were possible common,and mainly inherited from parent materials.
The relationship between total K,Na,Ca,Mg and Sr concentrations and TOC were evaluated by correlation analysis(Table 3).The content of TOC was significantly positively correlated with the total concentration of the five metals,thisphenomenon indicates that these five metals can easily complex with organic matters.
The metals elements speciation
The fractions of K,Na,Ca,Mg and Sr in sediments expressed as percentages of the sum of individual fractions were shown in Fig.1.The percentages for weak acid soluble,reducible,oxidizable and residual fractions were noted as f1,f2,f3and f4,respectively.The distribution patterns varied among the five metal elements,and were similar for a certain element among most of sample sites.K in most of the samples,residual fractions(f4)accounted for more than 85%of their total concentrations,f1 of Sr were about 80%for most of the samples.K in most of the samples,weak acid soluble fraction(f1)was less than 15%of their total concentrations,weak acid soluble fraction (f1)of Sr were more than 85%.The higher metal percentages of weak acid soluble fraction could result in greater metal bioavailability in sediments. Thus, the bioavailability of Sr in the area is higher than K.The order of bioavailability was Sr>Na>Ca>Mg>K.
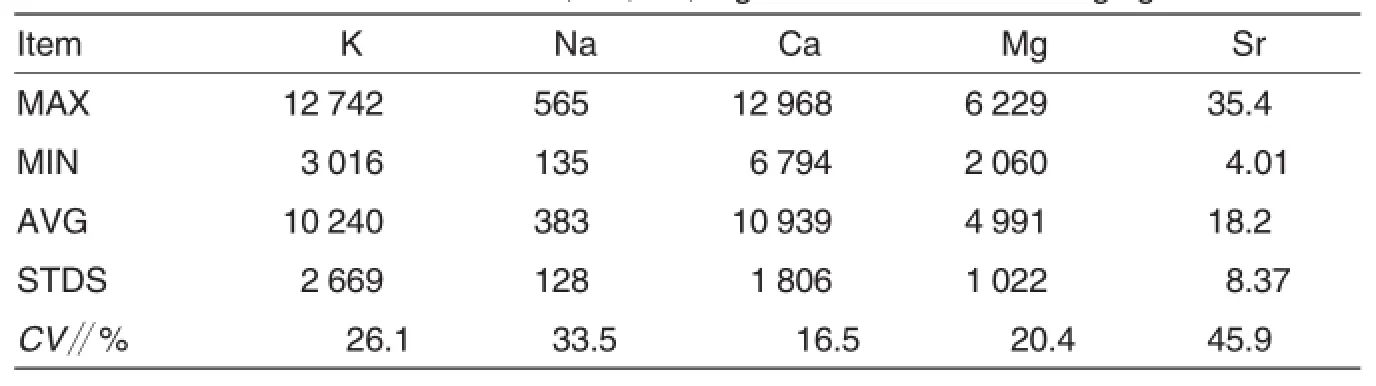
Table 1 The total concentrations of K,Na,Ca,Mg and Sr in sediments mg/kg

Table 2 Correlation matrixes for total K,Na,Ca,Mg and Sr concentrations in sediments
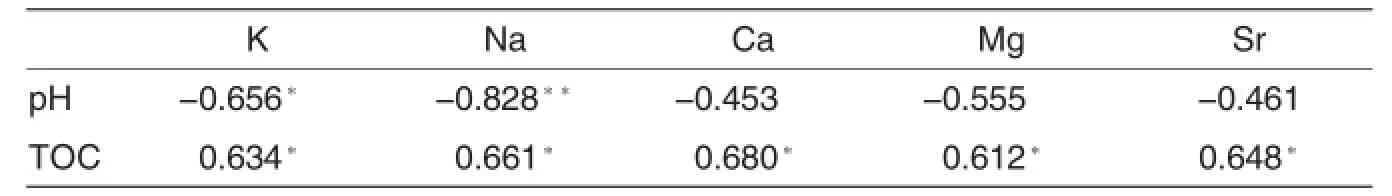
Table 3 Correlation between total K,Na,Ca,Mg and Sr concentrations and pH and TOC in sediments
Conclusions
Total concentrations of metal elements in sediments were in wide range,the ranges of K,Na,Ca,Mg and Sr were 3 016-12 742,135-565,6 794-12 968,2 060-6 229 and 4.01-35.4 mg/kg in sediments.K was dominated by the residual fraction,Sr was dominated by weak acid soluble fraction.The bioavailability of Sr in the area is higher than K.The order of bioavailability was Sr>Na>Ca>Mg>K.
[1]LI Q,WU Z,CHU B,et al.Heavy metals in coastal wetland sediments of the Pearl River Estuary,China[J].Environmental Pollution 2007,149:158-164.
[2]GU JG,HAN B,DUAN S.Initial transformation step of dicamba by a sulfatereducing consortium enriched from sediment of the Pearl River of China[J]. International Biodeterioration & Biodegradation 2008,62:455-459.
[3]NIU H,DENG W,WU Q,et al.Potential toxic risk of heavy metals from sediment of the Pearl River in South China[J]. JournalofEnvironmentalSciences 2009,21:1053-1058.
[4]YANG W,LAI Z,WEI T.Pollution and ecological hazard evaluation of sediment heavy metals in Pearl River Estuary[J].Journal of Zhejiang Ocean University (Natural Science)2009,28: 188-191.
[5]YE F,HUANG X,ZHANG D,et al.Distribution of heavy metals in sediments of the Pearl River Estuary,Southern China:Implications for sources and historical changes[J].Journal of Environmental Sciences 2012,24:579-588.
[6]XIAOTONG P,HUAIYANG Z,HUANXIN W,et al.Characteristics of major elements’constitutions and distributions in sediments of the Lingdingyang in the Pearl River Estuary and their geochemical implication[J].Journal of Zhejiang University(Science Edition)2003,30:697-702.
[7]YANG S,ZHOU D,YU H,et al.Distribution and speciation of metals(Cu,Zn,Cd,and Pb)in agricultural and nonagricultural soils near a stream upriver from the Pearl River,China[J].Environmental Pollution 2013,177:64-70.
[8]RAURET G,LOPEZ-SANCHEZ J,SAHUQUILLO A,et al.Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials[J].J Environ Monit 1999,1:57-61.
[9]URE A,QUEVAUVILLER P,MUNTAU H,et al.Speciation of heavy metals in soils and sediments.An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities[J].International journal of environmental analytical chemistry 1993,51:135-151.
[10]YANG S,JUNG H,LI C,et al.Major element geochemistry of sediments from Chinese and Kaorean rivers[J]. Geochimica 2004,33:99-105.
[11]YANG S,LI C,LI X,WANG A.Geochemical records of chemical weathering of the Xiashu Loess in the lower reches of the Changjiang River[J]. Geochimica 2001,30:402-406.
Responsible editor:Yong XU
Responsible proofreader:Xiaoyan WU
This Study was Supported by the Educational Commission of Yunnan Province of China(2013Y119),the Key Scientific Research Foundation of Southwest Forestry University(111417).
*Corresponding author.E-mail:swfcysl@126.com
Received:September 25,2015 Accepted:November 27,2015
杂志排行
Agricultural Science & Technology的其它文章
- Study on Engineering Characteristics and Application of Sticky Rice
- Periodical Development Trend of Vertical Greening
- Advances in Microbial Remediation on the Application of Heavy Metal Pollution in Agricultural Water Resources
- Analysis on Status quo and Future Development of Fruit and Vegetable Protreatment
- Effect of Three Treatment Measures on Harmless Seedling Raising of Pinus sylvestris var. mongolica Litv.
- Discussions and Recommendations for Supervision of Vegetable Quality and Safety in Miyun County
